Outcomes of a Near-Zero Fluoroscopy and Minimally Invasive Approach in Ablation of Right Free Wall Accessory Pathways in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Three-Dimensional Electro-Anatomical Mapping (EAM) and Electrophysiological Study (EPS)
2.3. TC Ablation Procedure
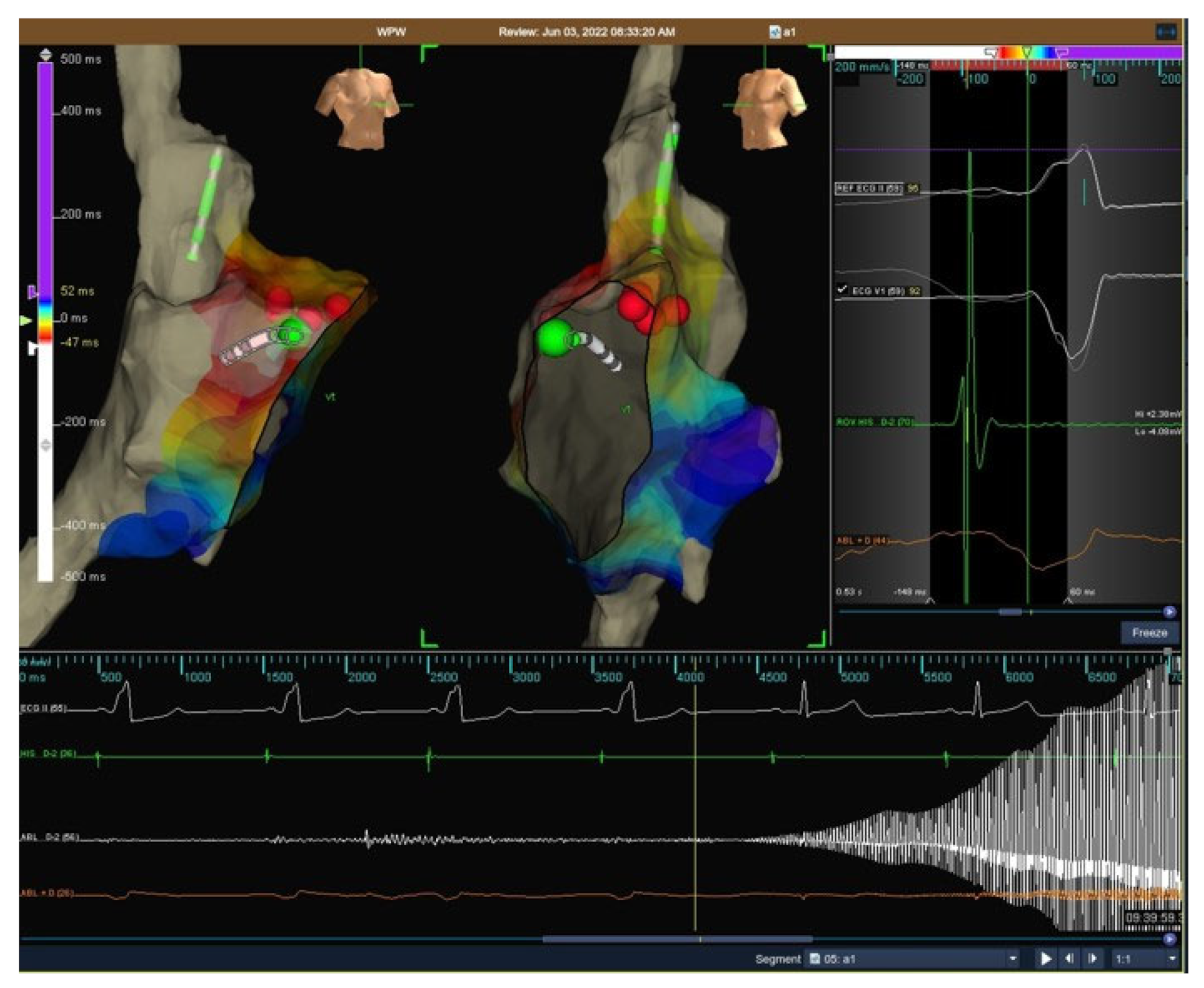
- In manifest APs, during cryomapping, the tip temperature was progressively reduced to –30 °C or step-by-step by 10 °C every 10 s. Cryomapping was considered positive when loss of ventricular pre-excitation was observed and cryoablation was delivered to create a permanent irreversible lesion (−75/80 °C for 480 s) (see Figure 1).
- In concealed APs, step-by-step cryomapping was performed at this site during tachycardia, and if a sudden retrograde interruption of AVRT was observed, cryoablation was performed to create a permanent lesion.
2.4. Post-Ablation Assessment and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Acute Results
3.2. Post-Procedural Follow-Up
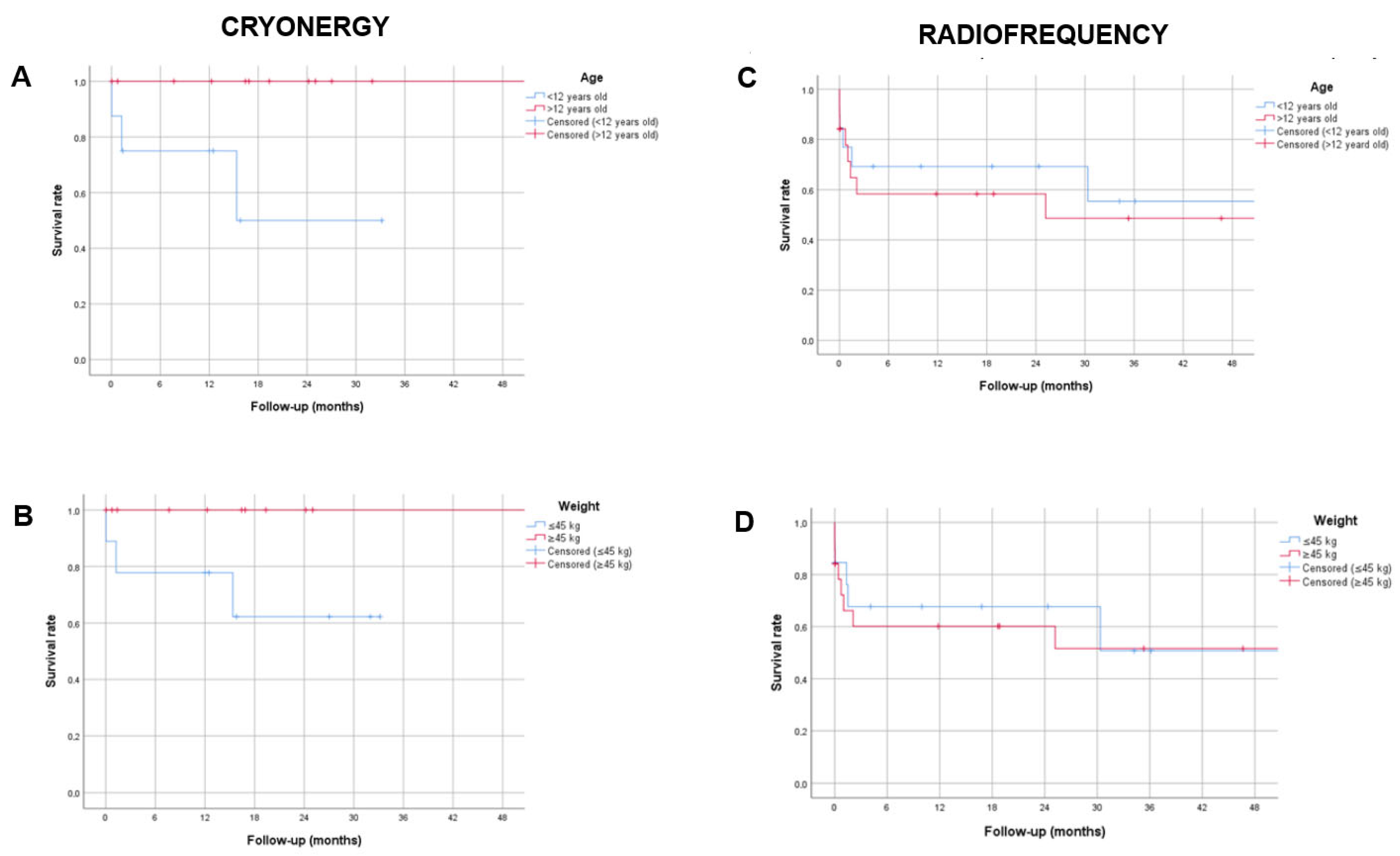
3.3. Outcomes of Patients with Recurrences
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, M.T.; Kiani, S.; Black, G.B.; Lu, M.L.R.; Bhatia, N.; Lloyd, M.; Shah, A.; Westerman, S.; Merchant, F.M.; El-Chami, M.F. Outcomes Of Manifest Right Free Wall Accessory Pathway Ablation: Data from a Single Center. J. Atr. Fibrillation 2021, 14, 20200462. [Google Scholar] [CrossRef]
- Telishevska, M.; Hebe, J.; Paul, T.; Nürnberg, J.H.; Krause, U.; Gebauer, R.; Gass, M.; Balmer, C.; Berger, F.; Molatta, S.; et al. Catheter ablation in ASymptomatic PEDiatric patients with ventricular preexcitation: Results from the multicenter ‘CASPED’ study. Clin. Res. Cardiol. 2019, 108, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Seslar, S.P.; Kugler, J.; Batra, A.S.; Collins, K.K.; Crosson, J.; Dubin, A.M.; Etheridge, S.; Kanter, R.; Papez, A.; Radbill, A.E.; et al. The Multicenter Pediatric and Adult Congenital EP Quality (MAP-IT) Initiative-Rationale and Design: Report from the Pediatric and Congenital Electrophysiology Society’s MAP-IT Taskforce. Congenit. Heart Dis. 2013, 8, 381–392. [Google Scholar] [CrossRef]
- Behjati Ardakani, M.; Dehghani, F.; Sarebanhassanabadi, M.; Yalameh, A.; Behjat, M.; Behjati Ardakani, M.; Shafiee, M.; Seyed Hosseini, S.-M. Impact of Accessory Pathway Location on Electrophysiologic Characteristics and Ablation Success. Crit. Pathw. Cardiol. A J. Evid. Based Med. 2020, 19, 94–97. [Google Scholar] [CrossRef]
- Jan, M.; Kalinšek, T.P.; Štublar, J.; Jelenc, M.; Pernat, A.; Žižek, D.; Lakič, N. Intra-cardiac ultrasound guided approach for catheter ablation of typical right free wall accessory pathways. BMC Cardiovasc. Disord. 2020, 20, 210. [Google Scholar] [CrossRef]
- Kaltman, J.R.; Tanel, R.E.; Wegrzynowicz, B.; Kozodoy, E.; Wieand, T.; Ennis, J.; Vetter, V.L.; Shah, M.J. Time and Temperature Profile of Catheter Cryoablation of Right Septal and Free Wall Accessory Pathways in Children. J. Cardiovasc. Electrophysiol. 2008, 19, 343–347. [Google Scholar] [CrossRef]
- Przybylski, R.; DeWitt, E.S.; Meziab, O.; Gauvreau, K.; Dionne, A.; O’Leary, E.T.; Alexander, M.E.; Walsh, E.P.; Mah, D.Y. Retroflexed catheter course reduces the risk of right free wall accessory pathway recurrence. J. Cardiovasc. Electrophysiol. 2023, 34, 1828–1834. [Google Scholar] [CrossRef]
- Krause, U.; Paul, T.; Della Bella, P.; Gulletta, S.; Gebauer, R.A.; Paech, C.; Kubus, P.; Janousek, J.; Ferrari, P.; De Filippo, P. Pediatric catheter ablation at the beginning of the 21st century: Results from the European Multicenter Pediatric Catheter Ablation Registry ‘EUROPA’. EP Eur. 2021, 23, 431–440. [Google Scholar] [CrossRef]
- Drago, F.; Flore, F.; Tamborrino, P.P.; Silvetti, M.S.; Maiolo, S.; Raponi, M. Trans-jugular approach for safe and successful cryoablation of para-Hisian/anterior-septal, anterior, and anterior-lateral accessory pathways in children. J. Interv. Card. Electrophysiol. 2024, 67, 1771–1780. [Google Scholar] [CrossRef]
- Park, J.K.; Halperin, B.D.; McAnulty, J.H.; Kron, J.; Silka, M.J. Comparison of radiofrequency catheter ablation procedures in children, adolescents, and adults and the impact of accessory pathway location. Am. J. Cardiol. 1994, 74, 786–789. [Google Scholar] [CrossRef]
- Van Hare, G.F.; Javitz, H.; Carmelli, D.; Saul, J.P.; Tanel, R.E.; Fischbach, P.S.; Schaffer, M.; Dunnigan, A.; Colan, S. Prospective assessment after pediatric cardiac ablation: Recurrence at 1 year after initially successful ablation of supraventricular tachycardia. Heart Rhythm 2004, 1, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Battipaglia, I.; Tamborrino, P.P.; Porco, L.; Calvieri, C.; Russo, M.S.; Pazzano, V.; Remoli, R.; Silvetti, M.S. 3D Non-Fluoroscopic Cryoablation of Right-Sided Accessory Pathways in Children: Monocentric Study and Literature Review. Congenit. Heart Dis. 2021, 16, 561–572. [Google Scholar] [CrossRef]
- Drago, F.; Silvetti, M.S.; DI Pino, A.; Grutter, G.; Bevilacqua, M.; Leibovich, S. Exclusion of Fluoroscopy During Ablation Treatment of Right Accessory Pathway in Children. J. Cardiovasc. Electrophysiol. 2002, 13, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Russo, M.S.; Marazzi, R.; Salerno-Uriarte, J.A.; Silvetti, M.S.; De Ponti, R. Atrial tachycardias in patients with congenital heart disease: A minimally invasive simplified approach in the use of three-dimensional electroanatomic mapping. Europace 2011, 13, 689–695. [Google Scholar] [CrossRef]
- Katritsis, D.G.; Boriani, G.; Cosio, F.G.; Hindricks, G.; Jaïs, P.; Josephson, M.E.; Keegan, R.; Kim, Y.-H.; Knight, B.P.; Kuck, K.-H.; et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). EP Eur. 2017, 19, 465–511. [Google Scholar] [CrossRef]
- Brugada, J.; Katritsis, D.G.; Arbelo, E.; Arribas, F.; Bax, J.J.; Blomström-Lundqvist, C.; Calkins, H.; Corrado, D.; Deftereos, S.G.; Diller, G.-P.; et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 655–720. [Google Scholar] [CrossRef]
- Saul, J.P.; Kanter, R.J.; Writing Committee; Abrams, D.; Asirvatham, S.; Bar-Cohen, Y.; Blaufox, A.D.; Cannon, B.; Clark, J.; Dick, M.; et al. PACES/HRS expert consensus statement on the use of catheter ablation in children and patients with congenital heart disease. Heart Rhythm 2016, 13, e251–e289. [Google Scholar] [CrossRef]
- Drago, F. Paediatric catheter cryoablation: Techniques, successes and failures. Curr. Opin. Cardiol. 2008, 23, 81–84. [Google Scholar] [CrossRef]
- Dubin, A.M.; Jorgensen, N.W.; Radbill, A.E.; Bradley, D.J.; Silva, J.N.; Tsao, S.; Kanter, R.J.; Tanel, R.E.; Trivedi, B.; Young, M.-L.; et al. What have we learned in the last 20 years? A comparison of a modern era pediatric and congenital catheter ablation registry to previous pediatric ablation registries. Heart Rhythm 2019, 16, 57–63. [Google Scholar] [CrossRef]
- Papagiannis, J.; Avramidis, D.; Alexopoulos, C.; Kirvassilis, G. Radiofrequency Ablation of Accessory Pathways in Children and Congenital Heart Disease Patients: Impact of a Nonfluoroscopic Navigation System. Pacing Clin. Electrophysiol. 2011, 34, 1288–1396. [Google Scholar] [CrossRef]
- Backhoff, D.; Klehs, S.; Müller, M.J.; Schneider, H.E.; Dieks, J.-K.; Paul, T.; Krause, U. Long-Term Follow-Up After Radiofrequency Catheter Ablation of Accessory Atrioventricular Pathways in Children. JACC Clin. Electrophysiol. 2018, 4, 448–455. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Puel, V.; Bekheit, S.; Dartigues, J.-F.; Egloff, P.; Metayer, T.L.E.; Clementy, J.; Warin, J.-F. Catheter ablation of accessory pathways in children. Eur. Heart J. 1994, 15, 200–205. [Google Scholar] [CrossRef]
- Morady, F.; Strickberger, S.A.; Man, K.C.; Daoud, E.; Niebauer, M.; Goyal, R.; Harvey, M.; Bogun, F. Reasons for prolonged or failed attempts at radiofrequency catheter ablation of accessory pathways. J. Am. Coll. Cardiol. 1996, 27, 683–689. [Google Scholar] [CrossRef]
- Corcia, M.C.G.; Stuart, G.; Walsh, M.; Radulescu, C.; Spera, F.; Tijskens, M.; Heidbuchel, H.; Sarkozy, A. Redo accessory pathway ablation in the pediatric population. J. Interv. Card. Electrophysiol. 2022, 63, 639–649. [Google Scholar] [CrossRef]
- Young, C.; Kwan, A.; Yepez, L.; McCarty, M.; Chan, A.; Hsu, D.; Han, J.; Taneja, T.; Park, S.; Hayward, R.; et al. Contemporary procedure characteristics and outcomes of accessory atrioventricular pathway ablations in an integrated community-based health care system using a tiered approach. BMC Cardiovasc. Disord. 2021, 21, 319. [Google Scholar] [CrossRef]
- Li, M.-M.; Li, J.-Y.; Sang, C.-H.; Jiang, C.-X.; Guo, X.-Y.; Zhao, X.; Li, S.-N.; Wang, W.; Tang, R.-B.; Long, D.-Y.; et al. Right free-wall accessory pathway with branched atrial insertions: Clinical, electrocardiographic, and electrophysiological characteristics. Heart Rhythm 2020, 17, 243–249. [Google Scholar] [CrossRef]
- Desai, V.C.A.; Kelton, C.M.L.; Czosek, R.J.; Heaton, P.C. Frequencies, Costs, and Complications of Catheter Ablation for Tachyarrhythmias in Children: 2000–2009. Pacing Clin. Electrophysiol. 2013, 36, 1468–1480. [Google Scholar] [CrossRef] [PubMed]
- Otomo, K.; Gonzalez, M.D.; Beckman, K.J.; Nakagawa, H.; Becker, A.E.; Shah, N.; Matsudaira, K.; Wang, Z.; Lazzara, R.; Jackman, W.M. Reversing the Direction of Paced Ventricular and Atrial Wavefronts Reveals an Oblique Course in Accessory AV Pathways and Improves Localization for Catheter Ablation. Circulation 2001, 104, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Kothari, S.; Gupta, A.K.; Lokhandwala, Y.Y.; Vora, A.M.; Kerkar, P.G.; Thakur, R.K. Atriofascicular Pathways: Where to Ablate? Pacing Clin. Electrophysiol. 2006, 29, 1226–1233. [Google Scholar] [CrossRef]
- Ergül, Y.; Özgür, S.; Şahin, G.T.; Kafalı, H.C.; Çelebi, S.B.; Bay, B.; Güzeltaş, A. The Transjugular Approach: An Alternative Route to Improve Ablation Success in Right Anteriorly and Anterolaterally-Located Supraventricular Tachycardia Substrates in Children. Pediatr. Cardiol. 2019, 40, 477–482. [Google Scholar] [CrossRef] [PubMed]

| Total Population N | Acutely Not Effective Ablation N (%) | Acutely Effective Ablation N (%) | p | |
|---|---|---|---|---|
| Gender | 1.000 | |||
| Female, n (%) | 36 | 6 (16.7) | 30 (83.3) | |
| Male, n (%) | 26 | 4 (15.4) | 22 (84.6) | |
| Age | 0.036 | |||
| <12 years old, n (%) | 29 | 8 (27.6) | 21 (72.4) | |
| >12 years old, n (%) | 33 | 2 (6.1) | 31 (93.9) | |
| Weight | 0.326 | |||
| ≤45 kg, n (%) | 28 | 6 (21.4) | 22 (78.6) | |
| ≥45 kg, n (%) | 34 | 4 (11.8) | 30 (88.2) | |
| Heart disease | 1.000 | |||
| Yes, n (%) | 6 | 1 (16.7) | 5 (83.3) | |
| No, n (%) | 56 | 9 (16.1) | 47 (83.9) | |
| VP | 0.431 | |||
| Concealed, n (%) | 14 | 1 (7.1) | 13 (92.9) | |
| Manifest, n (%) | 48 | 9 (18.7) | 39 (81.3) | |
| AP localization | 0.014 | |||
| RL, n (%) | 28 | 3 (10.7) | 25 (89.3) | |
| RAL, n (%) | 19 | 1 (5.3) | 18 (94.7) | |
| RPL, n (%) | 15 | 6 (40.0) | 9 (60.0) | |
| Palpitations | 0.052 | |||
| Yes, n (%) | 46 | 10 (21.7) | 36 (78.3) | |
| No, n (%) | 16 | 0 (0) | 16 (100) | |
| Therapy (before ablation) | 0.036 | |||
| Yes, n (%) | 29 | 8 (27.6) | 21 (72.4) | |
| No, n (%) | 33 | 2 (6.1) | 31 (93.9) | |
| Type of ablation | 0.506 | |||
| RF ablation, n (%) | 37 | 5 (13.5) | 32 (86.5) | |
| Cryoablation, n (%) | 25 | 5 (20.0) | 20 (80.0) | |
| Venous access | 0.185 | |||
| Femoral, n (%) | 51 | 10 (19.6) | 41 (80.4) | |
| Jugular, n (%) | 11 | 0 (0) | 11 (100) |
| Total Population (N = 62) | Cryoablation (N = 25) | Ablation with RF (N = 37) | p | |
|---|---|---|---|---|
| Gender | 0.068 | |||
| Male, n (%) | 26 (41.9) | 7 (28.0) | 19 (51.4) | |
| Female, n (%) | 36 (58.1) | 18 (72.0) | 18 (48.6) | |
| Age (y), mean (±SD) | 12.3 (±2.8) | 12.5 (±2.7) | 12.2 (±2.9) | 0.742 |
| Weight (kg), mean (±SD) | 50.3 (±16.1) | 53.8 (±20.2) | 47.9 (±12.4) | 0.201 |
| Height (cm), mean (±SD) | 155.24 (±14.3) | 156.4 (±14.7) | 154.4 (±14.1) | 0.592 |
| BSA (m2), mean (±SD) | 1.5 (±0.3) | 1.5 (±0.3) | 1.4 (±0.2) | 0.275 |
| Age | 0.874 | |||
| <12 years old, n (%) | 29 (46.8) | 12 (48.0) | 17 (45.9) | |
| >12 years old, n (%) | 33 (53.2) | 13 (52.0) | 20 (54.1) | |
| Weight | 0.880 | |||
| (45 kg, n (%) | 28 (45.2) | 11 (44.0) | 17 (45.9) | |
| (45 kg, n (%) | 34 (54.8) | 14 (56.0) | 20 (54.1) | |
| Heart disease | 1.000 | |||
| Yes, n (%) | 6 (9.7) | 2 (8.0) | 4 (10.8) | |
| No, n (%) | 56 (90.3) | 23 (92.0) | 33 (89.2) | |
| Accessory pathway | 0.031 | |||
| Concealed, n (%) | 14 (22.6) | 2 (8.0) | 12 (32.4) | |
| Manifest, n (%) | 48 (77.4) | 23 (92.0) | 25 (67.6) | |
| Right accessory pathway localization | 0.004 | |||
| Lateral, n (%) | 28 (45.2) | 10 (40.0) | 18 (48.6) | |
| Anterior–lateral, n (%) | 19 (30.6) | 13 (52.0) | 6 (16.2) | |
| Posterior–lateral, n (%) | 15 (24.2) | 2 (8.0) | 13 (35.1) | |
| Multiple accessory pathways | 0.678 | |||
| Yes, n (%) | 6 (9.7) | 3 (12.0) | 3 (8.1) | |
| No, n (%) | 56 (90.3) | 22 (88.0) | 34 (91.9) | |
| Therapy before ablation | 0.338 | |||
| Yes, n (%) | 29 (46.8) | 13 (52.0) | 16 (43.2) | |
| No, n (%) | 33 (53.2) | 12 (48.0) | 21 (56.8) | |
| Duration of ablation (hours), median (IQR) | 3.0 (2.0–3.8) | 3.5 (2.7–4.0) | 2.5 (1.7–3.5) | 0.001 |
| N° of lesions, median (IQR) | 6 (3.0–10.0) Missing in n = 2 | 6.5 (5.0–11.7) Missing in n = 1 | 4.5 (2.2–9.7) Missing in n = 1 | 0.089 |
| Effective lesion, median (IQR) | 2 (1.0–5.5) Missing in n = 5 | 1.0 (1.0–3.7) Missing in n = 5 | 3.0 (1.0–6.5) | 0.128 |
| Acutely effective ablation | 0.506 | |||
| Yes, n (%) | 52 (83.9) | 20 (80.0) | 32 (86.5) | |
| No, n (%) | 10 (16.1) | 5 (20.0) | 5 (13.5) | |
| Recurrences, n (%) Yes, n (%) No, n (%) | 16 (30.8) 36 (69.2) Performed in n = 52 | 3 (15.0) 17 (85.0) Performed in n = 20 | 13 (40.6) 19 (59.4) Performed in n = 32 | 0.068 |
| Dosage (μGy/m2), median (IQR) | 3.0 (1.0–19.4) Missing in n = 15 | 1.6 (0.2–20.0) Missing in n = 5 | 7.6 (1.1–18.0) Missing in n = 10 | 0.220 |
| Dosage Tot (mGy), median (IQR) | 0.15 (0.0–0.6) Missing in n = 14 | 0.0 (0.0–0.5) Missing in n = 4 | 0.3 (0.0–0.8) Missing in n = 10 | 0.218 |
| Time (min), median (IQR) | 0.4 (0.0–3.2) Missing in n = 3 | 0.2 (0.0–2.8) Missing in n = 1 | 0.5 (0.1–3.4) Missing in n = 2 | 0.319 |
| Venous access | 0.092 | |||
| Femoral, n (%) | 51 (82.3) | 23 (92.0) | 28 (75.7) | |
| Jugular, n (%) | 11 (17.7) | 2 (8.0) | 9 (24.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raimondo, C.; Flore, F.; Alberio, A.M.Q.; Garibaldi, S.; Blandino, R.; Cantarutti, N.; Di Mambro, C.; Silvetti, M.S.; Drago, F. Outcomes of a Near-Zero Fluoroscopy and Minimally Invasive Approach in Ablation of Right Free Wall Accessory Pathways in Children. J. Clin. Med. 2025, 14, 6204. https://doi.org/10.3390/jcm14176204
Raimondo C, Flore F, Alberio AMQ, Garibaldi S, Blandino R, Cantarutti N, Di Mambro C, Silvetti MS, Drago F. Outcomes of a Near-Zero Fluoroscopy and Minimally Invasive Approach in Ablation of Right Free Wall Accessory Pathways in Children. Journal of Clinical Medicine. 2025; 14(17):6204. https://doi.org/10.3390/jcm14176204
Chicago/Turabian StyleRaimondo, Cristina, Francesco Flore, Antonino Maria Quintilio Alberio, Silvia Garibaldi, Rita Blandino, Nicoletta Cantarutti, Corrado Di Mambro, Massimo Stefano Silvetti, and Fabrizio Drago. 2025. "Outcomes of a Near-Zero Fluoroscopy and Minimally Invasive Approach in Ablation of Right Free Wall Accessory Pathways in Children" Journal of Clinical Medicine 14, no. 17: 6204. https://doi.org/10.3390/jcm14176204
APA StyleRaimondo, C., Flore, F., Alberio, A. M. Q., Garibaldi, S., Blandino, R., Cantarutti, N., Di Mambro, C., Silvetti, M. S., & Drago, F. (2025). Outcomes of a Near-Zero Fluoroscopy and Minimally Invasive Approach in Ablation of Right Free Wall Accessory Pathways in Children. Journal of Clinical Medicine, 14(17), 6204. https://doi.org/10.3390/jcm14176204