Neurodevelopmental Outcomes in Children Born to Mothers Infected with SARS-CoV-2 During Pregnancy: A Narrative Review
Abstract
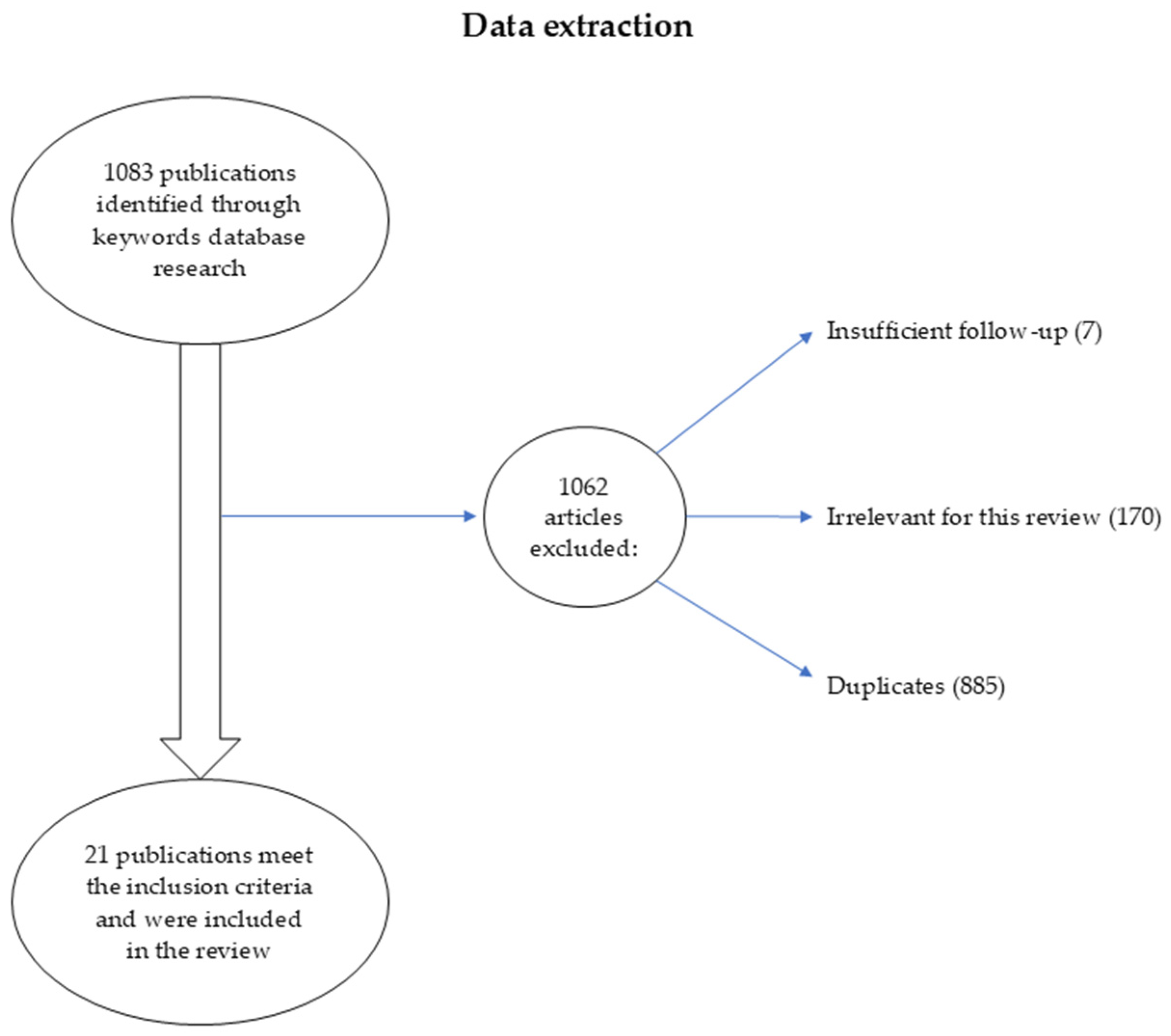
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
- Peer-reviewed observational studies (cohort or case–control).
- Neurodevelopment assessed via standardized tools (e.g., ASQ 3, Bayley scales) or clinical examination during infancy or early childhood (≤24 months), standardized parent-reported questionnaires (e.g., Ages and Stages Questionnaire), or direct neurological clinical examination during infancy or early childhood (≤24 months).
2.2. Exclusion Criteria
- Narrative reviews, animal studies (except mechanistic discussion), case reports without neurodevelopmental follow-up.
- Studies reporting exclusively biological outcomes (e.g., cytokine profiles, epigenetic changes) without separate reporting of standardized neurodevelopmental assessments.
2.3. Search Strategy, Selection and Data Extraction
2.4. Data Synthesis
3. Results
3.1. Motor Development Findings
3.2. Communication and Language Development Findings
3.3. Socioemotional and Behavioral Outcomes
3.4. Timing of Maternal Infection
3.5. Disease Severity
3.6. Studies Reporting No Significant Differences
3.7. Adjustment for Confounders
4. Discussion
4.1. Overall Pattern of Findings and Study Quality Considerations
4.2. Gestational Timing of SARS-CoV2 Infection
4.3. Infection Severity
4.4. Maternal Immune Activation
4.5. Specific Areas of Development Affected
4.6. Methodological Heterogeneity Impact on Interpretation
4.6.1. Assessment Tools and Their Clinical Implications
4.6.2. Follow-Up Duration and Developmental Windows
4.6.3. Distinguishing Viral Effects from Pandemic Context
4.6.4. Confounding and Study Quality Considerations
4.7. Similar Studies
4.8. Limitations
4.9. Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID 19 | Coronavirus disease 2019 |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| ASD | Autism spectrum disorders |
| CMV | Cytomegalovirus |
| EBV | Epstein–Barr virus |
| NOS | Newcastle–Ottawa Scale |
| OR | Odds ratio |
| ASQ-3 | Ages and Stages Questionnaire |
| BPSC | Baby Pediatric Symptom Checklist |
| GMA | General Movement Assessment |
| MOS-R | Motor Optimality Scores Revised |
| NBAS | Neonatal Behavioral Assessment Scale |
| ICD-10 | International Statistical Classification of Diseases and Related Health Problems, Tenth Revision |
| SWYC | Survey of Well-Being of Young Children |
| ASQ:SE-2 | Age and Stage Questionnaire Social-Emotional |
| MIA | Maternal Immune Activation |
| LBW | Low birth weight |
| GA | Gestational age |
| OR | Odds ratio |
References
- Fitzgerald, E.; Hor, K.; Drake, A.J. Maternal Influences on Fetal Brain Development: The Role of Nutrition, Infection and Stress, and the Potential for Intergenerational Consequences. Early Hum. Dev. 2020, 150, 105190. [Google Scholar] [CrossRef]
- Buss, C.; Entringer, S.; Wadhwa, P.D. Fetal Programming of Brain Development: Intrauterine Stress and Susceptibility to Psychopathology. A Presentation from the European Society for Paediatric Endocrinology (ESPE) New Inroads to Child Health (NICHe) Conference on Stress Response and Child Health in Heraklion, Crete, Greece, 18–20 May 2012. Sci. Signal 2012, 5, pt7. [Google Scholar] [CrossRef]
- Leibovitz, Z.; Lerman-Sagie, T.; Haddad, L. Fetal Brain Development: Regulating Processes and Related Malformations. Life 2022, 12, 809. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Choi, G.B.; Huh, J.R. Maternal Inflammation and Its Ramifications on Fetal Neurodevelopment. Trends Immunol. 2022, 43, 230–244. [Google Scholar] [CrossRef]
- Goeden, N.; Velasquez, J.; Arnold, K.A.; Chan, Y.; Lund, B.T.; Anderson, G.M.; Bonnin, A. Maternal Inflammation Disrupts Fetal Neurodevelopment via Increased Placental Output of Serotonin to the Fetal Brain. J. Neurosci. 2016, 36, 6041–6049. [Google Scholar] [CrossRef] [PubMed]
- Sokou, R.; Lianou, A.; Lampridou, M.; Panagiotounakou, P.; Kafalidis, G.; Paliatsiou, S.; Volaki, P.; Tsantes, A.G.; Boutsikou, T.; Iliodromiti, Z.; et al. Neonates at Risk: Understanding the Impact of High-Risk Pregnancies on Neonatal Health. Medicina 2025, 61, 1077. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, C.; Parisi, F.; Cetin, I. Impact of Maternal Environment and Inflammation on Fetal Neurodevelopment. Antioxidants 2024, 13, 453. [Google Scholar] [CrossRef]
- Lungu, N.; Popescu, D.-E.; Jura, A.M.C.; Zaharie, M.; Jura, M.-A.; Roșca, I.; Boia, M. Enhancing Early Detection of Sepsis in Neonates through Multimodal Biosignal Integration: A Study of Pulse Oximetry, Near-Infrared Spectroscopy (NIRS), and Skin Temperature Monitoring. Bioengineering 2024, 11, 681. [Google Scholar] [CrossRef]
- Sood, E.; Newburger, J.W.; Anixt, J.S.; Cassidy, A.R.; Jackson, J.L.; Jonas, R.A.; Lisanti, A.J.; Lopez, K.N.; Peyvandi, S.; Marino, B.S. Neurodevelopmental Outcomes for Individuals with Congenital Heart Disease: Updates in Neuroprotection, Risk-Stratification, Evaluation, and Management: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e997–e1022. [Google Scholar] [CrossRef]
- Doi, M.; Usui, N.; Shimada, S. Prenatal Environment and Neurodevelopmental Disorders. Front. Endocrinol. 2022, 13, 860110. [Google Scholar] [CrossRef]
- De Felice, A.; Ricceri, L.; Venerosi, A.; Chiarotti, F.; Calamandrei, G. Multifactorial Origin of Neurodevelopmental Disorders: Approaches to Understanding Complex Etiologies. Toxics 2015, 3, 89–129. [Google Scholar] [CrossRef]
- Năstase, L.; Cristea, O.; Diaconu, A.; Stoicescu, S.-M.; Mohora, R.; Pascu, B.M.; Tala, S.T.; Roșca, I. Two Cases of Congenital Hypothyroidism Revealing Thyroid Agenesis. Medicina 2023, 59, 1887. [Google Scholar] [CrossRef]
- Rosca, I.; Turenschi, A.; Nicolescu, A.; Constantin, A.T.; Canciu, A.M.; Dica, A.D.; Bratila, E.; Coroleuca, C.A.; Nastase, L. Endocrine Disorders in a Newborn with Heterozygous Galactosemia, Down Syndrome and Complex Cardiac Malformation: Case Report. Medicina 2023, 59, 856. [Google Scholar] [CrossRef]
- Bratan, C.A.; Gheorghe, M.; Ispas, I.; Franti, E.; Dascalu, M.; Stoicescu, S.M.; Rosca, I.; Gherghiceanu, F.; Dumitrache, D.; Nastase, L. Dunstan Baby Language Classification with CNN. In Proceedings of the 2021 International Conference on Speech Technology and Human-Computer Dialogue (SpeD), Bucharest, Romania, 13–15 October 2021; IEEE: New York, NY, USA; pp. 167–171. [Google Scholar]
- Pesch, M.H.; Leung, J.; Lanzieri, T.M.; Tinker, S.C.; Rose, C.E.; Danielson, M.L.; Yeargin-Allsopp, M.; Grosse, S.D. Autism Spectrum Disorder Diagnoses and Congenital Cytomegalovirus. Pediatrics 2024, 153, e2023064081. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Geng, X.; Zhang, X.; Xiao, Y.; Wang, W. Associations of Epstein-Barr Virus Infection with Attention Deficit Hyperactivity Disorder, Learning Disability, and Special Education in U.S. Children. Int. J. Gen. Med. 2021, 15, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Egorova, M.; Egorov, V.; Zabrodskaya, Y. Maternal Influenza and Offspring Neurodevelopment. Curr. Issues Mol. Biol. 2024, 46, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.M.; Connolly, M.G.; Gonzalez-Ricon, R.J.; Wang, S.S.; Allen, J.M.; Antonson, A.M. Influenza A Virus During Pregnancy Disrupts Maternal Intestinal Immunity and Fetal Cortical Development in a Dose- and Time-Dependent Manner. Mol. Psychiatry 2025, 30, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Guardado, K.; Varela-Cardoso, M.; Pérez-Roa, V.O.; Morales-Romero, J.; Zenteno-Cuevas, R.; Ramos-Ligonio, Á.; Guzmán-Martínez, O.; Sampieri, C.L.; Ortiz-Chacha, C.S.; Pérez-Varela, R.; et al. Evaluation of Anomalies and Neurodevelopment in Children Exposed to ZIKV During Pregnancy. Children 2022, 9, 1216. [Google Scholar] [CrossRef]
- Pesch, M.H.; Lauer, C.S.; Weinberg, J.B. Neurodevelopmental Outcomes of Children with Congenital Cytomegalovirus: A Systematic Scoping Review. Pediatr. Res. 2024, 95, 418–435. [Google Scholar] [CrossRef]
- Seblany, H.T.; Dinu, I.S.; Safer, M.; Plesca, D.A. FACTORS THAT HAVE A NEGATIVE IMPACT ON QUALITY OF LIFE IN CHILDREN WITH ADHD. Farmacia 2014, 62, 350–357. [Google Scholar]
- Fung, S.G.; Fakhraei, R.; Condran, G.; Regan, A.K.; Dimanlig-Cruz, S.; Ricci, C.; Foo, D.; Sarna, M.; Török, E.; Fell, D.B. Neuropsychiatric Outcomes in Offspring after Fetal Exposure to Maternal Influenza Infection During Pregnancy: A Systematic Review. Reprod. Toxicol. 2022, 113, 155–169. [Google Scholar] [CrossRef]
- Moreno, J.L.; Kurita, M.; Holloway, T.; López, J.; Cadagan, R.; Martínez-Sobrido, L.; García-Sastre, A.; González-Maeso, J. Maternal Influenza Viral Infection Causes Schizophrenia-Like Alterations of 5-HT2A and MGlu2 Receptors in the Adult Offspring. J. Neurosci. 2011, 31, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Selten, J.-P.; Termorshuizen, F. The Serological Evidence for Maternal Influenza as Risk Factor for Psychosis in Offspring Is Insufficient: Critical Review and Meta-Analysis. Schizophr. Res. 2017, 183, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Shook, L.L.; Sullivan, E.L.; Lo, J.O.; Perlis, R.H.; Edlow, A.G. COVID-19 in Pregnancy: Implications for Fetal Brain Development. Trends Mol. Med. 2022, 28, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Dubey, H.; Sharma, R.K.; Krishnan, S.; Knickmeyer, R. SARS-CoV-2 (COVID-19) as a Possible Risk Factor for Neurodevelopmental Disorders. Front. Neurosci. 2022, 16, 1021721. [Google Scholar] [CrossRef]
- Popescu, D.-E.; Cerbu, S.; Rosca, I.; Lungu, N.; Trușculescu, A.A.; Belengeanu, V.; Manea, A.M.; Dima, M.A.; Gorun, F.; Popa, Z.L.; et al. Comparative Analysis of Hematological and Biochemical Changes in Neonates among Women with and Without COVID-19 Infection During Pregnancy. Children 2023, 10, 1370. [Google Scholar] [CrossRef]
- De Luca, D.; Vauloup-Fellous, C.; Benachi, A.; Vivanti, A. Transmission of SARS-CoV-2 from Mother to Fetus or Neonate: What to Know and What to Do? Semin. Fetal Neonatal Med. 2023, 28, 101429. [Google Scholar] [CrossRef]
- Jeganathan, K.; Paul, A.B. Vertical Transmission of SARS-CoV-2: A Systematic Review. Obs. Med. 2022, 15, 91–98. [Google Scholar] [CrossRef]
- Lins, B. Maternal Immune Activation as a Risk Factor for Psychiatric Illness in the Context of the SARS-CoV-2 Pandemic. Brain Behav. Immun. Health 2021, 16, 100297. [Google Scholar] [CrossRef]
- Hofsink, N.; Groenink, L.; Plösch, T. The Fetal Programming Effect of Maternal Immune Activation (MIA) on the Offspring’s Immune System. Semin. Immunopathol. 2024, 46, 14. [Google Scholar] [CrossRef]
- Popescu, D.E.; Roșca, I.; Jura, A.M.C.; Cioca, A.; Pop, O.; Lungu, N.; Popa, Z.-L.; Rațiu, A.; Boia, M. Prompt Placental Histopathological and Immunohistochemical Assessment after SARS-CoV-2 Infection During Pregnancy—Our Perspective of a Small Group. Int. J. Mol. Sci. 2024, 25, 1836. [Google Scholar] [CrossRef]
- Kasaven, L.S.; Raynaud, I.; Jalmbrant, M.; Joash, K.; Jones, B.P. The Impact of the COVID-19 Pandemic on Perinatal Services and Maternal Mental Health in the UK. BJPsych Open 2023, 9, e13. [Google Scholar] [CrossRef] [PubMed]
- Kotlar, B.; Gerson, E.M.; Petrillo, S.; Langer, A.; Tiemeier, H. The Impact of the COVID-19 Pandemic on Maternal and Perinatal Health: A Scoping Review. Reprod. Health 2021, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Sun, M.; Hao, J.; Chen, Y.; Jiang, X.; Tao, R.; Huang, K.; Tao, F. Does Prenatal Maternal Stress Impair Cognitive Development and Alter Temperament Characteristics in Toddlers with Healthy Birth Outcomes? Dev. Med. Child. Neurol. 2014, 56, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Perelli, F.; Vidiri, A.; Palomba, G.; Franco, R.; Gallitelli, V.; Parasiliti, M.; Bisanti, M.; Spanò, A.; Silvagni, A.; Lopez, A.; et al. Preterm Birth and SARS-CoV-2: Does a Correlation Exist? Biomedicines 2025, 13, 282. [Google Scholar] [CrossRef]
- Smith, L.H.; Dollinger, C.Y.; VanderWeele, T.J.; Wyszynski, D.F.; Hernández-Díaz, S. Timing and Severity of COVID-19 During Pregnancy and Risk of Preterm Birth in the International Registry of Coronavirus Exposure in Pregnancy. BMC Pregnancy Childbirth 2022, 22, 775. [Google Scholar] [CrossRef]
- Klein, A.Z.; Kunatharaju, S.; Golder, S.; Levine, L.D.; Figueiredo, J.C.; Gonzalez-Hernandez, G. Association Between COVID-19 During Pregnancy and Preterm Birth by Trimester of Infection: A Retrospective Cohort Study Using Longitudinal Social Media Data. J. Med. Internet Res. 2025, 27, e66097. [Google Scholar] [CrossRef]
- Zamparini, J.; Saggers, R.; Buga, C.E. A Review of Coronavirus Disease 2019 in Pregnancy. Semin. Respir. Crit. Care Med. 2023, 44, 050–065. [Google Scholar] [CrossRef]
- Metz, T.D.; Clifton, R.G.; Hughes, B.L.; Sandoval, G.J.; Grobman, W.A.; Saade, G.R.; Manuck, T.A.; Longo, M.; Sowles, A.; Clark, K.; et al. Association of SARS-CoV-2 Infection with Serious Maternal Morbidity and Mortality From Obstetric Complications. JAMA 2022, 327, 748. [Google Scholar] [CrossRef]
- Zanardo, V.; Tortora, D.; Sandri, A.; Severino, L.; Mesirca, P.; Straface, G. COVID-19 Pandemic: Impact on Gestational Diabetes Mellitus Prevalence. Diabetes Res. Clin. Pract. 2022, 183, 109149. [Google Scholar] [CrossRef]
- Murphy, C.A.; O’Reilly, D.P.; Edebiri, O.; Donnelly, J.C.; McCallion, N.; Drew, R.J.; Ferguson, W. The Effect of COVID-19 Infection During Pregnancy; Evaluating Neonatal Outcomes and the Impact of the B.1.1.7. Variant. Pediatr. Infect. Dis. J. 2021, 40, e475–e481. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, G.S.M.A.; de Souza, R.C.; de Oliveira Azevedo, V.M.G.; Guimarães, N.S.; Pires, L.G.; Lemos, S.M.A.; Alves, C.R.L. Effects of Intrauterine Exposure to SARS-CoV-2 on Infants’ Development: A Rapid Review and Meta-Analysis. Eur. J. Pediatr. 2023, 182, 2041–2055. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sun, L.; Luo, J.; Qi, W.; Zuo, X.; Yang, Z. The Effect of Long-Term COVID-19 Infection on Maternal and Fetal Complications: A Retrospective Cohort Study Conducted at a Single Center in China. Sci. Rep. 2024, 14, 17273. [Google Scholar] [CrossRef] [PubMed]
- Goicea, L.; Buzoianu, E.; Davitoiu, A.M.; Chindris, S.; Zamfirescu, A.; Iancu, M.; Ghiorghiu, I.; Tincu, I.; Draganescu, A.; Dorobantu, A.; et al. Multisystemic Inflammatory Syndrome in Children Post-COVID-19: Clinical-Biological Characteristics of Patients in the First Year of the Pandemic. J. Pediatr. Neonatal Individ. Med. 2023, 12, e120210. [Google Scholar]
- Dunn, A.; Malhotra, A. Neurodevelopmental Risks of SARS-CoV-2 Infection in Utero. Pediatr. Res. 2025. [Google Scholar] [CrossRef]
- Buechel, C.; Friedmann, A.; Eber, S.; Behrends, U.; Mall, V.; Nehring, I. The Change of Psychosocial Stress Factors in Families with Infants and Toddlers During the COVID-19 Pandemic. A Longitudinal Perspective on the CoronabaBY Study from Germany. Front. Pediatr. 2024, 12, 1354089. [Google Scholar] [CrossRef]
- Nazzari, S.; Pili, M.P.; Günay, Y.; Provenzi, L. Pandemic Babies: A Systematic Review of the Association between Maternal Pandemic-Related Stress During Pregnancy and Infant Development. Neurosci. Biobehav. Rev. 2024, 162, 105723. [Google Scholar] [CrossRef]
- Penna, A.L.; de Aquino, C.M.; Pinheiro, M.S.N.; do Nascimento, R.L.F.; Farias-Antúnez, S.; Araújo, D.A.B.S.; Mita, C.; Machado, M.M.T.; Castro, M.C. Impact of the COVID-19 Pandemic on Maternal Mental Health, Early Childhood Development, and Parental Practices: A Global Scoping Review. BMC Public Health 2023, 23, 388. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 24 June 2025).
- Shuffrey, L.C.; Firestein, M.R.; Kyle, M.; Fields, A.; Alcántara, C.; Amso, D.; Austin, J.; Bain, J.M.; Barbosa, J.; Bence, M.; et al. Birth During the COVID-19 Pandemic, but Not Maternal SARS-CoV-2 Infection During Pregnancy, Is Associated with Lower Neurodevelopmental Scores at 6-Months. medRxiv 2021. [Google Scholar] [CrossRef]
- ACNP 60th Annual Meeting: Poster Abstracts P1–P275. Neuropsychopharmacology 2021, 46, 72–217. [CrossRef]
- Cheng, Y.; Teng, H.; Xiao, Y.; Yao, M.; Yin, J.; Sun, G. Impact of SARS-CoV-2 Infection During Pregnancy on Infant Neurobehavioral Development: A Case-Control Study. Front. Pediatr. 2021, 9, 762684. [Google Scholar] [CrossRef]
- Wu, T.; Chen, L.; Wang, Y.; Shi, H.; Niu, J.; Yin, X.; Li, M.; Tan, C.; Jiang, H.; Zheng, D.; et al. Effects of SARS-CoV-2 Infection During Late Pregnancy on Early Childhood Development: A Prospective Cohort Study. Front. Pediatr. 2021, 9, 750012. [Google Scholar] [CrossRef] [PubMed]
- Schuh, T.L.; Mithal, L.B.; Naureckas, S.; Miller, E.S.; Garfield, C.F.; Shah, M.D. Outcomes from Birth to 6 Months of Publicly Insured Infants Born to Mothers with Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States. J. Perinat. Med. 2022, 50, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Edlow, A.G.; Castro, V.M.; Shook, L.L.; Kaimal, A.J.; Perlis, R.H. Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 During Pregnancy. JAMA Netw. Open 2022, 5, e2215787. [Google Scholar] [CrossRef] [PubMed]
- Ayed, M.; Embaireeg, A.; Kartam, M.; More, K.; Alqallaf, M.; AlNafisi, A.; Alsaffar, Z.; Bahzad, Z.; Buhamad, Y.; Alsayegh, H.; et al. Neurodevelopmental Outcomes of Infants Born to Mothers with SARS-CoV-2 Infections During Pregnancy: A National Prospective Study in Kuwait. BMC Pediatr. 2022, 22, 319. [Google Scholar] [CrossRef]
- Buonsenso, D.; Costa, S.; Giordano, L.; Priolo, F.; Colonna, A.T.; Morini, S.; Sbarbati, M.; Pata, D.; Acampora, A.; Conti, G.; et al. Short- and Mid-Term Multidisciplinary Outcomes of Newborns Exposed to SARS-CoV-2 in Utero or During the Perinatal Period: Preliminary Findings. Eur. J. Pediatr. 2022, 181, 1507–1520. [Google Scholar] [CrossRef]
- Aldrete-Cortez, V.; Bobadilla, L.; Tafoya, S.A.; Gonzalez-Carpinteiro, A.; Nava, F.; Viñals, C.; Alvarado, E.; Mendizabal-Espinosa, R.; Gómez-López, M.E.; Ramirez-Garcia, L.A.; et al. Infants Prenatally Exposed to SARS-CoV-2 Show the Absence of Fidgety Movements and Are at Higher Risk for Neurological Disorders: A Comparative Study. PLoS ONE 2022, 17, e0267575. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Guo, J.; Zeng, C.; Cao, Y.; Ran, R.; Wu, T.; Yang, G.; Zhao, D.; Yang, P.; Yu, X.; et al. Transient Early Fine Motor Abnormalities in Infants Born to COVID-19 Mothers Are Associated with Placental Hypoxia and Ischemia. Front. Pediatr. 2022, 9, 793561. [Google Scholar] [CrossRef]
- Martenot, A.; Labbassi, I.; Delfils-Stern, A.; Deruelle, P.; Kuhn, P. Medical and Neurodevelopmental Outcomes at 10 Months of Age in Infants Born at 34 Weeks plus to Mothers with COVID-19. Acta Paediatr. 2023, 112, 296–297. [Google Scholar] [CrossRef]
- Fajardo Martinez, V.; Zhang, D.; Paiola, S.; Mok, T.; Cambou, M.C.; Kerin, T.; Rao, R.; Brasil, P.; Ferreira, F.; Fuller, T.; et al. Neuromotor Repertoires in Infants Exposed to Maternal COVID-19 During Pregnancy: A Cohort Study. BMJ Open 2023, 13, e069194. [Google Scholar] [CrossRef]
- Silva, P.Y.F.; Lima da Cruz, M.C.; Guerra Azevedo, I.; Moreira, R.S.; Sousa, K.G.; Pereira, S.A. Risk of Global Developmental Delay in Infants Born from Mothers with COVID-19: A Cross-Sectional Study. Int. J. Womens Health 2023, 15, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Ayesa-Arriola, R.; Castro Quintas, Á.; Ortiz-García de la Foz, V.; Miguel Corredera, M.; San Martín González, N.; Murillo-García, N.; Neergaard, K.; Fañanás Saura, L.; de las Cuevas-Terán, I. Exploring the Impact of COVID-19 on Newborn Neurodevelopment: A Pilot Study. Sci. Rep. 2023, 13, 2983. [Google Scholar] [CrossRef]
- Rood, M.; ten Kate, L.; Boeddha, N.P.; van‘t Kruys, K. Clinical Characteristics, Transmission Rate and Outcome of Neonates Born to COVID-19-Positive Mothers: A Prospective Case Series From a Resource-Limited Setting. Pediatr. Infect. Dis. J. 2023, 42, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Vrantsidis, D.M.; van de Wouw, M.; Hall, E.R.M.; Kuret, V.; Rioux, C.; Conrad, M.L.; Mesa, C.; Harris, A.; Lebel, C.; Tomfohr-Madsen, L.; et al. Neurodevelopment in the First 2 Years of Life Following Prenatal Exposure to Maternal SARS-CoV-2 Infection. JAMA Netw. Open 2024, 7, e2443697. [Google Scholar] [CrossRef] [PubMed]
- Jaswa, E.G.; Huddleston, H.G.; Lindquist, K.J.; Wu, A.H.B.; Bishop, S.L.; Kim, Y.-S.; Kaing, A.; Prahl, M.; Gaw, S.L.; Corley, J.; et al. In Utero Exposure to Maternal COVID-19 and Offspring Neurodevelopment Through Age 24 Months. JAMA Netw. Open 2024, 7, e2439792. [Google Scholar] [CrossRef]
- Hill, R.A.; Gibbons, A.; Suwakulsiri, W.; Taseska, A.; Darke, H.; Malhotra, A.; Yee, H.; Fahey, M.; Hunt, R.W.; Lim, I.; et al. Investigating the Impact of Severe Maternal SARS-CoV-2 Infection on Infant DNA Methylation and Neurodevelopment. Mol. Psychiatry 2025, 30, 1976–1984. [Google Scholar] [CrossRef]
- Silva, L.M.; Melo, M.L.dO.; Alpes, M.F.; Santos, C.M.D. Risk of Neurodevelopmental Changes in 18-Month-Old Children Born to Mothers Infected with SARS-CoV-2. Audiol. Commun. Res. 2025, 30, e2963. [Google Scholar] [CrossRef]
- Kehdi, R.C.; Silva, M.F.S.; Cavalcante, L.R.L.; Fiorenza, N.G.; Viana, M.; Leite, I.B.; dos Santos Silva, B.R.; de Assis, D.F.; Cortez, P.C.; Bezerra, D.L.; et al. Cord Blood Cytokines/Chemokines Linked to Delays in Toddlers Exposed to SARS-CoV-2 Prenatally. Pediatr. Res. 2025. [Google Scholar] [CrossRef]
- Berg, J.; Linden, K.; Zaigham, M.; Domellöf, M.; Ahlsson, F.; Elfvin, A.; Åden, U.; Abrahamsson, T.; Ohlin, A.; Berg, J.; et al. The Association between Antenatal SARS-CoV-2 Exposure and Infant Neurodevelopment at Four Months of Age: A Prospective Multicenter Cohort Survey within the COPE Study. Int. J. Infect. Dis. 2025, 158, 107973. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Miyazawa, H.; Miura, M. Neural Tube Closure and Embryonic Metabolism. Congenit. Anom. 2017, 57, 134–137. [Google Scholar] [CrossRef]
- Engelhardt, D.M.; Martyr, C.A.; Niswander, L. Pathogenesis of Neural Tube Defects: The Regulation and Disruption of Cellular Processes Underlying Neural Tube Closure. WIREs Mech. Dis. 2022, 14, e1559. [Google Scholar] [CrossRef] [PubMed]
- Goasdoué, K.; Miller, S.M.; Colditz, P.B.; Björkman, S.T. Review: The Blood-Brain Barrier; Protecting the Developing Fetal Brain. Placenta 2017, 54, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Bergdolt, L.; Dunaevsky, A. Brain Changes in a Maternal Immune Activation Model of Neurodevelopmental Brain Disorders. Prog. Neurobiol. 2019, 175, 1–19. [Google Scholar] [CrossRef] [PubMed]
- McEwan, F.; Glazier, J.D.; Hager, R. The Impact of Maternal Immune Activation on Embryonic Brain Development. Front. Neurosci. 2023, 17, 1146710. [Google Scholar] [CrossRef]
- Chhetri, P.K.; Das, J.M. Neuroanatomy, Neural Tube Development and Stages. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557414/ (accessed on 23 August 2025).
- Kostović, I.; Judaš, M. Embryonic and Fetal Development of the Human Cerebral Cortex. In Brain Mapping; Elsevier: Amsterdam, The Netherlands, 2015; pp. 167–175. [Google Scholar]
- National Research Council (US); Institute of Medicine (US). Committee on Integrating the Science of Early Childhood Development The Developing Brain. In From Neurons to Neighborhoods: The Science of Early Childhood Development; Shonkoff, J.P., Phillips, D.A., Eds.; National Academies Press (US): Washington, DC, USA, 2000. [Google Scholar]
- Jackson, R.; Cornish, R.; Daskalopoulou, Z.; Gale, C.; Hurd, M.; Johnson, S.; Knight, M.; Kurinczuk, J.J.; Woodward, K.; Chakkarapani, E.; et al. Association of Antenatal or Neonatal SARS-CoV-2 Exposure with Developmental and Respiratory Outcomes, and Healthcare Usage in Early Childhood: A National Prospective Cohort Study. EClinicalMedicine 2024, 72, 102628. [Google Scholar] [CrossRef]
- Hall, M.B.; Willis, D.E.; Rodriguez, E.L.; Schwarz, J.M. Maternal Immune Activation as an Epidemiological Risk Factor for Neurodevelopmental Disorders: Considerations of Timing, Severity, Individual Differences, and Sex in Human and Rodent Studies. Front. Neurosci. 2023, 17, 1135559. [Google Scholar] [CrossRef]
- Groh, J.; Feng, R.; Yuan, X.; Liu, L.; Klein, D.; Hutahaean, G.; Butz, E.; Wang, Z.; Steinbrecher, L.; Neher, J.; et al. Microglia Activation Orchestrates CXCL10-Mediated CD8+ T Cell Recruitment to Promote Aging-Related White Matter Degeneration. Nat. Neurosci. 2025, 28, 1160–1173. [Google Scholar] [CrossRef]
- Kummer, K.K.; Zeidler, M.; Kalpachidou, T.; Kress, M. Role of IL-6 in the Regulation of Neuronal Development, Survival and Function. Cytokine 2021, 144, 155582. [Google Scholar] [CrossRef]
- Gonzalez Caldito, N. Role of Tumor Necrosis Factor-Alpha in the Central Nervous System: A Focus on Autoimmune Disorders. Front. Immunol. 2023, 14, 1213448. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Bicanin Ilic, M.; Nikolic Turnic, T.; Ilic, I.; Nikolov, A.; Mujkovic, S.; Rakic, D.; Jovic, N.; Arsenijevic, N.; Mitrovic, S.; Spasojevic, M.; et al. SARS-CoV-2 Infection and Its Association with Maternal and Fetal Redox Status and Outcomes: A Prospective Clinical Study. J. Clin. Med. 2025, 14, 1555. [Google Scholar] [CrossRef]
- LaSalle, J.M. DNA Methylation Biomarkers of Intellectual/Developmental Disability across the Lifespan. J. Neurodev. Disord. 2025, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Walton, E.; Alemany, S.; Cecil, C.; González, J.R.; Jima, D.D.; Lahti, J.; Tuominen, S.T.; Barker, E.D.; Binder, E.; et al. Association between DNA Methylation and ADHD Symptoms from Birth to School Age: A Prospective Meta-Analysis. Transl. Psychiatry 2020, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Trajkova, S.; Kerkhof, J.; Rossi Sebastiano, M.; Pavinato, L.; Ferrero, E.; Giovenino, C.; Carli, D.; Di Gregorio, E.; Marinoni, R.; Mandrile, G.; et al. DNA Methylation Analysis in Patients with Neurodevelopmental Disorders Improves Variant Interpretation and Reveals Complexity. Hum. Genet. Genom. Adv. 2024, 5, 100309. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, M.; Parvez, M.S.A.; Yamawaki, Y.; Oe, S.; Liang, Y.; Wada, Y.; Hirahara, Y.; Koike, T.; Imai, H.; Oishi, N.; et al. Maternal Immune Activation Followed by Peripubertal Stress Combinedly Produce Reactive Microglia and Confine Cerebellar Cognition. Commun. Biol. 2025, 8, 296. [Google Scholar] [CrossRef]
- Hagberg, H.; Mallard, C.; Ferriero, D.M.; Vannucci, S.J.; Levison, S.W.; Vexler, Z.S.; Gressens, P. The Role of Inflammation in Perinatal Brain Injury. Nat. Rev. Neurol. 2015, 11, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.L.; Beeson, P.M.; Stark, A.J.; Rapcsak, S.Z. The Role of Left Perisylvian Cortical Regions in Spelling. Brain Lang 2007, 100, 44–52. [Google Scholar] [CrossRef]
- Ellul, P.; Maruani, A.; Vantalon, V.; Humeau, E.; Amestoy, A.; Anchordoqui, A.; Atzori, P.; Baleyte, J.-M.; Benmansour, S.; Bonnot, O.; et al. Maternal Immune Activation During Pregnancy Is Associated with More Difficulties in Socio-Adaptive Behaviors in Autism Spectrum Disorder. Sci. Rep. 2023, 13, 17687. [Google Scholar] [CrossRef]
- Quagliato, L.A.; de Matos, U.; Nardi, A.E. Maternal Immune Activation Generates Anxiety in Offspring: A Translational Meta-Analysis. Transl. Psychiatry 2021, 11, 245. [Google Scholar] [CrossRef]
- Murlanova, K.; Begmatova, D.; Weber-Stadlbauer, U.; Meyer, U.; Pletnikov, M.; Pinhasov, A. Double Trouble: Prenatal Immune Activation in Stress Sensitive Offspring. Brain Behav. Immun. 2022, 99, 3–8. [Google Scholar] [CrossRef]
- Duggan, C.; Irvine, A.D.; O’B Hourihane, J.; Kiely, M.E.; Murray, D.M. ASQ-3 and BSID-III’s Concurrent Validity and Predictive Ability of Cognitive Outcome at 5 Years. Pediatr. Res. 2023, 94, 1465–1471. [Google Scholar] [CrossRef]
- Lockhart, M.; Chaux, R.; Chevin, M.; Celle, M.; Raia-Barjat, T.; Patural, H.; Chabrier, S.; Giraud, A. Classification Performance of the Ages and Stages Questionnaire: Influence of Maternal Education Level. Children 2023, 10, 449. [Google Scholar] [CrossRef]
- Crowle, C.; Jackman, M.; Webb, A.; Morgan, C. Use of the Motor Optimality Score-Revised (MOS-R) to Predict Neurodevelopmental Outcomes in Infants with Congenital Anomalies. Early Hum. Dev. 2023, 187, 105876. [Google Scholar] [CrossRef] [PubMed]
- Einspieler, C.; Bos, A.F.; Spittle, A.J.; Bertoncelli, N.; Burger, M.; Peyton, C.; Toldo, M.; Utsch, F.; Zhang, D.; Marschik, P.B. The General Movement Optimality Score-Revised (GMOS-R) with Socioeconomically Stratified Percentile Ranks. J. Clin. Med. 2024, 13, 2260. [Google Scholar] [CrossRef] [PubMed]
- Örtqvist, M.; Marschik, P.B.; Toldo, M.; Zhang, D.; Fajardo-Martinez, V.; Nielsen-Saines, K.; Ådén, U.; Einspieler, C. Reliability of the Motor Optimality Score-Revised: A Study of Infants at Elevated Likelihood for Adverse Neurological Outcomes. Acta Paediatr. 2023, 112, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Ossmy, O.; Donati, G.; Kaur, A.; Sotoodeh, M.S.; Forrester, G. Towards Automatic Assessment of Atypical Early Motor Development? Brain Res. Bull. 2025, 224, 111311. [Google Scholar] [CrossRef]
- Kokkinaki, T.; Hatzidaki, E. COVID-19 Pandemic-Related Restrictions: Factors That May Affect Perinatal Maternal Mental Health and Implications for Infant Development. Front. Pediatr. 2022, 10, 846627. [Google Scholar] [CrossRef]
- Firestein, M.R.; Dumitriu, D.; Marsh, R.; Monk, C. Maternal Mental Health and Infant Development During the COVID-19 Pandemic. JAMA Psychiatry 2022, 79, 1040. [Google Scholar] [CrossRef]
- Hessami, K.; Norooznezhad, A.H.; Monteiro, S.; Barrozo, E.R.; Abdolmaleki, A.S.; Arian, S.E.; Zargarzadeh, N.; Shekerdemian, L.S.; Aagaard, K.M.; Shamshirsaz, A.A. COVID-19 Pandemic and Infant Neurodevelopmental Impairment. JAMA Netw. Open 2022, 5, e2238941. [Google Scholar] [CrossRef]
- Veloso, A.H.N.; Barbosa, A.d.M.; Ribeiro, M.F.M.; Gervásio, F.M. Neurodevelopment in the First Year of Children Exposed to SARS-CoV-2 During Intrauterine Period: Systematic Review. Rev. Gauch. Enferm. 2024, 45, e20240020. [Google Scholar] [CrossRef]
- Jackson, R.; Woodward, K.; Ireland, M.; Larkin, C.; Kurinczuk, J.J.; Knight, M.; Gale, C.; Johnson, S.; Cornish, R.; Chakkarapani, E. Antenatal and Neonatal Exposure to SARS-CoV-2 and Children’s Development: A Systematic Review and Meta-Analysis. Pediatr. Res. 2024, 96, 40–50. [Google Scholar] [CrossRef]
| Number | Study | Selection (Max 4) | Comparability (Max 2) | Outcome (Max 3) | Total Score | Risk Level |
|---|---|---|---|---|---|---|
| 1 | Shuffrey et al., 2021 [51] | 4 | 2 | 3 | 9 | Low |
| 2 | Roffman et al., 2021 [52] | 3 | 1 | 2 | 6 | Moderate |
| 3 | Cheng et al., 2021 [53] | 3 | 1 | 1 | 5 | Moderate |
| 4 | Wu et al., 2021 [54] | 3 | 1 | 2 | 6 | Moderate |
| 5 | Schuh et al., 2021 [55] | 2 | 1 | 1 | 4 | Moderate |
| 6 | Edlow et al., 2022 [56] | 4 | 2 | 2 | 8 | Low |
| 7 | Ayed et al., 2022 [57] | 3 | 2 | 2 | 7 | Low |
| 8 | Buonsenso et al., 2022 [58] | 3 | 1 | 1 | 5 | Moderate |
| 9 | Aldrete-Cortez et al., 2022 [59] | 3 | 1 | 2 | 6 | Moderate |
| 10 | Liu et al., 2022 [60] | 3 | 1 | 2 | 6 | Moderate |
| 11 | Martenot et al., 2022 [61] | 3 | 1 | 1 | 5 | Moderate |
| 12 | Martinez et al., 2023 [62] | 4 | 1 | 2 | 7 | Low |
| 13 | Silva et al., 2023 [63] | 3 | 1 | 2 | 6 | Moderate |
| 14 | Ayesa-Arriola et al., 2023 [64] | 2 | 1 | 2 | 5 | Moderate |
| 15 | Rood et al., 2023 [65] | 2 | 0 | 1 | 3 | High |
| 16 | Vrantsidis et al., 2024 [66] | 4 | 2 | 3 | 9 | Low |
| 17 | Jaswa et al., 2024 [67] | 4 | 2 | 3 | 9 | Low |
| 18 | Hill et al., 2024 [68] | 4 | 2 | 2 | 8 | Low |
| 19 | Silva et al., 2025 [69] | 3 | 1 | 2 | 6 | Moderate |
| 20 | Kehdi et al., 2025 [70] | 4 | 2 | 3 | 9 | Low |
| 21 | Berg et al., 2025 [71] | 4 | 2 | 3 | 9 | Low |
| Study | Design/N (Exposed) | Maternal Infection Timing (Trimester) | Maternal Disease Severity | Neurodevelopment Tool | Follow-Up Age | Key Findings | Adjustment for Confounders |
|---|---|---|---|---|---|---|---|
| Shuffrey et al., 2021 [51] | Cohort/N = 317 (114 exposed) | 1st: n = 42, 2nd: n = 90, 3rd: n = 67;) | 34% asymptomatic, 62% mild/moderate, 4% severe | ASQ-3 | 6 months | No major neurodevelopmental differences in infants born during the pandemic (exposed vs. non-exposed), but those born during the pandemic had significantly lower scores on gross motor, fine motor, and personal-social subdomains when compared to a cohort born prior to the onset of the pandemic. | Yes—adjusted for maternal education, race/ethnicity, infant sex, GA, mode of delivery, NICU admission |
| Roffman et al., 2021 [52] | Cohort/N = 34 (19 exposed) | Not reported (NR) | NR | ASQ-3, BPSC | 12 months | Gross motor delay and high irritability (p = 0.015). | NR |
| Cheng et al., 2021 [53] | Cohort/N = 18 (9 exposed) | All in the 3rd trimester | 100% mild/moderate | ASQ-3 | 8–10 months | The infants from SARS-CoV-2-exposed mothers had lower scores in communication, gross movement, fine movement, problem solving, and personal-social domains, but only fine motor movement was significantly lower (p = 0.03). | NR |
| Wu et al., 2021 [54] | Cohort/N = 135 (57 exposed) | 1st: n = 0, 2nd: n = 4, 3rd: n = 53 | 86% mild/moderate, 14% severe | ASQ-3, ASQ:SE-2 | 3 months | No significant neurodevelopmental differences between exposed and unexposed groups. | Yes—adjusted for mother–infant separation, low birth weight (LBW), infant sex, preterm birth, NICU admission, breastfeeding at 3 months |
| Schuh et al., 2021 [55] | Cohort/N = 15 (15 exposed) | NR | 100% severe | ASQ-3 | 6 months | All of them were reported as normal development. | NR |
| Edlow et al., 2022 [56] | Cohort/N = 7772 (222 exposed) | 1st: n = 1, 2nd: n = 61, 3rd: n = 160 | NR | ICD-10 codes | 12 months | Maternal SARS-CoV-2 positivity during pregnancy was associated with a greater rate of neurodevelopmental diagnoses (Odds ratio (OR) = 1.86, p = 0.04). | Yes—adjusted for maternal age, race/ethnicity, insurance status, gestational age (GA), birthweight, infant sex, |
| Ayed et al., 2022 [57] | Cohort/N = 298 (298 exposed) | 1st: n = 5, 2nd: n = 20, 3rd: n = 273 | 39.5% asymptomatic, 53.6% mild/moderate, 6.9% severe | ASQ-3 | 10–12 months | The rate of development delays was 10%. The risk of developmental delays was higher with first-trimester (OR: 8.2, p = 0.039) and second-trimester maternal SARS-CoV-2 infections (OR: 8.1, p = 0.001) than with third-trimester. | Yes—adjusted for maternal age, maternal infection timing, GA, birthweight, infant sex, parental education and type of feeding in the first 6 months |
| Buonsenso et al., 2022 [58] | Cohort/N = 199 (199 exposed) | 1st: n = 6, 2nd: n = 6, 3rd: n = 187 | 57.6% asymptomatic, 36.9% mild/moderate, 5.5% severe | Clinical exam | 3–6-9–12 months | All of them were reported as normal neurological development. | NR |
| Aldrete-Cortez et al., 2022 [59] | Cohort/N = 56 (28 exposed) | All in 3rd trimester | 100% mild/moderate | GMA | 3–5 months | The exposed group had a significantly reduced total MOS-R; the median was lower in the exposed group (21 vs. 25, p = 0.002). | Yes—adjusted for maternal/infant characteristics differing between groups: maternal age, marital status, education level, preeclampsia, hypothyroidism, gestational diabetes, GA, infant sex, type of delivery, Hyperbilirubinemia, APGAR score, days of hospitalization, birthweight, length at birth, birth head circumference |
| Liu et al., 2022 [60] | Cohort/N = 98 (31 exposed) | All in the 3rd trimester | 100% mild/moderate | Denver II | 9 months | Fine motor abnormalities higher in the exposed group (15.2% vs. 2.1%; p = 0.02). | Yes—adjusted using for age, sex, infection status |
| Martenot et al., 2022 [61] | Cohort/N = 24 (24 exposed) | All in the 3rd trimester | 4% asymptomatic, 96% symptomatic (severity NR) | ASQ-2 | 10 months | All of them were reported as normal neurological development. | NR |
| Martinez et al., 2023 [62] | Cohort/N = 239 (124 exposed) | 1st: n = 17, 2nd: n = 37, 3rd: n = 70 | 14.5% asymptomatic, 67.7% mild/moderate, 17.7% severe | GMA, MOS-R, clinical exam | 3–8 months | Suboptimal neuromotor development in exposed infants. The median of MOS-R was lower in the exposed group (23 vs. 25, p < 0.001), and 16 exposed infants had MOS-R scores <20 vs. 0 controls (p < 0.001). At 6–8 months clinical exam, 13 exposed vs. 0 controls had developmental delay. | Yes—adjusted for COVID-19 severity, trimester of infection, neonatal and maternal comorbidities, fetal sex, maternal age, maternal fever during COVID-19 and preterm birth |
| Silva et al., 2023 [63] | Cross-sectional study, N = 54 (27 exposed); | NR | NR | SWYC | From 1 to 12 months | Motor developmental delay in COVID-19-exposed infants (OR = 6.3, p = 0.01); socioemotional developmental delay (OR = 4.0; p = 0.02); Inflexibility (OR = 14.0; p = 0.02); Parental concerns about behavior, learning, or development of the infant (OR = 9.7; p = 0.01). | Yes—adjusted for sex, GA and family context. |
| Ayesa-Arriola et al., 2023 [64] | Cohort/N = 42 (21 exposed) | 1st: n = 3, 2nd: n = 8, 3rd: n = 10 | 95.2% mild/moderate, 4.8% severe | NBAS (0–3 mo) | 6 weeks | Lower affectionate response in the exposed group (p = 0.009), especially those exposed in the third trimester (p = 0.043). | Yes—for maternal age, GA, trimester of infection, infant age at assessment and infant sex |
| Rood et al., 2023 [65] | Cohort/N = 13 (13 exposed) | All in 3rd trimester | 46% mild/moderate, 54% severe | Van Wiechen Scheme | 3 months | Follow-up similar to children born to COVID-19-negative mothers, mild neurodevelopmental delay in 2 (15.3%). | NR |
| Vrantsidis et al., 2024 [66] | Cohort/N = 896 (96 exposed) | 1st: n = 21, 2nd: n = 45, 3rd: n = 30 | 1% asymptomatic, 74% mild/moderate, 25% severe | ASQ-3 | 6–24 months | No significant neurodevelopmental differences between the exposed and unexposed groups | Yes—for maternal comorbidities and household socioeconomic status |
| Jaswa et al., 2024 [67] | Cohort/N = 2003 (217 exposed) | 1st: n = 122, 2nd: n = 42, 3rd: n = 53 | NR | ASQ-3 | 12–24 months | No significant neurodevelopmental differences between exposed and unexposed groups, or by the trimester of infection in the exposed group. | Yes—for maternal age, race, education level, income, maternal generalized anxiety and depression symptoms at baseline. |
| Hill et al., 2024 [68] | Cohort/N= 30 (16 expoed) | 1st: n = 0, 2nd: n = 4, 3rd: n = 12 | 100% severe | ASQ-3 | 12 months | Lower ASQ-3 scores in children exposed to severe SARS-CoV-2 maternal infection in communication, problem solving and personal-social domains (p < 0.005); correlations with cytokine profiles and epigenetic markers. | NR |
| Silva et al., 2025 [69] | Cohort/N = 41 (41 exposed) | 1st: n = 5, 2nd: n = 9, 3rd: n = 27 | NR | ASQ-3 | 18 months | 22 (53%) children performed below the cutoff value in communication and 19 (46%) in gross motor coordination. | Yes—for maternal age, GA, type of delivery, sex, Apgar score, birth weight, the child’s need for prolonged hospitalization after birth, and trimester infection |
| Kehdi et al., 2025 [70] | Cohort/N = 41 (18 exposed) | All in 3rd trimester | 55% mild/moderate, 55% severe | Bayley-III | 6–24 months | At 6 and 24 months, up to 36% cognitive, 64% communication, and 57% motor delays were observed. Specific cord blood cytokines correlated with respective domain delays. | No adjustment for confounders; BSID-III scores corrected for gestational age |
| Berg et al., 2025 [71] | Cohort/N = 1446 (555 exposed) | 1st: n = 63, 2nd: n = 278, 3rd: n = 214 | 95.5% mild/asymptomatic, 4.5% severe | ASQ-3 | 4 months | There was no group difference in ASQ total mean scores, but those exposed to severe maternal COVID-19 had an increased risk of total ASQ scores below the cutoff (exposed: 16.0% vs. unexposed: 6.1%; OR 3.57; 95% CI, 1.14–11.24). | Yes—for mother BMI, education, country of origin, maternal age, pre-pregnancy comorbidity, GA, APGAR, type of delivery, birthweight, type of feeding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Păcurar, D.; Dinulescu, A.; Prejmereanu, A.; Palcău, A.C.; Dijmărescu, I.; Pavelescu, M.-L. Neurodevelopmental Outcomes in Children Born to Mothers Infected with SARS-CoV-2 During Pregnancy: A Narrative Review. J. Clin. Med. 2025, 14, 6202. https://doi.org/10.3390/jcm14176202
Păcurar D, Dinulescu A, Prejmereanu A, Palcău AC, Dijmărescu I, Pavelescu M-L. Neurodevelopmental Outcomes in Children Born to Mothers Infected with SARS-CoV-2 During Pregnancy: A Narrative Review. Journal of Clinical Medicine. 2025; 14(17):6202. https://doi.org/10.3390/jcm14176202
Chicago/Turabian StylePăcurar, Daniela, Alexandru Dinulescu, Ana Prejmereanu, Alexandru Cosmin Palcău, Irina Dijmărescu, and Mirela-Luminița Pavelescu. 2025. "Neurodevelopmental Outcomes in Children Born to Mothers Infected with SARS-CoV-2 During Pregnancy: A Narrative Review" Journal of Clinical Medicine 14, no. 17: 6202. https://doi.org/10.3390/jcm14176202
APA StylePăcurar, D., Dinulescu, A., Prejmereanu, A., Palcău, A. C., Dijmărescu, I., & Pavelescu, M.-L. (2025). Neurodevelopmental Outcomes in Children Born to Mothers Infected with SARS-CoV-2 During Pregnancy: A Narrative Review. Journal of Clinical Medicine, 14(17), 6202. https://doi.org/10.3390/jcm14176202






