Standardized Myocardial T1 and T2 Relaxation Times: Defining Age- and Comorbidity-Adjusted Reference Values for Improved CMR-Based Tissue Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Inclusion Criteria
- Age > 18 years;
- Full legal capacity to provide consent;
- Cardiovascular health defined as the absence of ischemia or myocardial scarring in CMR;
- Clinical indication for stress CMR, justifying the use of a myocardial ischemia test;
- Suitability for regadenoson administration, without contraindications such as severe obstructive pulmonary disease or acute heart failure.
2.3. Exclusion Criteria
- Presence of ferromagnetic implants, such as pacemakers, implantable cardioverter defibrillators (ICD), or vascular clips, posing a safety risk during CMR;
- Pregnancy or lactation, due to insufficient data on potential effects of the magnetic field;
- Claustrophobia preventing completion of the CMR examination;
- Poor image quality due to motion or technical artifacts;
- Withdrawal of consent or the development of contraindications during the examination.
2.4. Comorbidities
2.5. CMR Examination Protocol
2.6. Cardiac Function Analysis
2.6.1. T1 Mapping–MOLLI
2.6.2. T2 Mapping–GRASE
2.7. Image Analysis and Data Processing
2.8. Statistical Analysis
3. Results
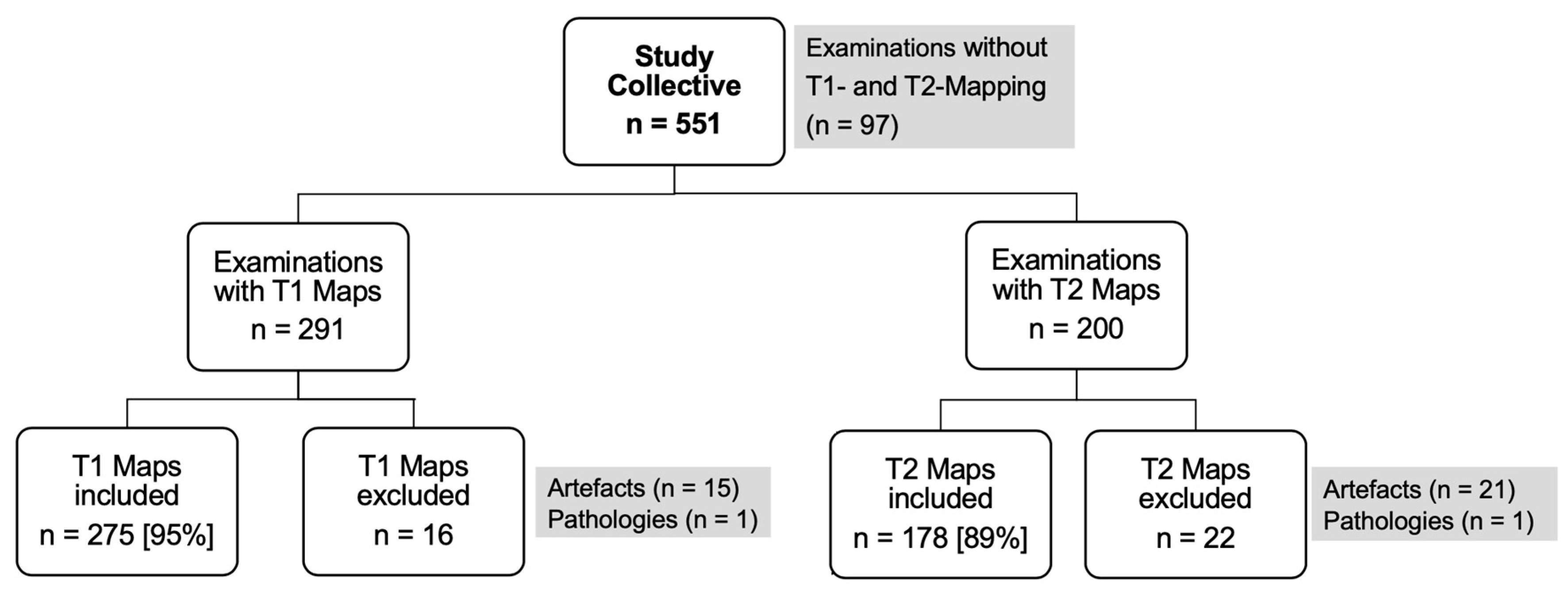
3.1. Study Population and Clinical Characteristics
3.2. Mean Values
3.2.1. T1 Relaxation Time
3.2.2. T2 Relaxation Time
3.3. Intra- and Inter-Observer Variability
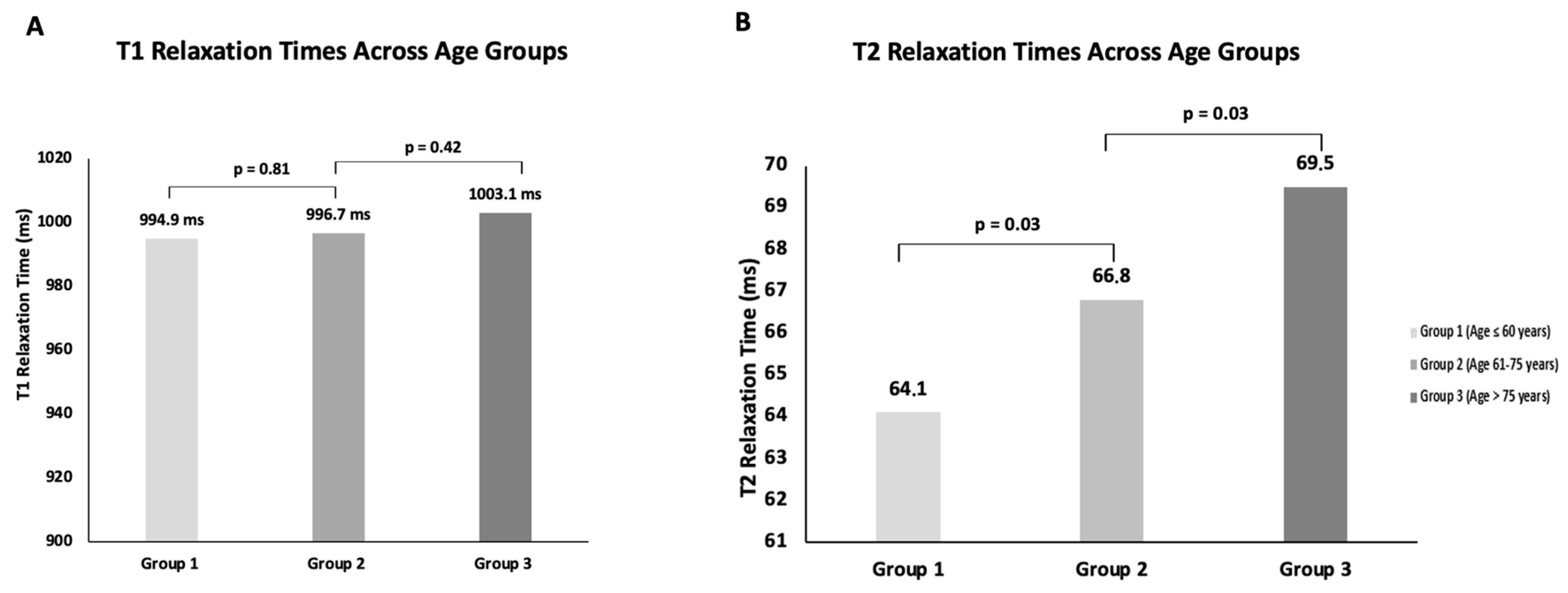
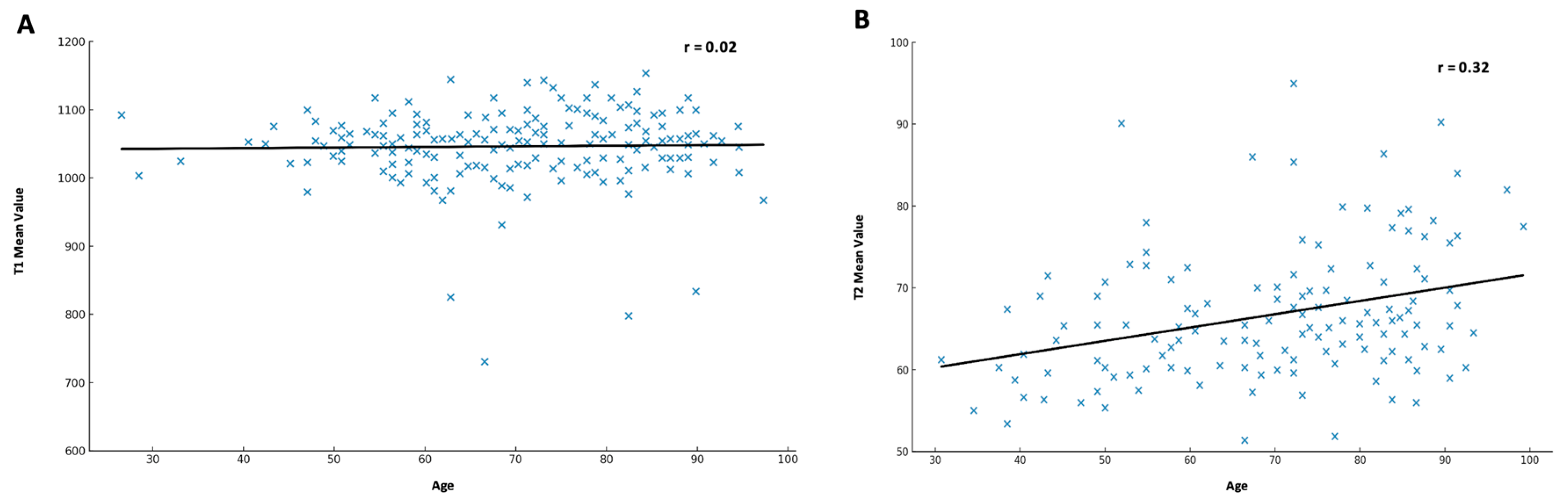
3.4. Influence of Age
3.4.1. T1 Relaxation Time
3.4.2. T2 Relaxation Time
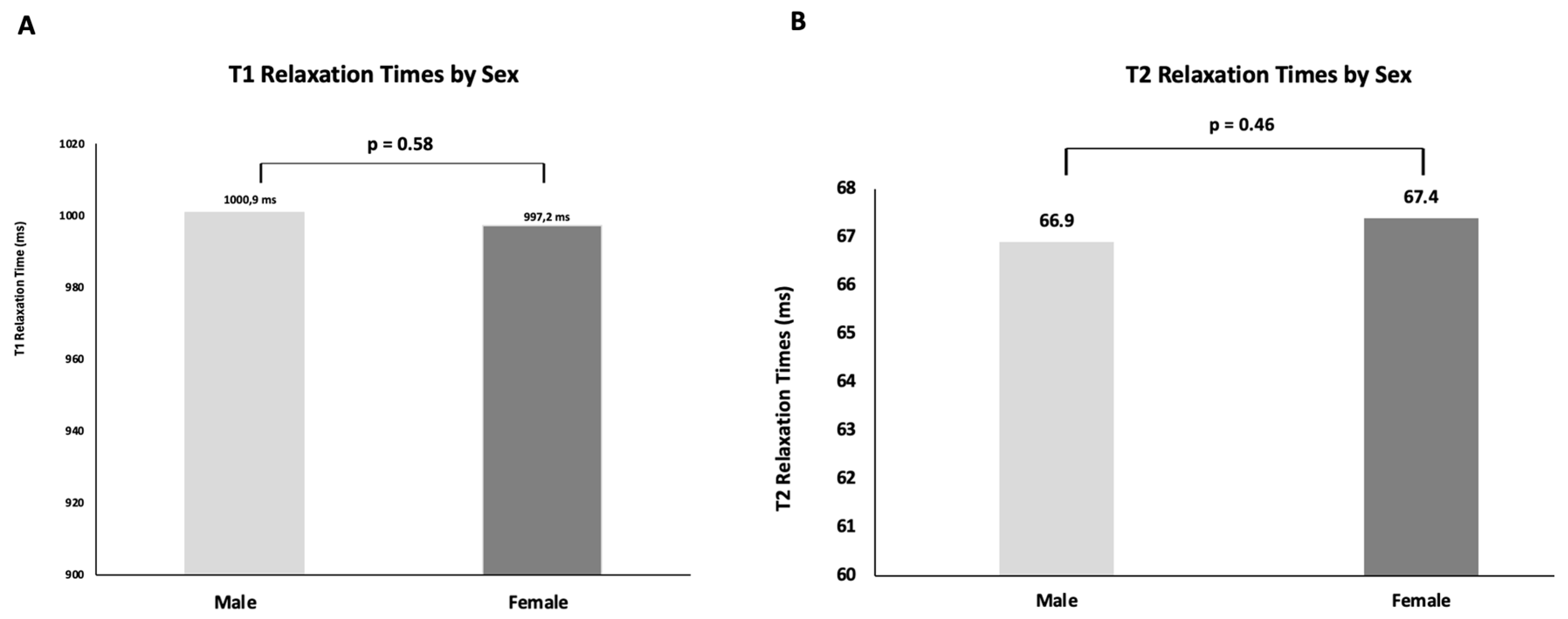
3.5. Influence of Gender
3.5.1. T1 Relaxation Time
3.5.2. T2 Relaxation Time
3.6. Influence of Comorbidities
3.6.1. T1 Relaxation Time
3.6.2. T2 Relaxation Time
3.7. Summary of Findings
4. Discussion
4.1. T1 and T2 Relaxation Times in the Myocardium of a Representative Clinical Cohort
4.2. Influence of Comorbidities on T1 Relaxation Times
4.3. Age and Sex Influence on T1 Relaxation Times
4.4. T2 Relaxation Times and Their Determinants
4.5. Generalizability of Results
4.6. Clinical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AI | Artificial intelligence |
| b-SSFP | Balanced steady-state free precession |
| CAD | Coronary Artery Disease |
| CCS | Canadian Cardiovascular Society |
| COPD | Chronic obstructive pulmonary disease |
| CMR | Cardiac magnetic resonance imaging |
| CVD | Cardiovascular disease |
| DGK | German Cardiac Society |
| EACVI | European Association for Cardiovascular Imaging |
| EPI | Echo planar imaging |
| ESC | European Society of Cardiology |
| GRaSE | Gradient and spin-echo sequence |
| ICD | Implantable cardioverter defibrillator |
| LGE | Late gadolinium enhancement |
| LVEF | Left ventricular ejection fraction |
| MOLLI | Modified Look-Locker Inversion Recovery |
| MR | Mitral regurgitation |
| NYHA | New York Heart Association |
| SCMR | Society for Cardiovascular Magnetic Resonance |
| TSE | Turbo spin echo |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Rampidis, G.P.; Kampaktsis, P.; Kouskouras, K.; Samaras, A.; Benetos, G.; Giannopoulos, A.; Karamitsos, T.; Kallifatidis, A.; Samaras, A.; Vogiatzis, I.; et al. Role of cardiac CT in the diagnostic evaluation and risk stratification of patients with myocardial infarction and non-obstructive coronary arteries (MINOCA): Rationale and design of the MINOCA-GR study. BMJ Open 2022, 12, e054698. [Google Scholar] [CrossRef]
- Channon, K.M.; Newby, D.E.; Nicol, E.D.; Deanfield, J. Cardiovascular computed tomography imaging for coronary artery disease risk: Plaque, flow and fat. Heart 2022, 108, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.A.; Chow, K.; Salerno, M. Myocardial T1 and ECV Measurement: Underlying Concepts and Technical Considerations. JACC Cardiovasc. Imaging 2019, 12 Pt 2, 2332–2344. [Google Scholar] [CrossRef]
- Kim, P.K.; Hong, Y.J.; Im, D.J.; Suh, Y.J.; Park, C.H.; Kim, J.Y.; Chang, S.; Lee, H.-J.; Hur, J.; Kim, Y.J.; et al. Myocardial T1 and T2 Mapping: Techniques and Clinical Applications. Korean J. Radiol. 2017, 18, 113–131. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Gaizauskiene, K.; Leketaite, K.; Glaveckaite, S.; Valeviciene, N. Diagnostic Value of Cardiovascular Magnetic Resonance T1 and T2 Mapping in Acute Myocarditis: A Systematic Literature Review. Medicina 2024, 60, 1162. [Google Scholar] [CrossRef]
- Carrabba, N.; Amico, M.A.; Guaricci, A.I.; Carella, M.C.; Maestrini, V.; Monosilio, S.; Pedrotti, P.; Ricci, F.; Monti, L.; Figliozzi, S.; et al. CMR Mapping: The 4th-Era Revolution in Cardiac Imaging. J. Clin. Med. 2024, 13, 337. [Google Scholar] [CrossRef]
- Haberkorn, S.M.; Spieker, M.; Jacoby, C.; Flögel, U.; Kelm, M.; Bönner, F. State of the Art in Cardiovascular T2 Mapping: On the Way to a Cardiac Biomarker? Curr. Cardiovasc. Imaging Rep. 2018, 11, 15. [Google Scholar] [CrossRef]
- Bhatt, N.; Ramanan, V.; Orbach, A.; Biswas, L.; Ng, M.; Guo, F.; Qi, X.; Guo, L.; Jimenez-Juan, L.; Roifman, I.; et al. A Deep Learning Segmentation Pipeline for Cardiac T1 Mapping Using MRI Relaxation-based Synthetic Contrast Augmentation. Radiol. Artif. Intell. 2022, 4, e210294. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; 3rd Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Grazzini, G.; Pradella, S.; Bani, R.; Fornaciari, C.; Cappelli, F.; Perfetto, F.; Cozzi, D.; Giovannelli, S.; Sica, G.; Miele, V. The Role of T2 Mapping in Cardiac Amyloidosis. Diagnostics 2024, 14, 1048. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Monastero, S.; Todiere, G.; Barison, A.; De Gori, C.; Grigoratos, C.; Parisella, M.L.; Faggioni, L.; Cioni, D.; Lencioni, R.; et al. Diagnostic Role of Native T1 Mapping Compared to Conventional Magnetic Resonance Techniques in Cardiac Disease in a Real-Life Cohort. Diagnostics 2023, 13, 2461. [Google Scholar] [CrossRef] [PubMed]
- Kawel-Boehm, N.; Hetzel, S.J.; Ambale-Venkatesh, B.; Captur, G.; Francois, C.J.; Jerosch-Herold, M.; Salerno, M.; Teague, S.D.; Valsangiacomo-Buechel, E.; van der Geest, R.J.; et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 87. [Google Scholar] [CrossRef]
- Pica, S.; Sado, D.M.; Maestrini, V.; Fontana, M.; White, S.K.; Treibel, T.; Captur, G.; Anderson, S.; Piechnik, S.K.; Robson, M.D.; et al. Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2014, 16, 99. [Google Scholar] [CrossRef]
- Mordi, I.; Carrick, D.; Bezerra, H.; Tzemos, N. T1 and T2 mapping for early diagnosis of dilated non-ischaemic cardiomyopathy in middle-aged patients and differentiation from normal physiological adaptation. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 797–803. [Google Scholar] [CrossRef]
- Staerk, L.; Sherer, J.A.; Ko, D.; Benjamin, E.J.; Helm, R.H. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ. Res. 2017, 120, 1501–1517. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, S.M.; Cho, S.J.; Choe, Y.H. Longitudinal Changes in the Myocardial T1 Relaxation Time, Extracellular Volume Fraction, and Left Ventricular Function in Asymptomatic Men. J. Cardiovasc. Dev. Dis. 2023, 10, 252. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Ma, X.; Greiser, A.; Zhang, T.; An, J.; Bai, R.; Dong, J.; Fan, Z. Systolic MOLLI T1 mapping with heart-rate-dependent pulse sequence sampling scheme is feasible in patients with atrial fibrillation. J. Cardiovasc. Magn. Reson. 2016, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Chehab, O.; Roberts-Thomson, R.; Ng Yin Ling, C.; Marber, M.; Prendergast, B.D.; Rajani, R.; Redwood, S.R. Secondary mitral regurgitation: Pathophysiology, proportionality and prognosis. Heart 2020, 106, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.; Stromp, T.A.; Hui, Z.; Vandsburger, M. Myocardial native-T1 times are elevated as a function of hypertrophy, HbA1c, and heart rate in diabetic adults without diffuse fibrosis. Magn. Reson. Imaging 2019, 61, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Myhr, K.A.; Andres-Jensen, L.; Larsen, B.S.; Kunkel, J.B.; Kristensen, C.B.; Vejlstrup, N.; Køber, L.; Pecini, R. Sex-and age-related variations in myocardial tissue composition of the healthy heart: A native T1 mapping cohort study. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Bonner, F.; Janzarik, N.; Jacoby, C.; Spieker, M.; Schnackenburg, B.; Range, F.; Butzbach, B.; Haberkorn, S.; Westenfeld, R.; Neizel-Wittke, M.; et al. Myocardial T2 mapping reveals age- and sex-related differences in volunteers. J. Cardiovasc. Magn. Reson. 2015, 17, 9. [Google Scholar] [CrossRef]
- Ruiz-Meana, M.; Bou-Teen, D.; Ferdinandy, P.; Gyongyosi, M.; Pesce, M.; Perrino, C.; Schulz, R.; Sluijter, J.P.G.; Tocchetti, C.G.; Thum, T.; et al. Cardiomyocyte ageing and cardioprotection: Consensus document from the ESC working groups cell biology of the heart and myocardial function. Cardiovasc. Res. 2020, 116, 1835–1849. [Google Scholar] [CrossRef]
- Markousis-Mavrogenis, G.; Belegrinos, A.; Giannakopoulou, A.; Papavasiliou, A.; Koulouri, V.; Marketos, N.; Patsilinakou, E.; Lazarioti, F.; Bacopoulou, F.; Mavragani, C.P.; et al. Cardiovascular Magnetic Resonance Demonstrates Myocardial Inflammation of Differing Etiologies and Acuities in Patients with Genetic and Inflammatory Myopathies. J. Clin. Med. 2023, 12, 1575. [Google Scholar] [CrossRef]
- Busse, A.; Rajagopal, R.; Yucel, S.; Beller, E.; Oner, A.; Streckenbach, F.; Cantré, D.; Ince, H.; Weber, M.A.; Meinel, F.G. Cardiac MRI-Update 2020. Radiologe 2020, 60 (Suppl. 1), 33–40. [Google Scholar] [CrossRef]
| T1-Group n = 291 [in %] | T2-Group n = 200 [in %] | p-Value | |
|---|---|---|---|
| Age [years] | 70.8 [±14.4] | 68.1 [±16.3] | 0.056 |
| Female sex | 107 [37%] | 60 [30%] | 0.120 |
| BMI > 30 kg/m2 | 32 [11%] | 19 [10%] | 0.968 |
| Chronic Coronary Disease (CAD) | 144 [49%] | 92 [47%] | 0.657 |
| Single-vessel disease (SVD) | 24 [8%] | 20 [10%] | 0.440 |
| Two-vessel disease (2VD) | 30 [10%] | 23 [12%] | 0.594 |
| Three-vessel disease (3VD) | 83 [29%] | 44 [22%] | 0.152 |
| Chronic Kidney Disease (CKD) | 27 [9%] | 16 [8%] | 0.696 |
| Diastolisc Dysfunction | 52 [18%] | 39 [20%] | 0.538 |
| EF < 55% | 72 [25%] | 53 [27%] | 0.526 |
| Cardiomyopathy | 17 [6%] | 19 [10%] | 0.104 |
| Atrial Fibrilation (AF) | 53 [18%] | 28 [14%] | 0.319 |
| COPD | 21 [7%] | 13 [7%] | 0.828 |
| Hypertension | 184 [63%] | 117 [60%] | 0.517 |
| Nicotin usage | 61 [21%] | 36 [19%] | 0.517 |
| Dyslipoprotinemia | 107 [37%] | 72 [37%] | 0.939 |
| Diabetes Mellitus | 35 [12%] | 24 [12%] | 0.910 |
| NYHA Status | |||
| O | 122 [42%] | 81 [40%] | 0.681 |
| I | 49 [17%] | 38 [19%] | 0.503 |
| II | 79 [27%] | 55 [27%] | 0.861 |
| III | 41 [14%] | 24 [12%] | 0.464 |
| IV | 1 [0%] | 2 [1%] | 0.319 |
| CCS Status | |||
| O | 224 [77%] | 158 [79%] | 0.674 |
| I | 9 [3%] | 10 [5%] | 0.219 |
| II | 26 [9%] | 12 [6%] | 0.162 |
| III | 17 [6%] | 6 [3%] | 0.204 |
| IV | 12 [4%] | 14 [7%] | 0.243 |
| Severe Aortic Senosis | 19 [7%] | 14 [8%] | 0.679 |
| Severe Aortic Regurgitation | 31 [11%] | 31 [17%] | 0.058 |
| Severe Mitral Stenosis | 7 [2%] | 2 [1%] | 0.298 |
| Severe Mitral Regurgitation | 63 [22%] | 44 [24%] | 0.615 |
| Severe Tricuspid Regurgitation | 38 [13%] | 29 [16%] | 0.442 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, M.; Koch, V.; Martin, S.; Vogl, T.; Ochs, M.M.; Leistner, D.M.; Haberkorn, S.M. Standardized Myocardial T1 and T2 Relaxation Times: Defining Age- and Comorbidity-Adjusted Reference Values for Improved CMR-Based Tissue Characterization. J. Clin. Med. 2025, 14, 6198. https://doi.org/10.3390/jcm14176198
Rana M, Koch V, Martin S, Vogl T, Ochs MM, Leistner DM, Haberkorn SM. Standardized Myocardial T1 and T2 Relaxation Times: Defining Age- and Comorbidity-Adjusted Reference Values for Improved CMR-Based Tissue Characterization. Journal of Clinical Medicine. 2025; 14(17):6198. https://doi.org/10.3390/jcm14176198
Chicago/Turabian StyleRana, Mukaram, Vitali Koch, Simon Martin, Thomas Vogl, Marco M. Ochs, David M. Leistner, and Sebastian M. Haberkorn. 2025. "Standardized Myocardial T1 and T2 Relaxation Times: Defining Age- and Comorbidity-Adjusted Reference Values for Improved CMR-Based Tissue Characterization" Journal of Clinical Medicine 14, no. 17: 6198. https://doi.org/10.3390/jcm14176198
APA StyleRana, M., Koch, V., Martin, S., Vogl, T., Ochs, M. M., Leistner, D. M., & Haberkorn, S. M. (2025). Standardized Myocardial T1 and T2 Relaxation Times: Defining Age- and Comorbidity-Adjusted Reference Values for Improved CMR-Based Tissue Characterization. Journal of Clinical Medicine, 14(17), 6198. https://doi.org/10.3390/jcm14176198








