Hair Transplantation for Lichen Planopilaris: A Case Series of Five Patients
Abstract
1. Introduction
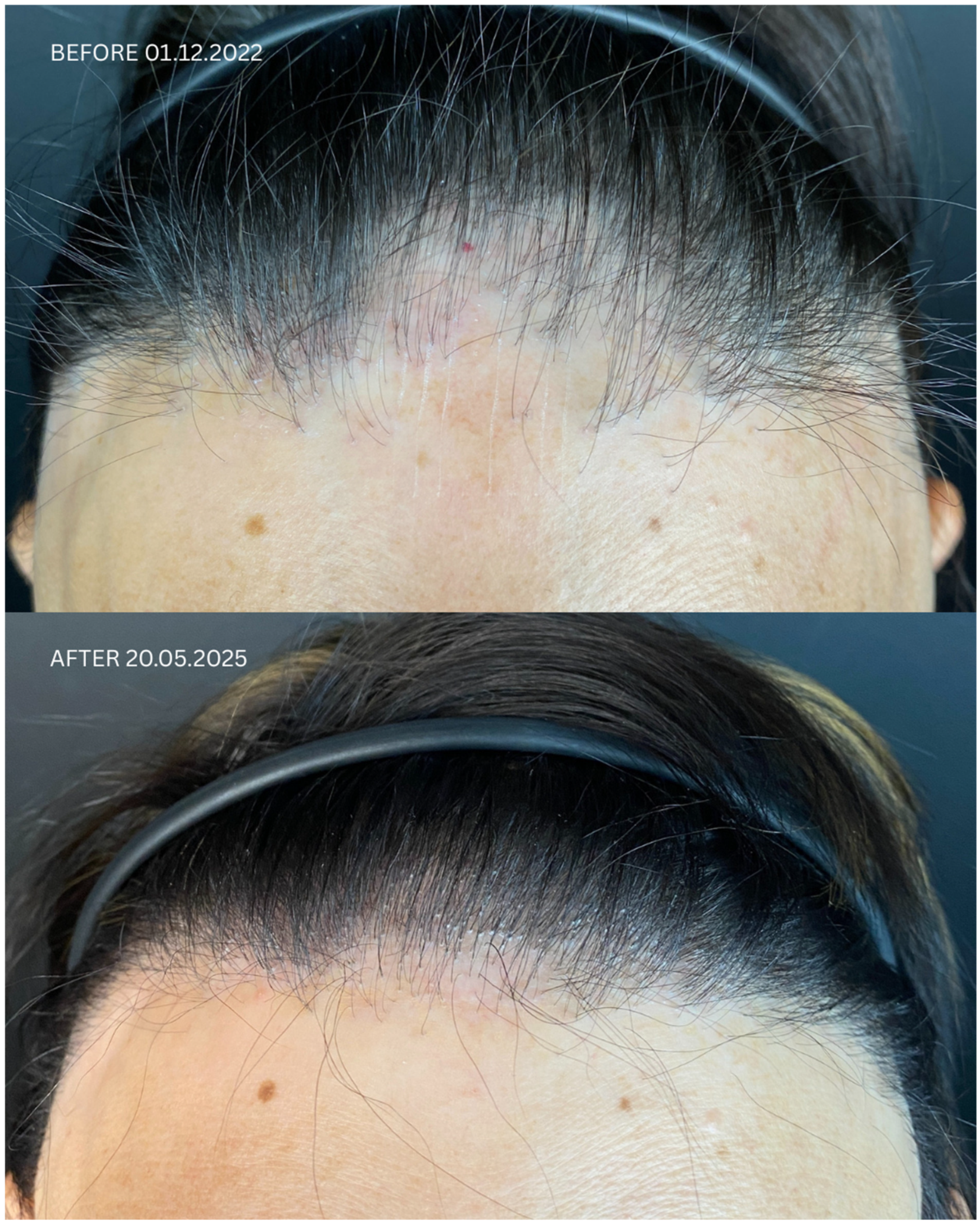
2. Case Presentation
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naeini, F.F.; Saber, M.; Faghihi, G. Lichen planopilaris: A review of evaluation methods. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 442–445. [Google Scholar] [CrossRef]
- Cardoso, C.O.; Tolentino, S.; Gratieri, T.; Cunha-Filho, M.; Lopez, R.F.; Gelfuso, G.M. Topical treatment for scarring and Non-Scarring alopecia: An overview of the current evidence. Clin. Cosmet. Investig. Dermatol. 2021, 14, 485–499. [Google Scholar] [CrossRef]
- Aukerman, E.L.; Jafferany, M. The psychological consequences of androgenetic alopecia: A systematic review. J. Cosmet. Dermatol. 2023, 22, 89–95. [Google Scholar] [CrossRef]
- Ujiie, H.; Rosmarin, D.; Schön, M.P.; Ständer, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Muthuvel, K. Role of Hair Transplantation in Scarring Alopecia-To Do or Not to Do. Indian J. Plast. Surg. 2021, 54, 501–506. [Google Scholar] [CrossRef]
- Crisóstomo, M.R.; Crisóstomo, M.G.R.; Crisóstomo, M.C.C.; Gondim, V.J.T.; Benevides, A.N. Hair loss due to lichen planopilaris after hair transplantation: A report of two cases and a literature review. An. Bras. Dermatol. 2011, 86, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J. Lichen planopilaris after hair transplantation: Report of 17 cases. Dermatol. Surg. 2012, 38, 1998–2004. [Google Scholar] [CrossRef]
- Chiang, Y.Z.; Tosti, A.; Chaudhry, I.H.; Lyne, L.; Farjo, B.; Farjo, N.; Cadore de Farias, D.; Griffiths, C.; Paus, R.; Harries, M. Lichen planopilaris following hair transplantation and face-lift surgery. Br. J. Dermatol. 2012, 166, 666–670. [Google Scholar] [CrossRef]
- Ekelem, C.; Pham, C.; Atanaskova Mesinkovska, N. A Systematic Review of the Outcome of Hair Transplantation in Primary Scarring Alopecia. Skin. Appendage Disord. 2019, 5, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Levy, D.A.; Patel, K.G.; Brennan, E.; Oyer, S.L. Hair Transplantation in Frontal Fibrosing Alopecia and Lichen Planopilaris: A Systematic Review. Laryngoscope 2021, 131, 59–66. [Google Scholar] [CrossRef]
- Ueki, H. Koebner phenomenon in lupus erythematosus with special consideration of clinical findings. Autoimmun. Rev. 2005, 4, 219–223. [Google Scholar] [CrossRef]
- Errichetti, E.; Figini, M.; Croatto, M.; Stinco, G. Therapeutic management of classic lichen planopilaris: A systematic review. Clin. Cosmet. Investig. Dermatol. 2018, 11, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Ajuchi, O.; Lukowiak, T.M.; Rao, B.K. Off-label use of biologics and janus kinase (JAK) inhibitors for scarring alopecias: A narrative review. Arch. Dermatol. Res. 2025, 317, 803. [Google Scholar] [CrossRef]
- Ahmed, H.; Petkar, M.; Steinhoff, M. Successful treatment of rare linear lichen planopilaris with Ixekizumab. J. Dermatolog. Treat. 2023, 34, 2201364.4. [Google Scholar] [CrossRef]
- Peters, G.; Pina, K.; Posligua, A.; Durkin, J. 53358 Ixekizumab in Patients with Lichen Planopilaris—An Open-Label Study. J. Am. Acad. Dermatol. 2024, 91 (Suppl. S3), AB246. [Google Scholar] [CrossRef]
- Takahashi, T.; Yamasaki, K.; Terui, H.; Omori, R.; Tsuchiyama, K.; Fujimura, T.; Aiba, S. Perifolliculitis capitis abscedens et suffodiens treatment with tumor necrosis factor inhibitors: A case report and review of published cases. J. Dermatol. 2019, 46, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; LaBelle, B. Treatment of lichen planopilaris with adalimumab in a patient with hidradenitis suppurativa and rheumatoid arthritis. JAAD Case Rep. 2020, 6, 219–221. [Google Scholar] [CrossRef]
- Workman, K.; Kindred, C. Hair regrowth in a patient with central centrifugal cicatricial alopecia after a 2-month trial of baricitinib. JAAD Case Rep. 2023, 39, 109–111. [Google Scholar] [CrossRef]
- Kumar, A.R.; Ishii, L.E. Hair transplantation for scarring alopecia. Facial Plast. Surg. Clin. N. Am. 2020, 28, 177–179. [Google Scholar] [CrossRef]
- Avram, M.R.; Finney, R.; Rogers, N. Hair transplantation controversies. Derm. Surg. 2017, 43 (Suppl. S2), S158–S162. [Google Scholar] [CrossRef]
- Darchini-Maragheh, E.; Moussa, A.; Yoong, N.; Bokhari, N.; Jones, L.; Sinclair, R. Alopecia Areata–Specific Patient-Reported Outcome Measures: A Systematic Review. JAMA Dermatol. 2025, 161, 421–429. [Google Scholar] [CrossRef]
- Sattur, S.S.; Sattur, S.I. Patient Counselling and Medicolegal Aspects of Hair Transplant Surgery. Indian J. Plast. Surg. 2021, 54, 441–445. [Google Scholar] [CrossRef]
- Desai, V. Managing an Unhappy Patient. Indian J. Plast. Surg. 2021, 54, 495–500. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Qu, Q.; Fan, Z.-X.; Miao, Y.; Hu, Z.-Q. Evaluating the Satisfaction of Patients Undergoing Hair Transplantation Surgery Using the FACE-Q Scales. Aesth. Plast. Surg. 2019, 43, 376–382. [Google Scholar] [CrossRef]
- Paichitrojjana, A.; Paichitrojjana, A. Platelet Rich Plasma and Its Use in Hair Regrowth: A Review. Drug Des. Devel Ther. 2022, 16, 635–645. [Google Scholar] [CrossRef]
- Behrangi, E.; Akbarzadehpasha, A.; Dehghani, A.; Zare, S.; Ghassemi, M.; Zeinali, R.; Goodarzi, A.; Lotfi, Z. Platelet-rich plasma as a new and successful treatment for lichen planopilaris: A controlled blinded randomized clinical trial. J. Cosmet. Dermatol. 2024, 23, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Saxena, D.K.; Savant, S.S. Successful Hair Transplant Outcome in Cicatricial Lichen Planus of the Scalp by Combining Scalp and Beard Hair Along With Platelet Rich Plasma. J. Cut. Aesth. Surg. 2016, 9, 51–55. [Google Scholar]
- Garg, S.; Pandya, I.; Bhatt, S. Follicular Unit Extraction (FUE) Hair Transplantation in Combination with Platelet Rich Plasma for the Treatment of Scarring Alopecia: A Case Series. Arch. Clin. Med. Case Rep. 2019, 3, 299–308. [Google Scholar] [CrossRef]
- Garg, S.; Manchanda, S. Platelet-rich plasma-an ‘Elixir’ for treatment of alopecia: Personal experience on 117 patients with review of literature. Stem Cell Investig. 2017, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Kossard, S.; Shiell, R.C. Frontal fibrosing alopecia developing after hair transplantation for androgenetic alopecia. Int. J. Dermatol. 2005, 44, 321–323. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, L.; Jiang, L.; Fu, C.; Huang, J.; Hu, Y.; Zhu, L.; Zhang, F.; Chen, J.; Zeng, Q. Characteristics and pathogenesis of Koebner phenomenon. Exp. Dermatol. 2023, 32, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Long, H.; Lai, W. TRPA1 may contribute to the exacerbation of oral lichen planus through Koebner phenomenon. Oral. Dis. 2017, 23, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Popescu, F.M.; Filip, L.; Popescu, M.; Florescu, I.P. Stem cell therapy prior to follicular unit hair transplantation on scarred tissue: A novel approach to a successful procedure. J. Med. Life 2024, 17, 582–587. [Google Scholar] [CrossRef]
- Rajan, A.; Rudnicka, L.; Szepietowski, J.C.; Lallas, A.; Rokni, G.R.; Grabbe, S.; Goldust, M. Differentiation of frontal fibrosing alopecia and Lichen planopilaris on trichoscopy: A comprehensive review. J. Cosmet. Dermatol. 2022, 21, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
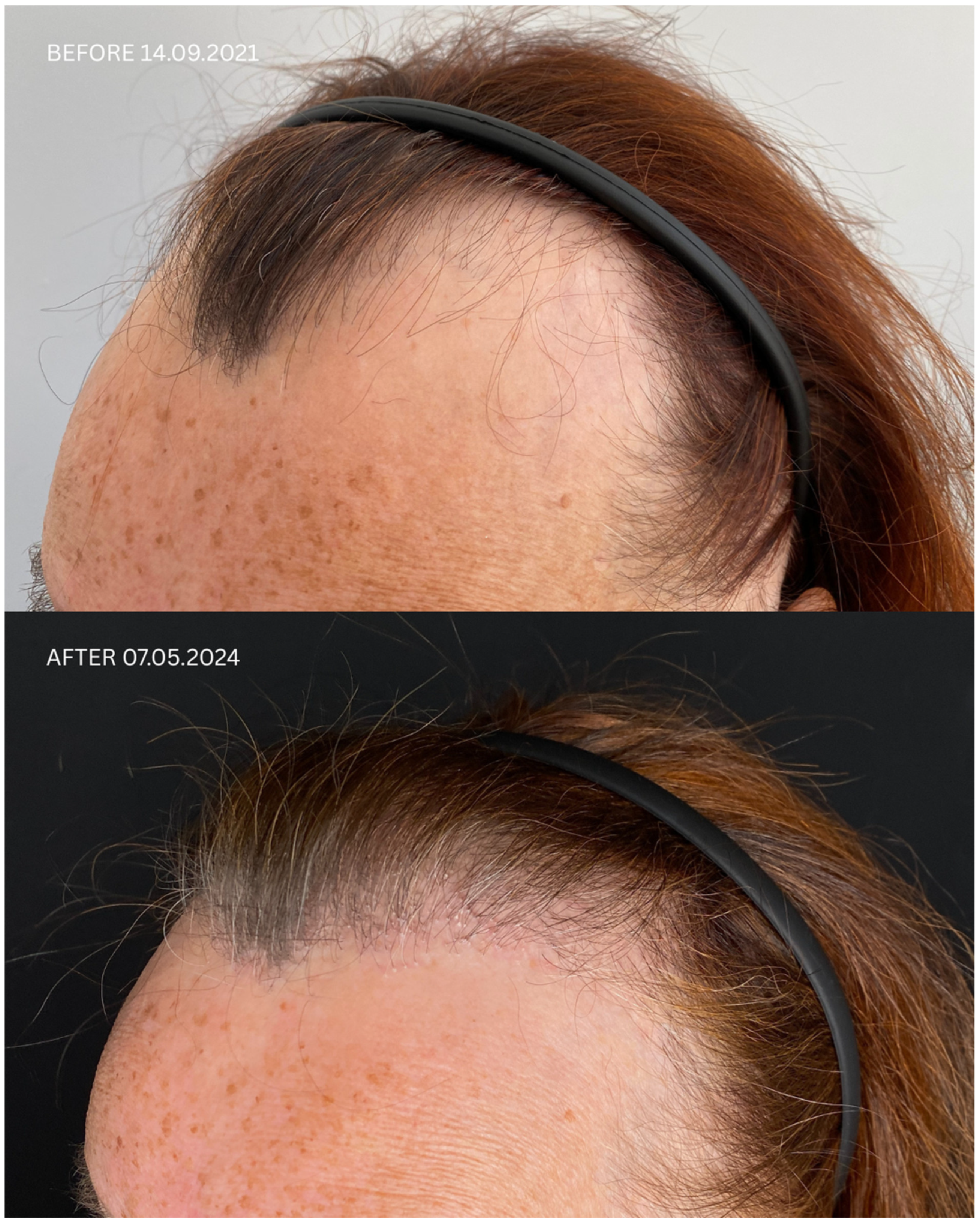
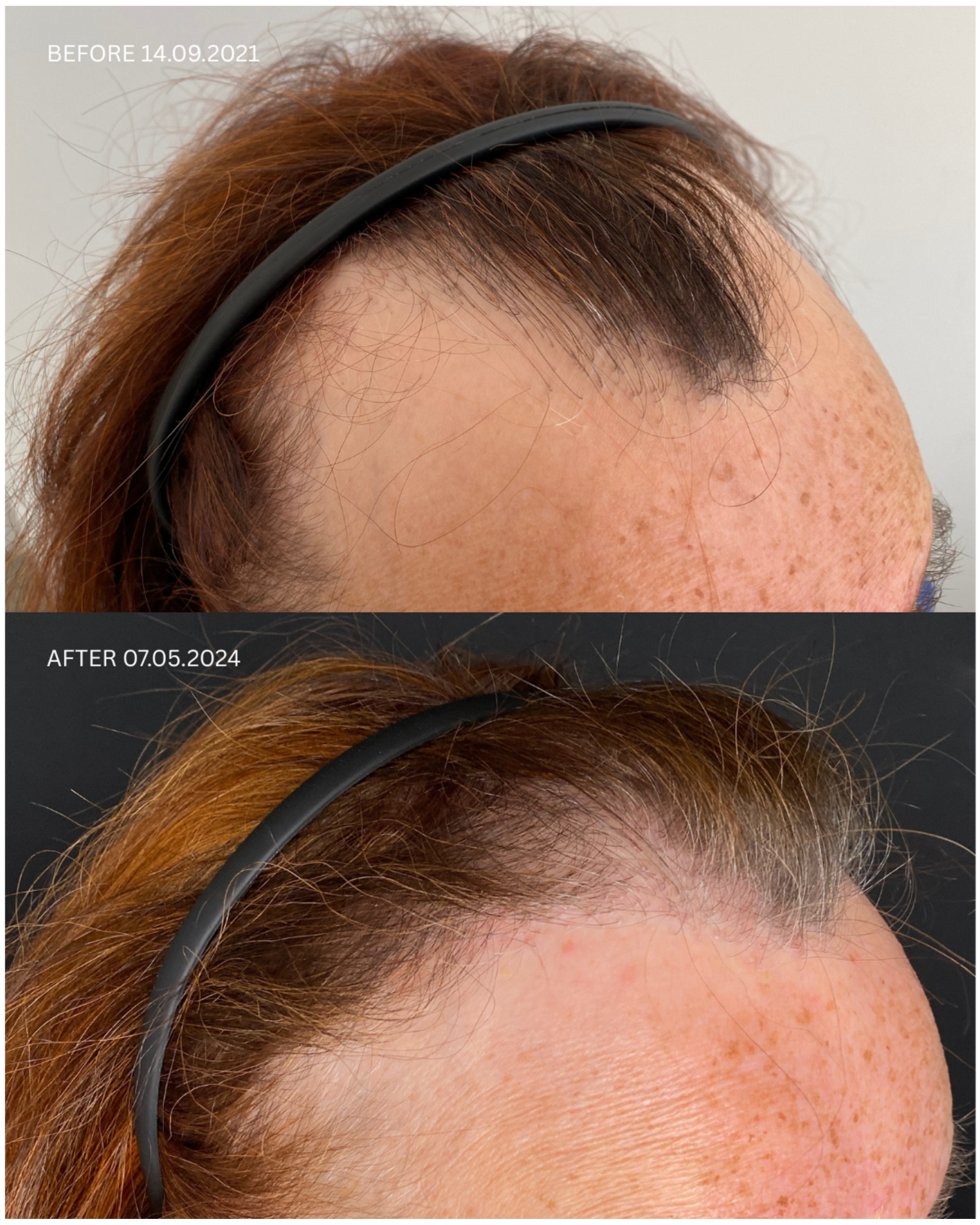
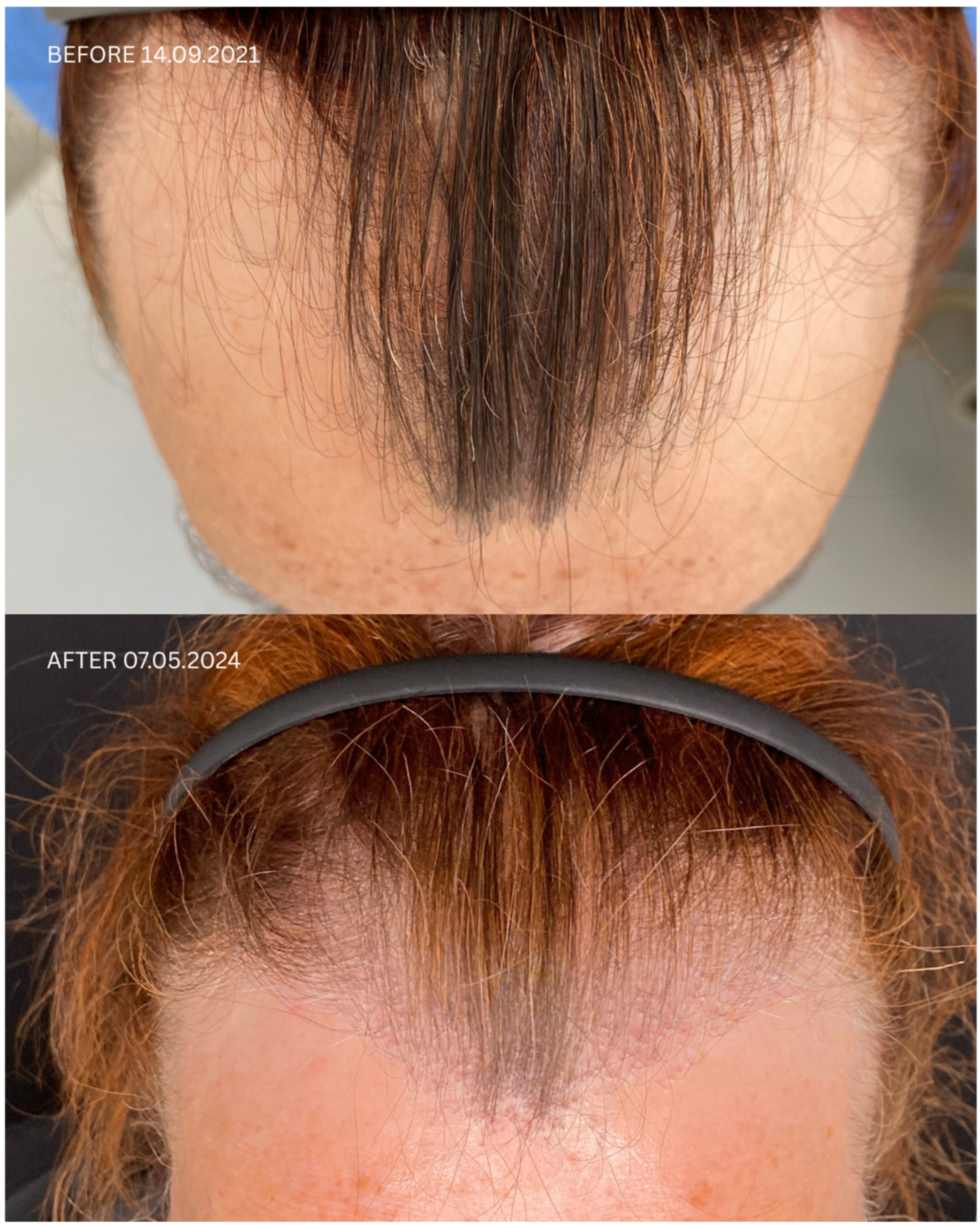
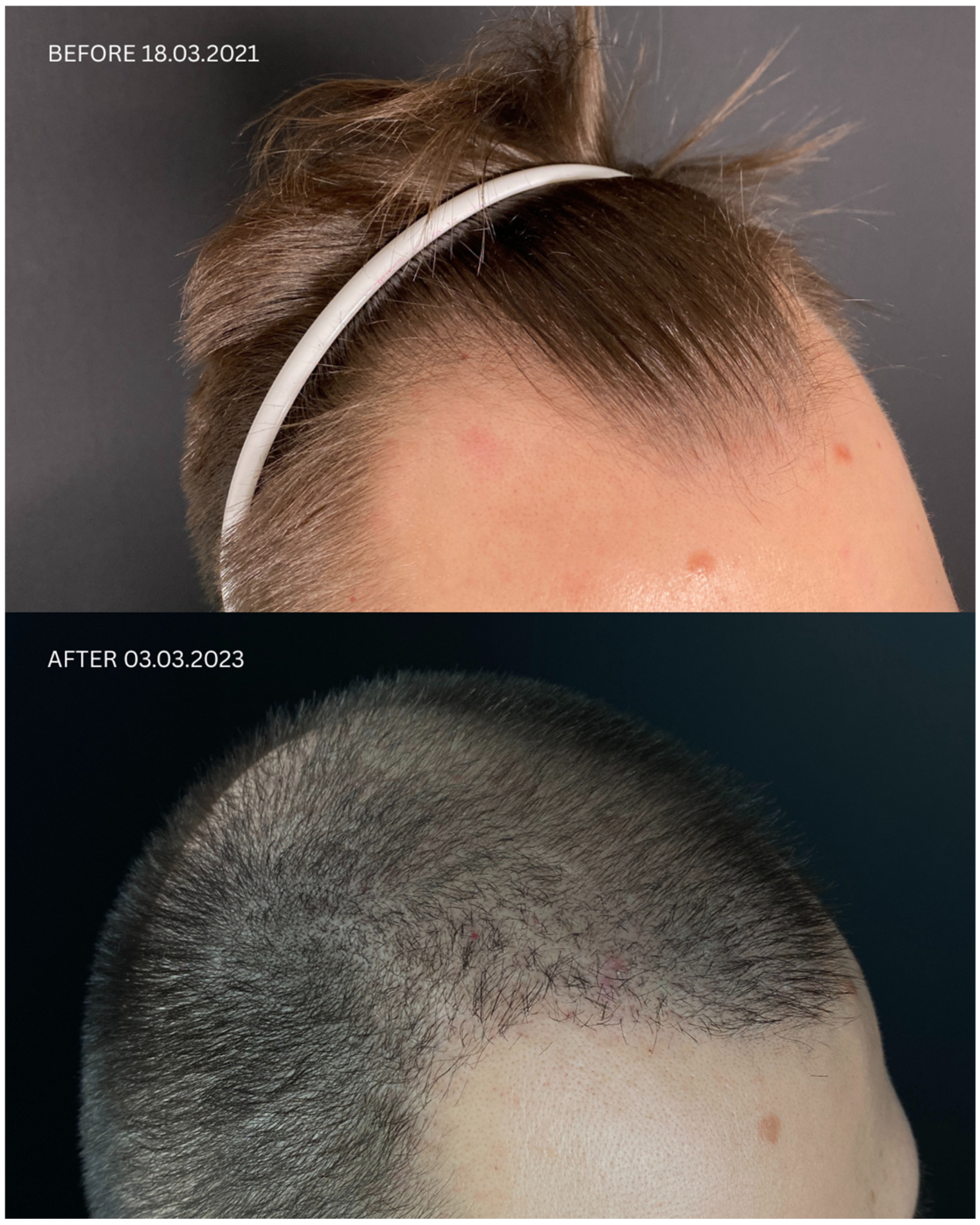


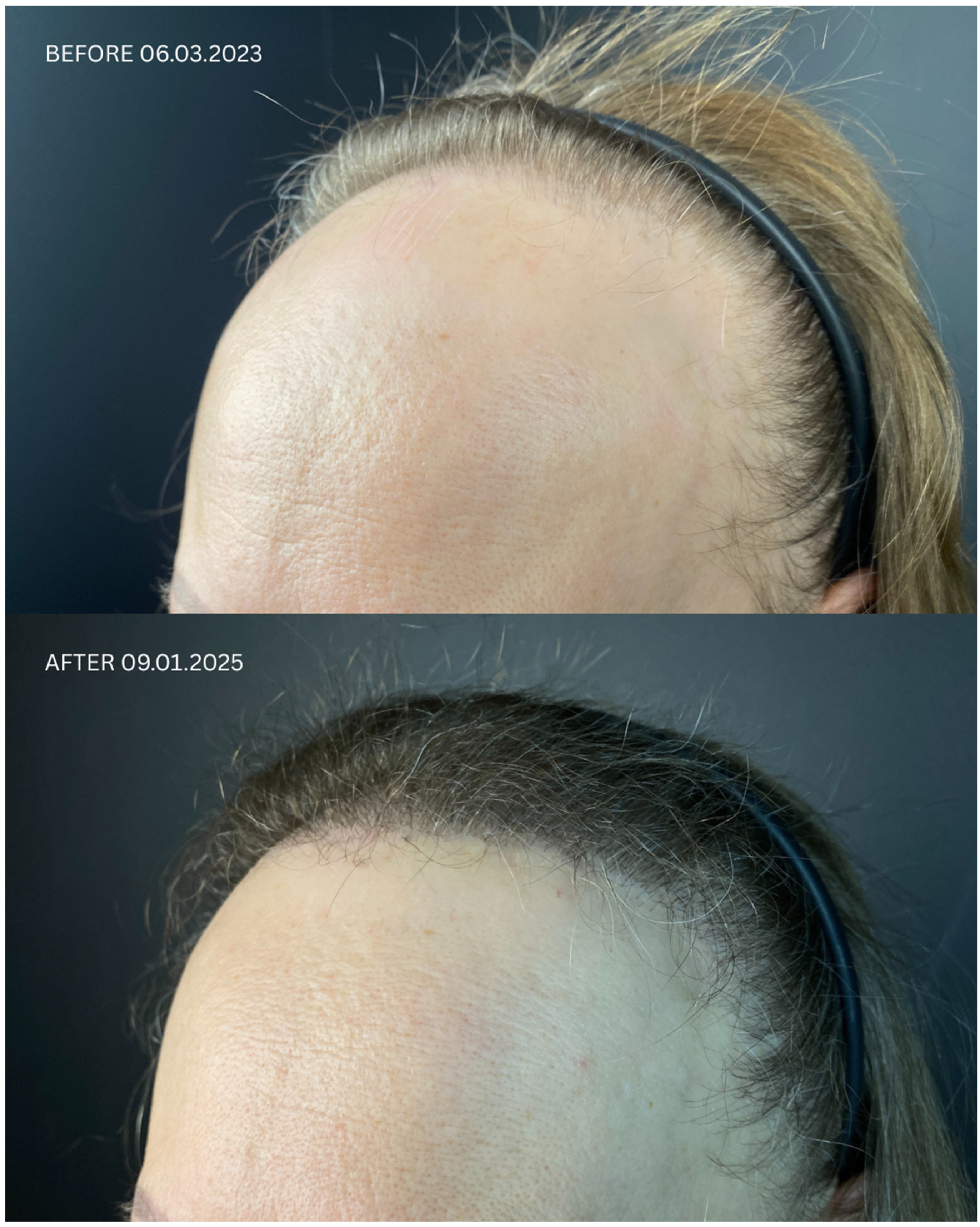
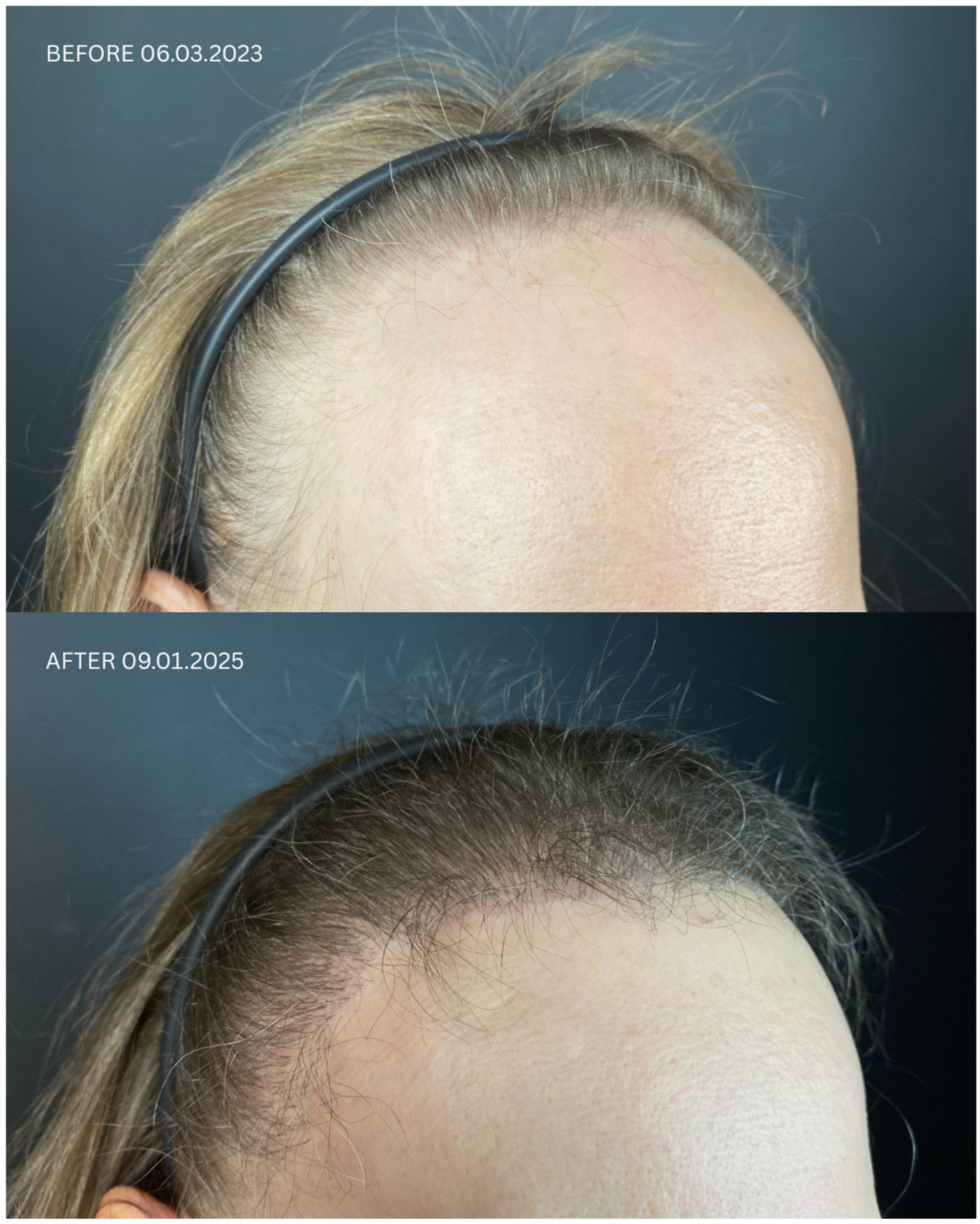
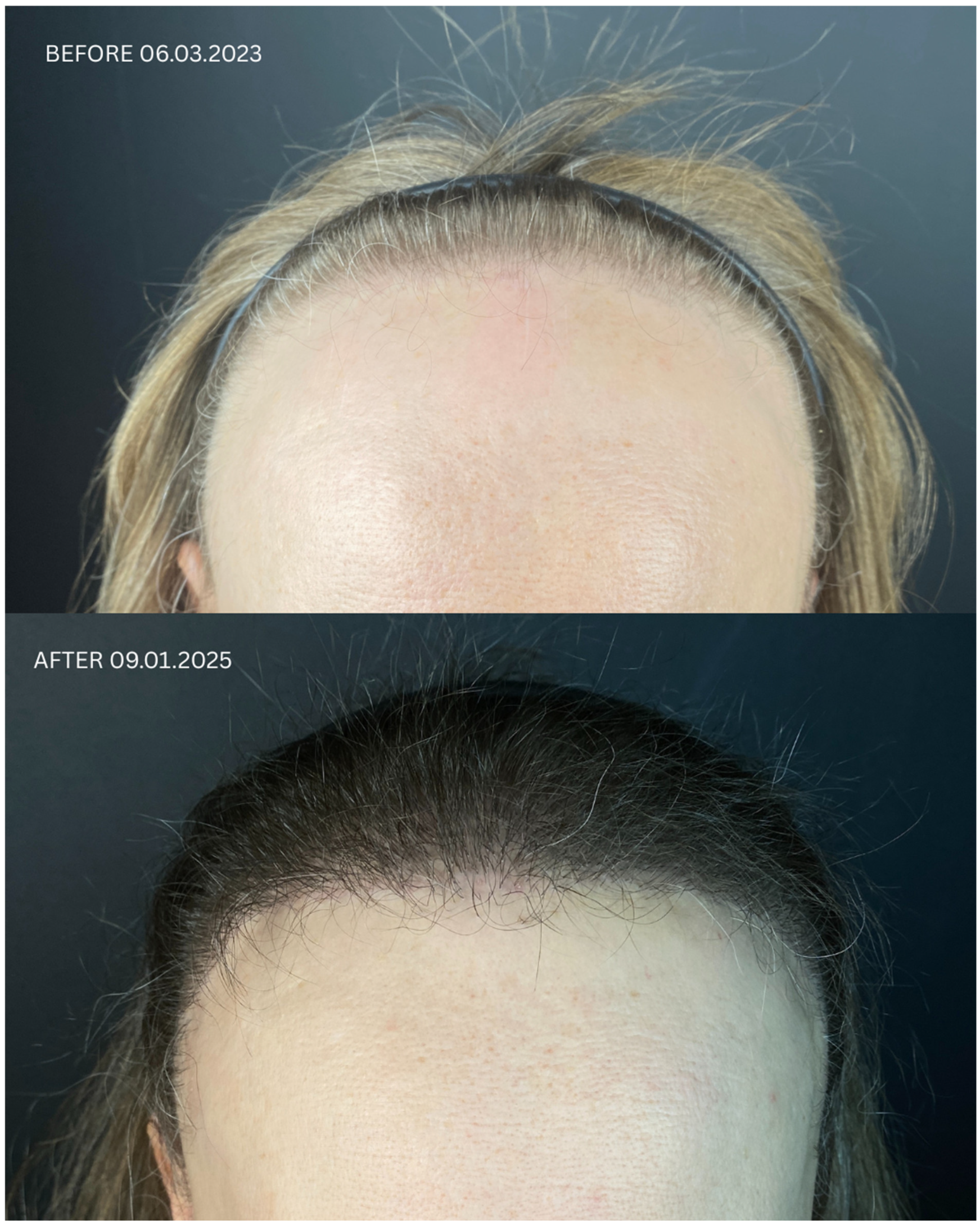

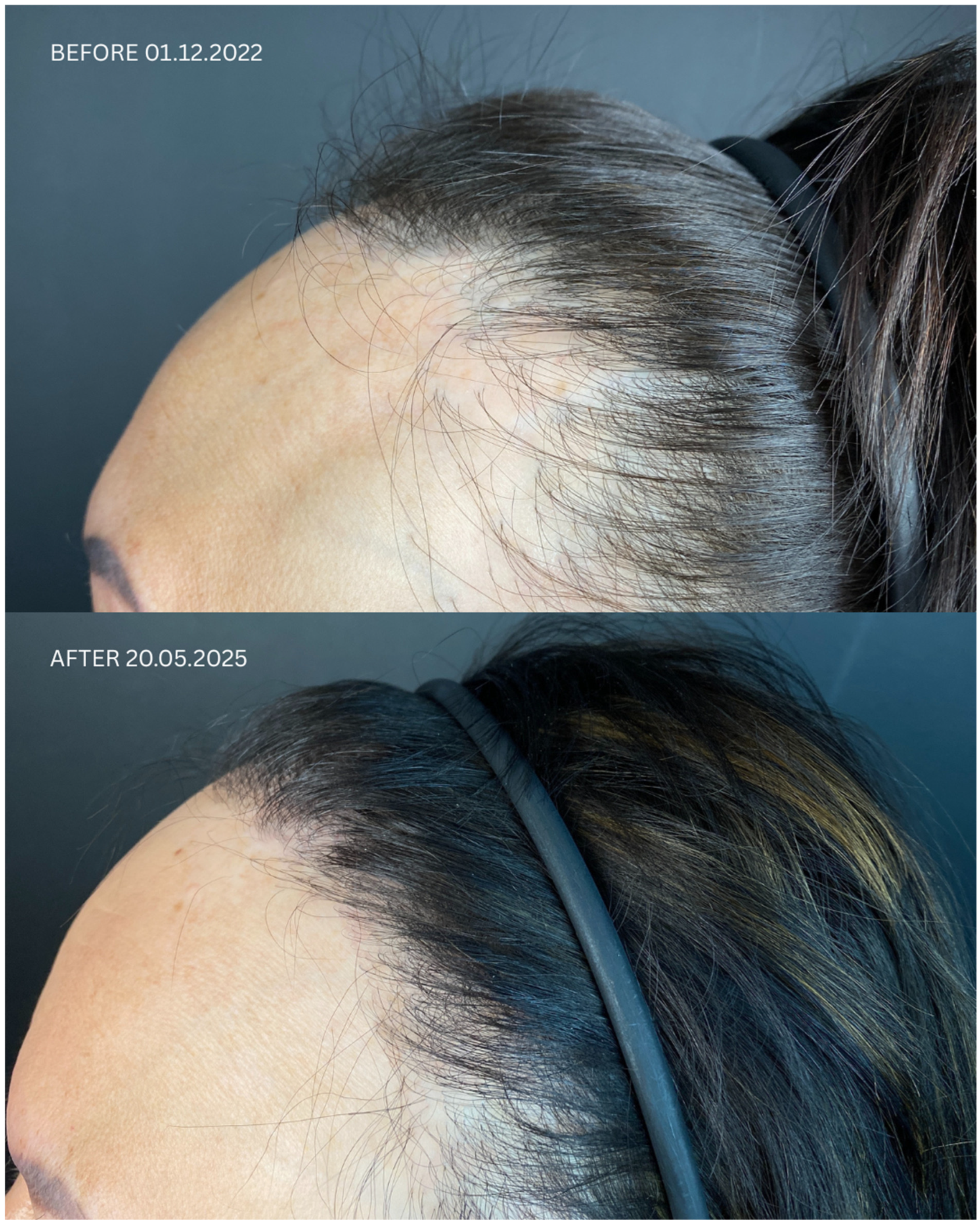
| Patient ID | Sex | Date of Diagnosis | Diagnostic Basis | Lesions in a Location Other Than the Scalp | Number of Months in Remission Before HT | Treatment in 14-Day Post-Operative Phase | Permanent Treatment After HT | Remission Duration Since the Day of HT | Date of HT | Number of Grafts | Post-Operative Procedures | Number of Follow-Up Visits from HT to Data Cut-Off Date | FU Visit, 12 Months After HT | Follow-Up in 2025 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before HT | After HT | ||||||||||||||
| 1 | F | FFA 21 January 2021 | HP+ TS+ | No | No | 8 months | Solacutan Pirolam Clobex Finahit Minoxidil 1.25 mg Phototherapy (UV) once daily | Minoxidili 1.25 mg Lactosi gs M.F. pulvis D.t.d. No. 30 PRP | 26 months Second procedure on 18 October 2022 | 14 September 2021 | 1700 + 2900 + 3200 | Tri-Wave MD Lamp, red light (633 nm, 20 min), + mesotherapy with PRP: 3 administrations every 30 days, the 4th administration 6 months after transplantation | 4 | Remission of LLP, no recurrence of LLP disease on clinical and trichoscopic examination Maintenance treatment: minoxidili 1.25 mg Lactosi gs M.F. pulvis D.t.d. No. 40, Finaster/Adadut | Remission of LLP, no recurrence of LLP disease on clinical and trichoscopic examination |
| 2 | M | 23 October 2020 | HP+ TS+ | Close to the scar | No | 5 months | Finahit Metronidasol Phototherapy (UV) | Finapil Curacn Alopexy PRP | 32 months (second procedure on 11 February 2022) | 18 March 2021 | 2500 + 2900 | Tri-Wave MD Lamp, red light (633 nm, 20 min) + mesotherapy with PRP: 3 administrations every 30 days, the 4th administration 6 months after transplantation | 4 | Remission of LLP, no recurrence of LLP disease on clinical and trichoscopic examination Maintenance treatment: Minoxidili 0.125 mg Lactosi gs M.F. pulvis D.t.d. No. 30, Finapil | Treatment finished |
| 3 | F | 16 November 2019 | HP+ TS+ | No | No | 6 months | Finasteridum Bluefish, Spironol, Minoxidil, Alpicort E, PRP | 6 March 2023 | 1500 | Tri-Wave MD Lamp, red light (633 nm, 20 min) + mesotherapy with PRP: 3 administrations every 30 days, the 4th administration 6 months after transplantation | 1 | ||||
| 4 | F | FFA 16 November 2019 | HP+ TS+ | No | No | 40 months | Finasteride Bluefish Minoksidili 0.125 mg Phototherapy (UV) | Finasteridum Bluefish, Minoksidil 0.125 mg PRP | 19 months | 6 March 2023 | 1500 | Tri-Wave MD Lamp, red light (633 nm, 20 min) + mesotherapy with PRP: 3 administrations every 30 days, the 4th administration 6 months after transplantation | 2 | Remission of LLP, no recurrence of LLP disease on clinical and trichoscopic examination Maintenance treatment: Minoxidili 0.00125 Lactosi gs M.F. pulvis D.t.d. No. 40, Finaster | Remission of LLP, no recurrence of LLP disease on clinical and trichoscopic examination |
| 5 | F | FFA 23. June 2021 | HP+ TS+ | No | No | 6 months | Axotret Dermovate Dostinex Finasteride Stada Phototherapy (UV) | Axotret PRP | 3 months (second procedure on 24 July 2024) | 1 December 2022 | 1655 | Tri-Wave MD Lamp, red light (633 nm, 20 min) + mesotherapy with PRP: 3 administrations every 30 days, the 4th administration 6 months after transplantation | 3 | Remission of LLP, no recurrence of LLP disease on clinical and trichoscopic examination Maintenance treatment: Minoxidili 1.25 mg Lactosi gs M.F. pulvis D.t.d. No. 40, Spironol | Remission of LLP, no recurrence of LLP disease on clinical and trichoscopic examination |
| Patient | Gender | Age | Time from Onset | Patient Satisfaction | |
|---|---|---|---|---|---|
| Early | After 12 Months | ||||
| Patient 1 | female | 75 | 8 years | 4 | 4–5 |
| Patient 2 | male | 33 | 10 years | 3 | 4–5 |
| Patient 3 | female | 56 | Since adolescence | 1 | 4–5 |
| Patient 4 | female | 46 | 2 years | 1 | 4–5 |
| Patient 5 | female | 43 | 2 years | 5 | 5 |
| Stage | Suggestion |
|---|---|
| Before surgery | Dermatoscopic evaluation to determine the cause of hair loss. |
| Donor area assessment Determine hair density, quality, and the number of follicular units that can be safely harvested without significantly worsening the donor area’s appearance. | |
| Recipient area assessment: Thoroughly check that the recipient area is completely free of LPP signs, even at a subclinical level. LPP can also affect the donor area, which would lead to damage or loss of extracted grafts after transplantation. | |
| Laboratory tests and confirmation of stable remission: Hair transplantation in active LPP is contraindicated. In that case, the transplanted hair follicles will be destroyed by the ongoing inflammatory process, and the procedure itself may even trigger disease reactivation. | |
| Managing patient expectations: Clearly communicate to the patient that the graft survival rate in scarred areas may be lower than in standard transplants, and density may be limited. Multiple procedures are often needed to achieve a satisfactory result. Explain that the cosmetic outcome may be inferior to transplants into healthy skin, and that initial effects will take at least 6 months, with the full effect expected no sooner than 12 months. | |
| Individualized hairline design tailored to facial features and age | |
| Planning immunosuppressive treatment before, during, and after surgery. | |
| During surgery | Recommended technique is Follicular Unit Extraction as it is less invasive and carries a lower risk of additional scarring in the donor area. |
| Platelet-Rich Plasma (PRP): PRP application may enhance procedure effectiveness by improving graft survival and scar tissue vascularization. | |
| Precise micro-incisions in the scarred areas are crucial for minimizing trauma and optimizing blood supply | |
| Lower graft density in scarred areas: Due to poorer blood supply, grafts should be implanted at a lower density in scarred areas than in standard transplants (e.g., 30–40 units/cm2 instead of 50–70 units/cm2) to prevent competition among follicles for limited blood supply and to avoid graft necrosis. | |
| After surgery | Ongoing dermatological follow-up: The patient must remain under constant dermatological care to monitor for potential LPP reactivation and implement appropriate treatment. |
| For scientific studies: Evaluations at 6 and 12 months are too infrequent to monitor effects with the sensitivity needed to capture inter-individual differences required for drawing scientific conclusions. For research purposes, follow-up visits should be conducted more frequently. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osipowicz, K.; Turkowski, P.; Kowalewski, C.; Pach, J.; Regulski, P.; Wozniak, K. Hair Transplantation for Lichen Planopilaris: A Case Series of Five Patients. J. Clin. Med. 2025, 14, 6199. https://doi.org/10.3390/jcm14176199
Osipowicz K, Turkowski P, Kowalewski C, Pach J, Regulski P, Wozniak K. Hair Transplantation for Lichen Planopilaris: A Case Series of Five Patients. Journal of Clinical Medicine. 2025; 14(17):6199. https://doi.org/10.3390/jcm14176199
Chicago/Turabian StyleOsipowicz, Katarzyna, Piotr Turkowski, Cezary Kowalewski, Janusz Pach, Piotr Regulski, and Katarzyna Wozniak. 2025. "Hair Transplantation for Lichen Planopilaris: A Case Series of Five Patients" Journal of Clinical Medicine 14, no. 17: 6199. https://doi.org/10.3390/jcm14176199
APA StyleOsipowicz, K., Turkowski, P., Kowalewski, C., Pach, J., Regulski, P., & Wozniak, K. (2025). Hair Transplantation for Lichen Planopilaris: A Case Series of Five Patients. Journal of Clinical Medicine, 14(17), 6199. https://doi.org/10.3390/jcm14176199






