The Efficacy of 22 °C Static Subnormothermic Preservation with an Extracellular-Type Solution for 2 h Warm-Ischemic Porcine Kidneys
Abstract
1. Introduction
2. Materials and Methods
2.1. Rationale and Clinical Relevance
2.2. Animals
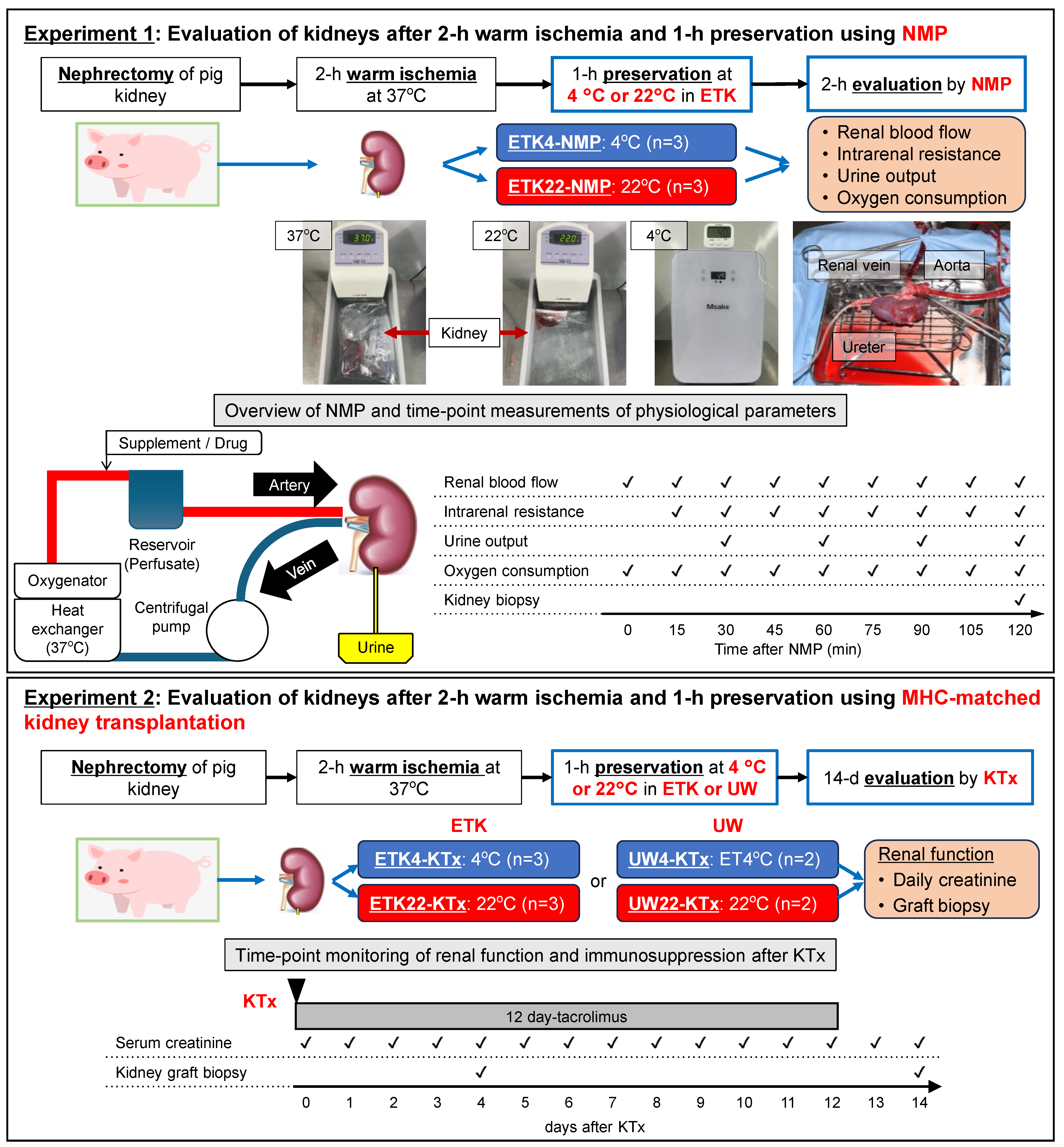
2.3. Experimental Design
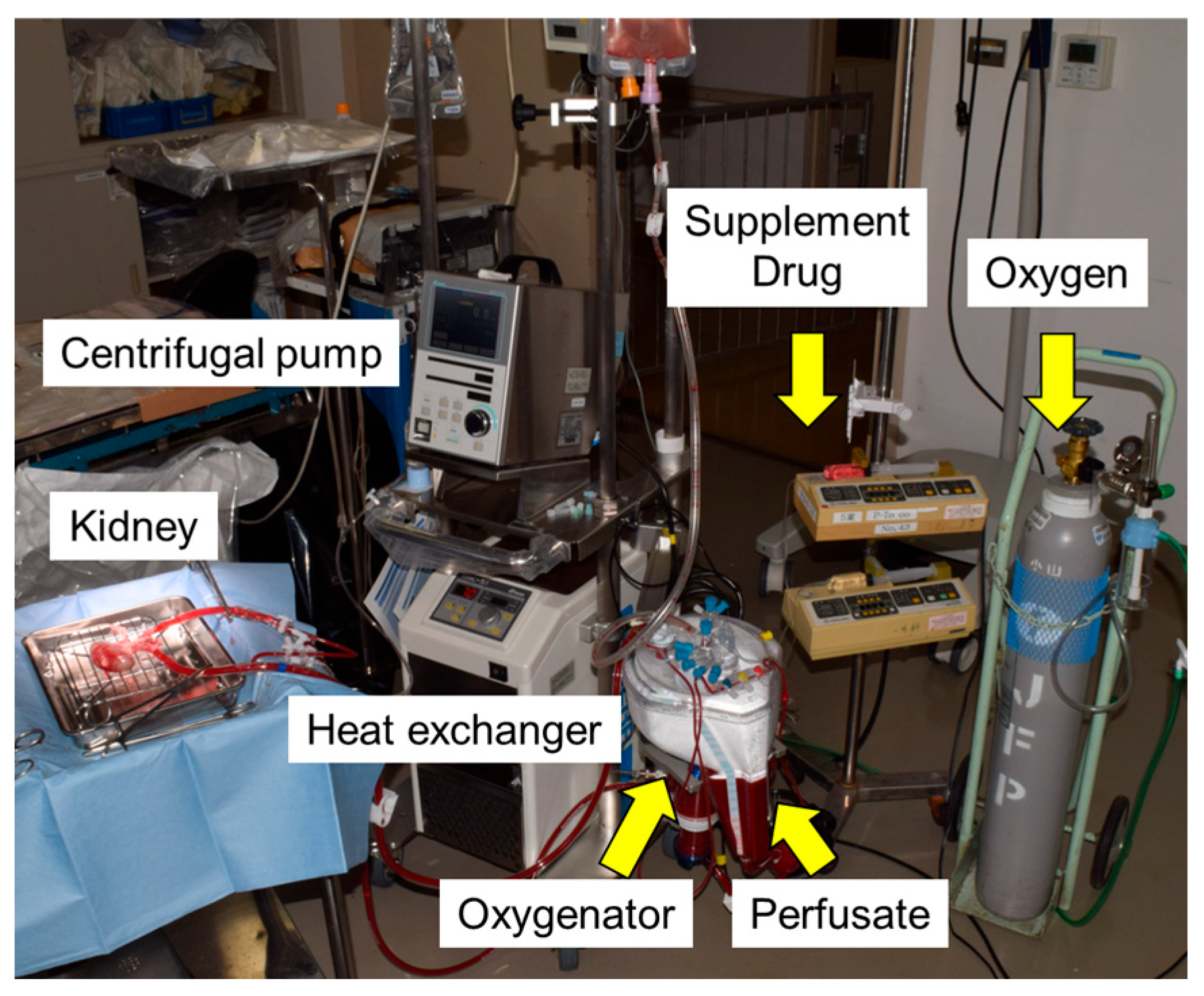
2.4. Normothermic Machine Perfusion (NMP)
2.5. Surgeries (Catheter Placement, Kidney Transplantation, and Kidney Graft Biopsy)
2.6. Immunosuppression
2.7. Assessment of Kidney Injury via Serum Analysis and Histological Examination
2.8. Statistical Analysis
3. Results
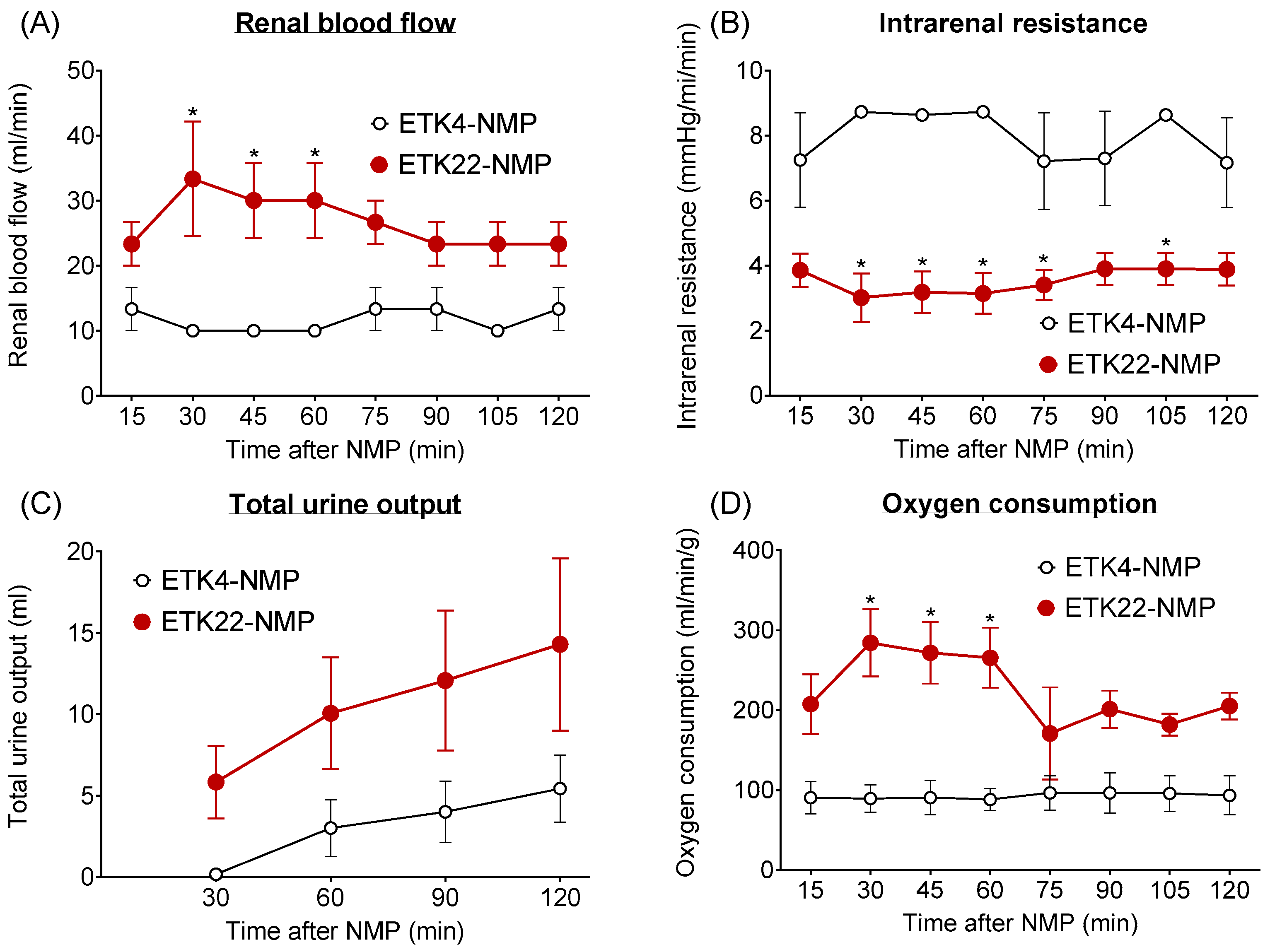
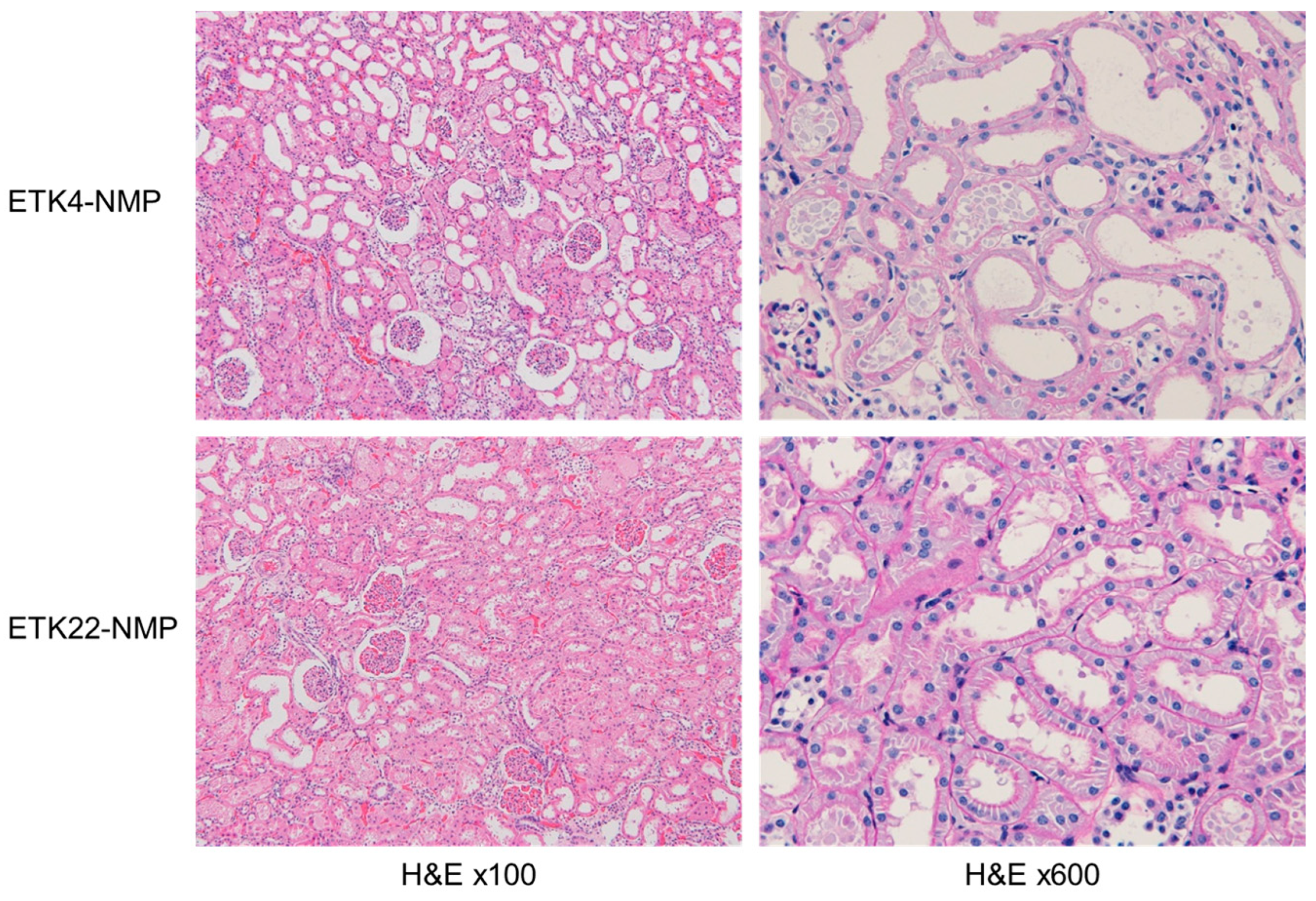
3.1. NMP Evaluation Revealed Superior Condition of 2 h Warm-Ischemic Kidneys Preserved at 22 °C Versus 4 °C (Experiment 1)
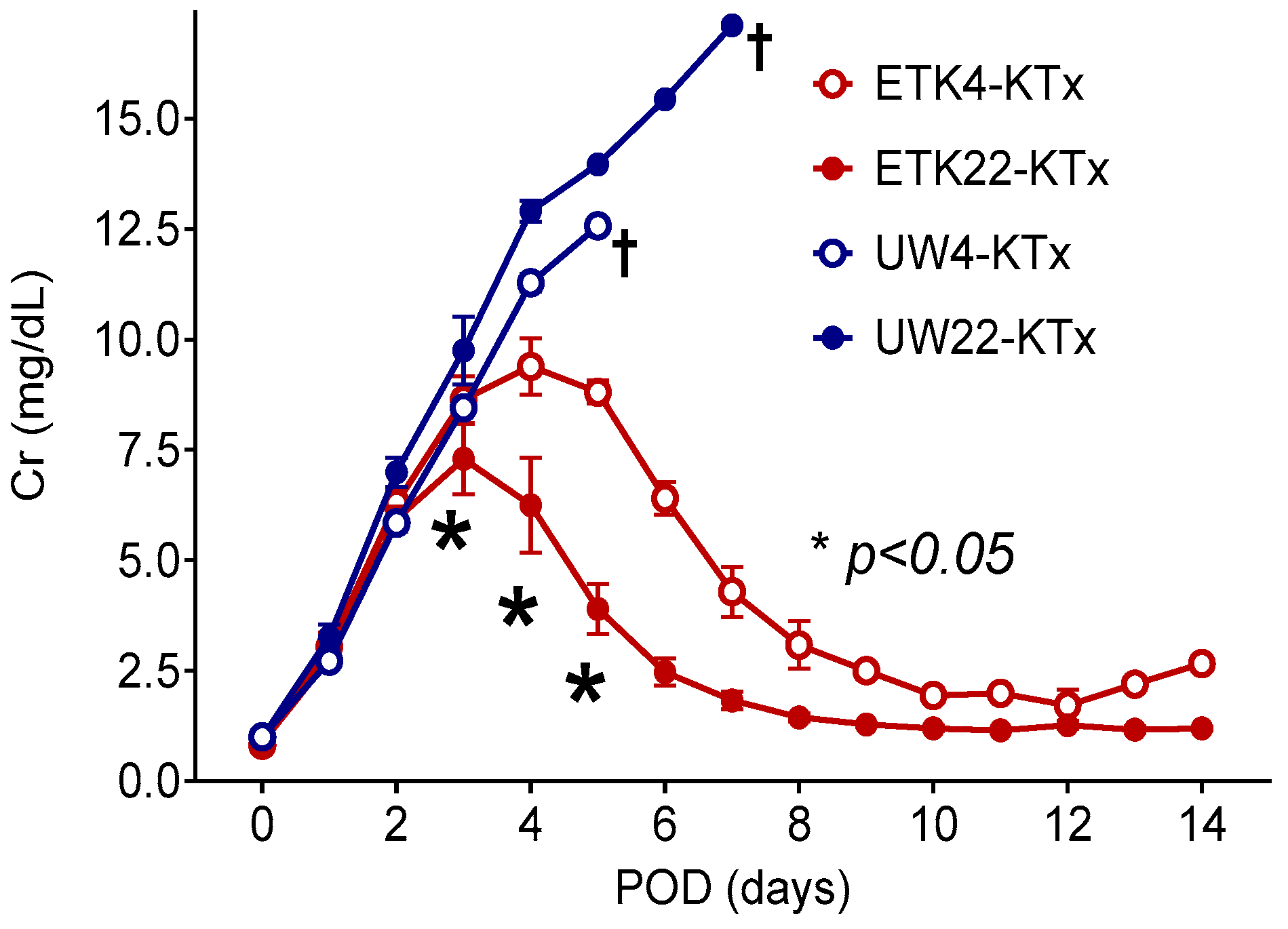
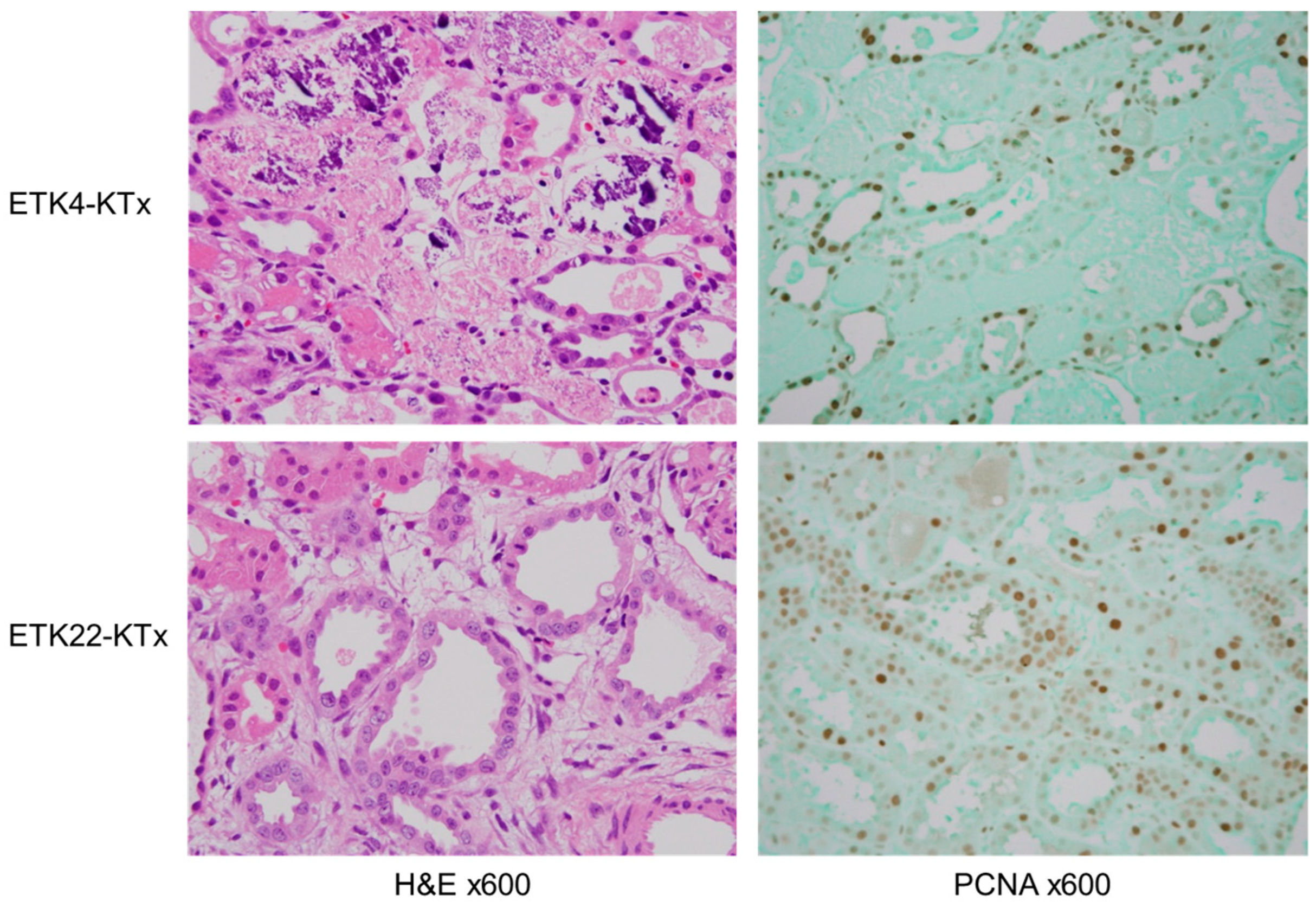
3.2. Preservation of 2 h Warm-Ischemic Kidneys at 22 °C Resulted in Lower Serum Cr After MHC-Matched Kidney Transplantation (Experiment 2)
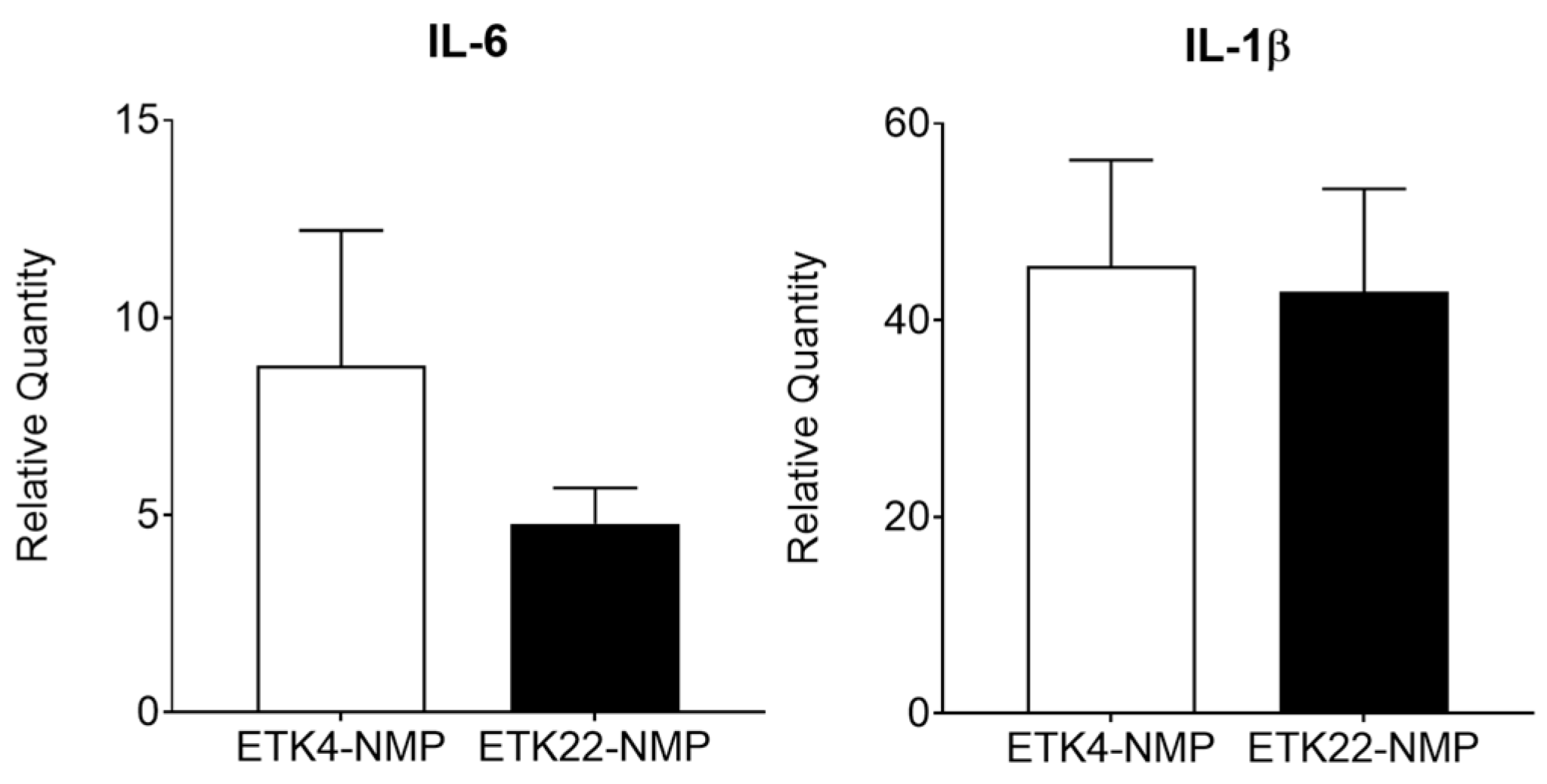
3.3. Intracellular-Type Preservation Solution Could Not Preserve 2 h Warm-Ischemic Kidneys
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATN | Acute tubular necrosis |
| ATP | Adenosine triphosphate |
| DCD | Donation after circulatory death |
| ETK | Extracellular trehalose Kyoto |
| IRI | Ischemia–reperfusion injury |
| KTx | Kidney transplantation |
| MHC | Major histocompatibility complex |
| NMP | Normothermic machine perfusion |
| PCNA | Proliferating cell nuclear antigen |
| POD | Postoperative day |
| RT-PCR | Real-time PCR |
| SCS | Static cold storage |
| SSS | Static subnormothermic storage |
| PGD | Primary graft dysfunction |
| UW | University of Wisconsin |
| WIT | Warm ischemia time |
References
- Summers, D.M.; Watson, C.J.; Pettigrew, G.J.; Johnson, R.J.; Collett, D.; Neuberger, J.M.; Bradley, J.A. Kidney donation after circulatory death (DCD): State of the art. Kidney Int. 2015, 88, 241–249. [Google Scholar] [CrossRef]
- Kwon, J.H.; Blanding, W.M.; Shorbaji, K.; Scalea, J.R.; Gibney, B.C.; Baliga, P.K.; Kilic, A. Waitlist and Transplant Outcomes in Organ Donation After Circulatory Death: Trends in the United States. Ann. Surg. 2023, 278, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Tilney, N.L.; Guttmann, R.D. Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation 1997, 64, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Nankivell, B.J.; Chapman, J.R. Chronic allograft nephropathy: Current concepts and future directions. Transplantation 2006, 81, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Fresneda, I.; Torras, J.; Cruzado, J.M.; Condom, E.; Vidal, A.; Riera, M.; Lloberas, N.; Alsina, J.; Grinyo, J.M. Do alloreactivity and prolonged cold ischemia cause different elementary lesions in chronic allograft nephropathy? Am. J. Pathol. 2003, 162, 127–137. [Google Scholar] [CrossRef][Green Version]
- Lentine, K.L.; Smith, J.M.; Lyden, G.R.; Miller, J.M.; Dolan, T.G.; Bradbrook, K.; Larkin, L.; Temple, K.; Handarova, D.K.; Weiss, S.; et al. OPTN/SRTR 2022 Annual Data Report: Kidney. Am. J. Transplant. 2024, 24, S19–S118. [Google Scholar] [CrossRef]
- Southard, J.H.; Belzer, F.O. Organ preservation. Annu. Rev. Med. 1995, 46, 235–247. [Google Scholar] [CrossRef]
- Timsit, M.O.; Tullius, S.G. Hypothermic kidney preservation: A remembrance of the past in the future? Curr. Opin. Organ. Transplant. 2011, 16, 162–168. [Google Scholar] [CrossRef]
- Salahudeen, A.K.; Huang, H.; Joshi, M.; Moore, N.A.; Jenkins, J.K. Involvement of the mitochondrial pathway in cold storage and rewarming-associated apoptosis of human renal proximal tubular cells. Am. J. Transplant. 2003, 3, 273–280. [Google Scholar] [CrossRef]
- Schilling, M.; Saunder, A.; Ametani, M.; Southard, J.H.; Belzer, F.O. Prolonged kidney preservation by inhibition of arachidonic acid metabolism. Transplant. Proc. 1993, 25, 1629–1630. Available online: https://www.ncbi.nlm.nih.gov/pubmed/8442216 (accessed on 1 July 2025).
- Sayegh, M.H. Why do we reject a graft? Role of indirect allorecognition in graft rejection. Kidney Int. 1999, 56, 1967–1979. [Google Scholar] [CrossRef]
- Summers, D.M.; Johnson, R.J.; Hudson, A.; Collett, D.; Watson, C.J.; Bradley, J.A. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: A cohort study. Lancet 2013, 381, 727–734. [Google Scholar] [CrossRef]
- Kay, M.D.; Hosgood, S.A.; Harper, S.J.; Bagul, A.; Waller, H.L.; Nicholson, M.L. Normothermic versus hypothermic ex vivo flush using a novel phosphate-free preservation solution (AQIX) in porcine kidneys. J. Surg. Res. 2011, 171, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Elliott, T.R.; Nicholson, M.L.; Hosgood, S.A. Normothermic kidney perfusion: An overview of protocols and strategies. Am. J. Transplant. 2021, 21, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Iwai, S.; Kikuchi, T.; Kasahara, N.; Teratani, T.; Yokoo, T.; Sakonju, I.; Okano, S.; Kobayashi, E. Impact of normothermic preservation with extracellular type solution containing trehalose on rat kidney grafting from a cardiac death donor. PLoS ONE 2012, 7, e33157. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.P.; Gallinat, A.; Swoboda, S.; Wohlschlager, J.; Rauen, U.; Paul, A.; Minor, T. Subnormothermic machine perfusion for preservation of porcine kidneys in a donation after circulatory death model. Transpl. Int. 2014, 27, 1097–1106. [Google Scholar] [CrossRef]
- Bhattacharjee, R.N.; Ruthirakanthan, A.; Sun, Q.; Richard-Mohamed, M.; Luke, S.; Jiang, L.; Aquil, S.; Sharma, H.; Tun-Abraham, M.E.; Alharbi, B.; et al. Subnormothermic Oxygenated Perfusion Optimally Preserves Donor Kidneys Ex Vivo. Kidney Int. Rep. 2019, 4, 1323–1333. [Google Scholar] [CrossRef]
- Agius, T.; Songeon, J.; Klauser, A.; Allagnat, F.; Longchamp, G.; Ruttimann, R.; Lyon, A.; Ivaniesevic, J.; Meier, R.; Deglise, S.; et al. Subnormothermic Ex Vivo Porcine Kidney Perfusion Improves Energy Metabolism: Analysis Using (31)P Magnetic Resonance Spectroscopic Imaging. Transplant. Direct. 2022, 8, e1354. [Google Scholar] [CrossRef]
- Abraham, N.; Gao, Q.; Kahan, R.; Alderete, I.S.; Wang, B.; Howell, D.N.; Anwar, I.J.; Ladowski, J.M.; Nakata, K.; Jarrett, E.; et al. Subnormothermic Oxygenated Machine Perfusion (24 h) in DCD Kidney Transplantation. Transplant. Direct. 2024, 10, e1633. [Google Scholar] [CrossRef]
- Hosgood, S.; Harper, S.; Kay, M.; Bagul, A.; Waller, H.; Nicholson, M.L. Effects of arterial pressure in an experimental isolated haemoperfused porcine kidney preservation system. Br. J. Surg. 2006, 93, 879–884. [Google Scholar] [CrossRef]
- Sekijima, M.; Sahara, H.; Miki, K.; Villani, V.; Ariyoshi, Y.; Iwanaga, T.; Tomita, Y.; Yamada, K. Hydrogen sulfide prevents renal ischemia-reperfusion injury in CLAWN miniature swine. J. Surg. Res. 2017, 219, 165–172. [Google Scholar] [CrossRef]
- Oku, M.; Okumi, M.; Shimizu, A.; Sahara, H.; Setoyama, K.; Nishimura, H.; Sada, M.; Scalea, J.; Ido, A.; Sachs, D.H.; et al. Hepatocyte growth factor sustains T regulatory cells and prolongs the survival of kidney allografts in major histocompatibility complex-inbred CLAWN-miniature swine. Transplantation 2012, 93, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Yamada, K.; Sachs, D.H.; Colvin, R.B. Persistent rejection of peritubular capillaries and tubules is associated with progressive interstitial fibrosis. Kidney Int. 2002, 61, 1867–1879. [Google Scholar] [CrossRef] [PubMed]
- Sahara, H.; Shimizu, A.; Setoyama, K.; Okumi, M.; Oku, M.; Samelson-Jones, E.; Yamada, K. Carbon monoxide reduces pulmonary ischemia-reperfusion injury in miniature swine. J. Thorac. Cardiovasc. Surg. 2010, 139, 1594–1601. [Google Scholar] [CrossRef]
- Manara, A.R.; Murphy, P.G.; O’Callaghan, G. Donation after circulatory death. Br. J. Anaesth. 2012, 108 (Suppl. S1), i108–i121. [Google Scholar] [CrossRef]
- Minor, T.; Paul, A. Hypothermic reconditioning in organ transplantation. Curr. Opin. Organ Transplant. 2013, 18, 161–167. [Google Scholar] [CrossRef]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus. Med. Hemother. 2011, 38, 125–142. [Google Scholar] [CrossRef]
- Healy, D.A.; Daly, P.J.; Docherty, N.G.; Murphy, M.; Fitzpatrick, J.M.; Watson, R.W. Heat shock-induced protection of renal proximal tubular epithelial cells from cold storage and rewarming injury. J. Am. Soc. Nephrol. 2006, 17, 805–812. [Google Scholar] [CrossRef]
- Tveita, T.; Johansen, K.; Lien, A.H.; Myklebust, R.; Lindal, S. Morphologic changes in tubular cells from in situ kidneys following experimental hypothermia and rewarming. APMIS 2005, 113, 13–20. [Google Scholar] [CrossRef]
- van de Kerkhove, M.P.; Hoekstra, R.; van Nooijen, F.C.; Spoelstra, F.O.; Doorschodt, B.M.; van Wijk, A.C.; Poyck, P.P.; Chamuleau, R.A.; van Gulik, T.M. Subnormothermic preservation maintains viability and function in a porcine hepatocyte culture model simulating bioreactor transport. Cell Transplant. 2006, 15, 161–168. [Google Scholar] [CrossRef]
- Liu, Q.; Berendsen, T.; Izamis, M.L.; Uygun, B.; Yarmush, M.L.; Uygun, K. Perfusion defatting at subnormothermic temperatures in steatotic rat livers. Transplant. Proc. 2013, 45, 3209–3213. [Google Scholar] [CrossRef]
- Karangwa, S.A.; Dutkowski, P.; Fontes, P.; Friend, P.J.; Guarrera, J.V.; Markmann, J.F.; Mergental, H.; Minor, T.; Quintini, C.; Selzner, M.; et al. Machine Perfusion of Donor Livers for Transplantation: A Proposal for Standardized Nomenclature and Reporting Guidelines. Am. J. Transplant. 2016, 16, 2932–2942. [Google Scholar] [CrossRef]
- Clausen, T.; Van Hardeveld, C.; Everts, M.E. Significance of cation transport in control of energy metabolism and thermogenesis. Physiol. Rev. 1991, 71, 733–774. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R.F.; Guarrera, J.V. Preservation solutions for static cold storage of abdominal allografts: Which is best? Curr. Opin. Organ Transplant. 2014, 19, 100–107. [Google Scholar] [CrossRef]
- Corwin, W.L.; Baust, J.M.; Baust, J.G.; Van Buskirk, R.G. Implications of differential stress response activation following non-frozen hepatocellular storage. Biopreserv. Biobank. 2013, 11, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Okuno, H.; Kamoto, T.; Habuchi, T.; Toda, Y.; Hasegawa, S.; Nakamura, T.; Wada, H.; Ogawa, O.; Yamamoto, S. Comparison of the effectiveness of ET-Kyoto with Euro-Collins and University of Wisconsin solutions in cold renal storage. Transplantation 2002, 74, 1231–1236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramella, S.G.; Hadj-Aissa, A.; Barbieux, A.; Steghens, J.P.; Colpart, J.J.; Zech, P.; Pozet, N. Evaluation of a high sodium-low potassium cold-storage solution by the isolated perfused rat kidney technique. Nephrol. Dial. Transplant. 1995, 10, 842–846. Available online: https://www.ncbi.nlm.nih.gov/pubmed/7566614 (accessed on 1 July 2025).[Green Version]
- Faure, J.P.; Baumert, H.; Han, Z.; Goujon, J.M.; Favreau, F.; Dutheil, D.; Petit, I.; Barriere, M.; Tallineau, C.; Tillement, J.P.; et al. Evidence for a protective role of trimetazidine during cold ischemia: Targeting inflammation and nephron mass. Biochem. Pharmacol. 2003, 66, 2241–2250. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Chapman, D. Preservation of membranes in anhydrobiotic organisms: The role of trehalose. Science 1984, 223, 701–703. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef]
- Zhu, B.; Furuki, T.; Okuda, T.; Sakurai, M. Natural DNA mixed with trehalose persists in B-form double-stranding even in the dry state. J. Phys. Chem. B 2007, 111, 5542–5544. [Google Scholar] [CrossRef]
| Perfusate Component | Quantity |
|---|---|
| Red blood cells | 200 mL |
| Ringer’s solution | 400 mL |
| 20% D-Mannitol | 15 mL |
| Dexamethasone | 8 mg |
| 7% Sodium bicarbonate | 30 mL |
| Heparin sodium | 2000 IU |
| Circuit supplement or drug | Dosing (initial dose or infusion rate) |
| Prostacyclin | 25 mL/h |
| 5% Glucose | 15 mL/h |
| 7% Sodium bicarbonate | 15 mL/h |
| Insulin | 50 units |
| Multivitamins | 5 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondo, A.; Okumi, M.; Ariyoshi, Y.; Sekijima, M.; Kawai, A.; Iwanaga, T.; Takeuchi, K.; Miura, K.; Miura, S.; Iwamoto, A.; et al. The Efficacy of 22 °C Static Subnormothermic Preservation with an Extracellular-Type Solution for 2 h Warm-Ischemic Porcine Kidneys. J. Clin. Med. 2025, 14, 6156. https://doi.org/10.3390/jcm14176156
Kondo A, Okumi M, Ariyoshi Y, Sekijima M, Kawai A, Iwanaga T, Takeuchi K, Miura K, Miura S, Iwamoto A, et al. The Efficacy of 22 °C Static Subnormothermic Preservation with an Extracellular-Type Solution for 2 h Warm-Ischemic Porcine Kidneys. Journal of Clinical Medicine. 2025; 14(17):6156. https://doi.org/10.3390/jcm14176156
Chicago/Turabian StyleKondo, Akira, Masayoshi Okumi, Yuichi Ariyoshi, Mitsuhiro Sekijima, Akihiro Kawai, Takehiro Iwanaga, Kazuhiro Takeuchi, Kohei Miura, Shiori Miura, Akiyuki Iwamoto, and et al. 2025. "The Efficacy of 22 °C Static Subnormothermic Preservation with an Extracellular-Type Solution for 2 h Warm-Ischemic Porcine Kidneys" Journal of Clinical Medicine 14, no. 17: 6156. https://doi.org/10.3390/jcm14176156
APA StyleKondo, A., Okumi, M., Ariyoshi, Y., Sekijima, M., Kawai, A., Iwanaga, T., Takeuchi, K., Miura, K., Miura, S., Iwamoto, A., Shimizu, K., Ichinari, Y., Shimizu, A., Kusaka, M., & Sahara, H. (2025). The Efficacy of 22 °C Static Subnormothermic Preservation with an Extracellular-Type Solution for 2 h Warm-Ischemic Porcine Kidneys. Journal of Clinical Medicine, 14(17), 6156. https://doi.org/10.3390/jcm14176156






