Effect of Ovarian Stimulation and Trigger Protocols on Oocyte and Embryo Numbers—Real World Experience
Abstract
1. Introduction
2. Materials and Methods
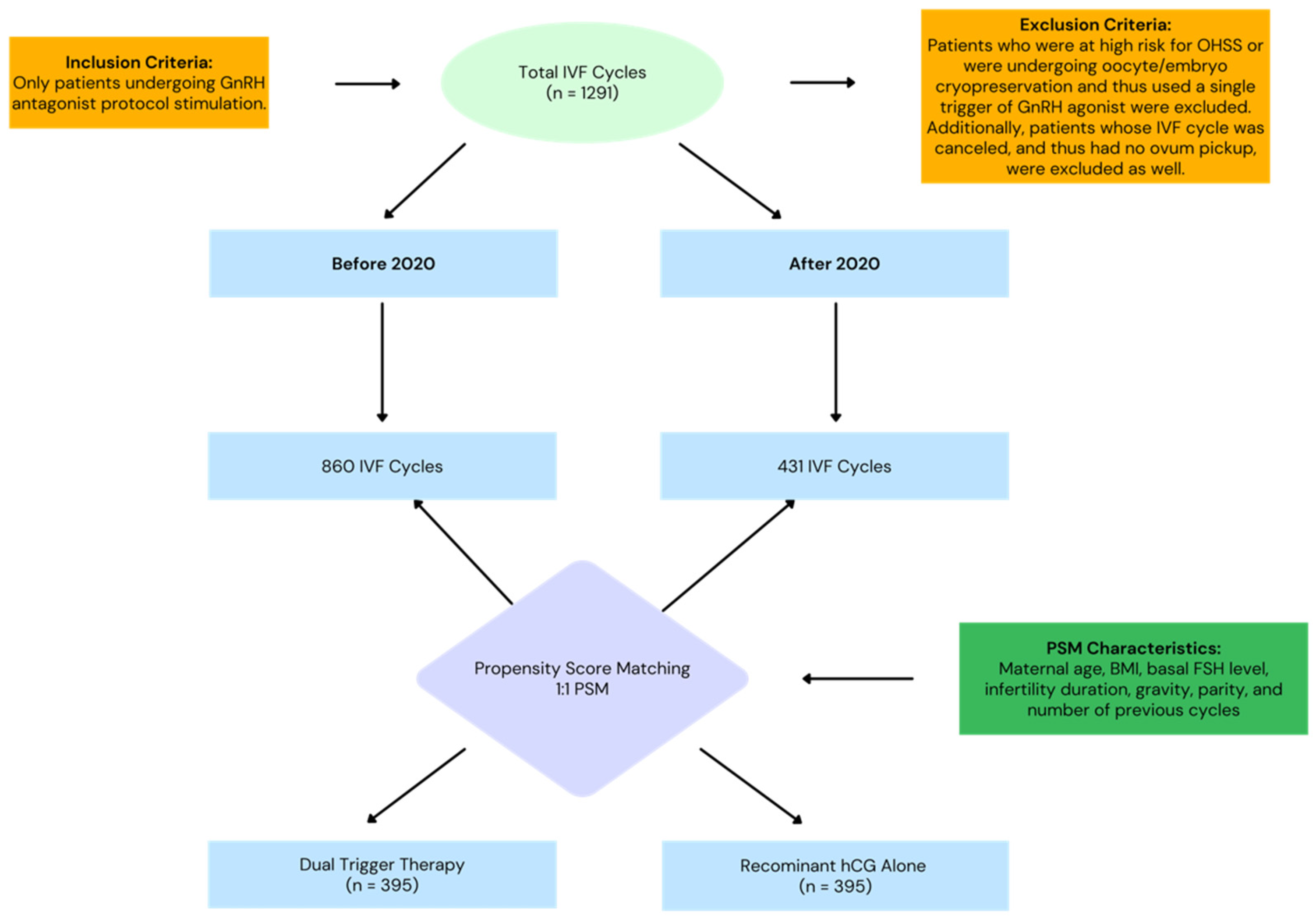
2.1. Study Design and Participants
2.2. Inclusion and Exclusion Criteria
2.3. Treatment Protocol
2.4. Outcome Variables
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ART | Assisted Reproductive Technology |
| BMI | Body Mass Index |
| FSH | Follicle Stimulating Hormone |
| GnRH | Gonadotropin-Releasing Hormone |
| hCG | Human Chorionic Gonadotropin |
| ICSI | Intracytoplasmic Sperm Injection |
| IRB | Institutional Review Board |
| IVF | In Vitro Fertilization |
| OHSS | Ovarian Hyperstimulation Syndrome |
| PSM | Propensity Score Matching |
| 2PN | 2 Pronuclei |
References
- Edwards, R.G.; Steptoe, P.C.; Purdy, J.M. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br. J. Obstet. Gynaecol. 1980, 87, 737–756. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.A.; Abou-Setta, A.M.; Lam, W.S. Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles. Cochrane Database Syst. Rev. 2016, 4, CD003719. [Google Scholar] [CrossRef] [PubMed]
- Gonen, Y.; Balakier, H.; Powell, W.; Casper, R.F. Use of gonadotropin-releasing hormone agonist to trigger follicular maturation for in vitro fertilization. J. Clin. Endocrinol. Metab. 1990, 71, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Itskovitz, J.; Boldes, R.; Levron, J.; Erlik, Y.; Kahana, L.; Brandes, J.M. Induction of preovulatory luteinizing hormone surge and prevention of ovarian hyperstimulation syndrome by gonadotropin-releasing hormone agonist. Fertil. Steril. 1991, 56, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Itskovitz-Eldor, J.; Kol, S.; Mannaerts, B. Use of a single bolus of GnRH agonist triptorelin to trigger ovulation after GnRH antagonist ganirelix treatment in women undergoing ovarian stimulation for assisted reproduction, with special reference to the prevention of ovarian hyperstimulation syndrome: Preliminary report: Short communication. Hum. Reprod. 2000, 15, 1965–1968. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, P.G.; Bosdou, J.K.; Lainas, G.T.; Lainas, T.G.; Grimbizis, G.F.; Kolibianakis, E.M. How frequent is severe ovarian hyperstimulation syndrome after GnRH agonist triggering in high-risk women? A systematic review and meta-analysis. Reprod. Biomed. Online 2021, 42, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Kol, S.; Humaidan, P. LH (as HCG) and FSH surges for final oocyte maturation: Sometimes it takes two to tango? Reprod. Biomed. Online 2010, 21, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Wu, F.S.-Y.; Lee, R.K.-K.; Li, S.-H.; Lin, S.-Y.; Hwu, Y.-M. Dual trigger with combination of gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves the live-birth rate for normal responders in GnRH-antagonist cycles. Fertil. Steril. 2013, 100, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Orvieto, R. Triggering final follicular maturation--hCG, GnRH-agonist or both, when and to whom? J. Ovarian Res. 2015, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- He, F.-F.; Hu, W.; Yong, L.; Li, Y.-M. Triggering of ovulation for GnRH-antagonist cycles in normal and low ovarian responders undergoing IVF/ICSI: A systematic review and meta-analysis of randomized trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 289, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Hsia, L.-H.; Lee, T.-H.; Lin, Y.-H.; Huang, Y.-Y.; Chang, H.-J.; Liu, Y.-L. Dual trigger improves the pregnancy rate in fresh in vitro fertilization (IVF) cycles compared with the human chorionic gonadotropin (hCG) trigger: A systematic review and meta-analysis of randomized trials. J. Assist. Reprod. Genet. 2023, 40, 2063–2077. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, A.M.; Johnston, L.A.; Munson, L.; Brown, J.L.; Wildt, D.E. Influence of gonadotropin treatment interval on follicular maturation, in vitro fertilization, circulating steroid concentrations, and subsequent luteal function in the domestic cat. Biol. Reprod. 1992, 46, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, E.; Mizrachi, Y.; Farhi, J.; Raziel, A.; Weissman, A. Does the interval between the last GnRH antagonist dose and the GnRH agonist trigger affect oocyte recovery and maturation rates? Reprod. Biomed. Online 2020, 41, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Smitz, J.; Platteau, P. Influence of human chorionic gonadotrophin during ovarian stimulation: An overview. Reprod. Biol. Endocrinol. 2020, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Beebeejaun, Y.; Copeland, T.; Duffy, J.M.N.; Sarris, I.; Showell, M.; Wang, R.; Sunkara, S.K. Triggering oocyte maturation in in vitro fertilization treatment in healthy responders: A systematic review and network meta-analysis. Fertil Steril. 2025, 123, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Bassil, R.; Samara, N.; Zilberberg, E.; Mehta, C.; Orvieto, R.; Casper, R.F. GnRH agonist and hCG (dual trigger) versus hCG trigger for final follicular maturation: A double-blinded, randomized controlled study. Hum. Reprod. 2020, 35, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, X.; Wang, Y.; Xi, J.; Pan, H.; Wang, M.; Zhou, Y.; Xiao, Y. Ovulation triggering with hCG alone, GnRH agonist alone or in combination? A randomized controlled trial in advanced-age women undergoing IVF/ICSI cycles. Hum. Reprod. 2022, 37, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Lian, F.; Wu, H.; Xiang, S.; Li, Y.; Wei, C.; Yu, X.; Xin, X. Reproductive outcomes of dual trigger with combination GnRH agonist and hCG versus trigger with hCG alone in women undergoing IVF/ICSI cycles: A retrospective cohort study with propensity score matching. BMC Pregnancy Childbirth 2022, 22, 583. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, Y.; Fu, M.; Zhang, Q.; Ren, Y.; Shen, H.; Han, H. Effect of a “Dual Trigger” Using a GnRH Agonist and hCG on the Cumulative Live-Birth Rate for Normal Responders in GnRH-Antagonist Cycles. Front. Med. 2021, 8, 683210. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, S.K. Number of oocytes and IVF outcomes: Real-world evidence. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 89, 102341. [Google Scholar] [CrossRef] [PubMed]
| Baseline | rhCG n = 395 | rhCG + GnRH Agonist n = 395 | p-Value |
|---|---|---|---|
| Age | 35.68 + 5.85 | 35.58 ± 6.00 | p = 0.82 |
| BMI | 27.20 ± 6.03 | 27.35 ± 5.91 | p = 0.73 |
| FSH | 1.47 ± 1.15 | 1.39 ± 1.15 | p = 0.35 |
| Number of Previous Cycles | 1.81 ± 2.42 | 1.89 ± 2.71 | p = 0.69 |
| Years of Infertility | 1.63 ± 1.10 | 1.63 ± 1.09 | p = 1.0 |
| Gravidity | 1.42 ± 1.72 | 1.18 ± 1.64 | p = 0.052 |
| Parity | 0.58 ± 0.72 | 0.54 ± 0.69 | p = 0.44 |
| Results | rhCG n = 395 | rhCG + GnRH Agonist n = 395 | p-Value |
|---|---|---|---|
| Total Number of Oocytes | n = 2417 | n = 2962 | p < 0.001 |
| Average Number of Oocytes | 6.12 ± 4.23 | 7.50 ± 5.23 | p < 0.001 |
| Number of Cycles with no Oocytes | 15/395 (3.8%) | 5/382 (1.3%) | p = 0.015 |
| Number of Mature Oocytes | 5.01 ± 3.13 | 5.67 ± 3.87 | p = 0.047 |
| Maturation Rate | 71.53 ± 24.75 | 72.52 ± 26.91 | p = 0.37 |
| Number of Fertilizations (2PN) | 2.92 ± 2.97 | 3.73 ± 3.30 | p < 0.001 |
| Fertilization Rate in IVF | 52.29 ± 32.11 | 55.14 ± 30.72 | p = 0.18 |
| Fertilization Rate in ICSI | 52.22 ± 34.12 | 64.93 ± 33.5 | p = 0.003 |
| Total Number of Embryos | 2.00 ± 1.93 | 2.43 ± 1.90 | p = 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somer, S.; Nothman, S.; Baram, S.; Izhaki, I.; Sela, N.D.; Beck-Fruchter, R. Effect of Ovarian Stimulation and Trigger Protocols on Oocyte and Embryo Numbers—Real World Experience. J. Clin. Med. 2025, 14, 6096. https://doi.org/10.3390/jcm14176096
Somer S, Nothman S, Baram S, Izhaki I, Sela ND, Beck-Fruchter R. Effect of Ovarian Stimulation and Trigger Protocols on Oocyte and Embryo Numbers—Real World Experience. Journal of Clinical Medicine. 2025; 14(17):6096. https://doi.org/10.3390/jcm14176096
Chicago/Turabian StyleSomer, Shmuel, Simon Nothman, Shira Baram, Ido Izhaki, Nitzan Dana Sela, and Ronit Beck-Fruchter. 2025. "Effect of Ovarian Stimulation and Trigger Protocols on Oocyte and Embryo Numbers—Real World Experience" Journal of Clinical Medicine 14, no. 17: 6096. https://doi.org/10.3390/jcm14176096
APA StyleSomer, S., Nothman, S., Baram, S., Izhaki, I., Sela, N. D., & Beck-Fruchter, R. (2025). Effect of Ovarian Stimulation and Trigger Protocols on Oocyte and Embryo Numbers—Real World Experience. Journal of Clinical Medicine, 14(17), 6096. https://doi.org/10.3390/jcm14176096









