Visual Snow Syndrome: Therapeutic Implications
Abstract
1. Introduction
| (A) | |||
|---|---|---|---|
| Category | Percentage Reported * | Symptom | Description |
| Visual | 100% | Visual Snow | Persistent grainy or pixelated visual disturbance across the entire field of vision, similar to TV static. |
| Visual | 85% | Afterimages (Palinopsia) | Lingering visual traces of objects, sometimes with a trailing, comet-like effect. |
| Visual | 74% | Light Sensitivity (Photosensitivity) | Discomfort or intolerance to bright lights or specific lighting conditions. |
| Visual | 51% | Enhanced Entoptic Phenomena | Heightened perception of floaters or other ocular particles typically not visible. |
| Visual | 33% | Night Vision Difficulty (Nyctalopia) | Impaired ability to see in low-light or night-time conditions. |
| Visual | 30% | Flashes of Light (Photopsia) | Random, spontaneous perceptions of light flashes without external stimuli. |
| (B) | |||
| Category | Percentage Reported * | Symptom | Description |
| Non-Visual | 26% | Balance Issues | Sensations of physical unsteadiness or difficulty maintaining balance. |
| Non-Visual | 15% | Sound Sensitivity (Hyperacusis) | Increased sensitivity to sounds, where normal noises feel overly intense. |
| Non-Visual | 26% | Sound-Related Fear (Phonophobia) | Excessive fear or distress triggered by common environmental sounds. |
| Non-Visual | 26% | Migraine Headaches | Severe, often one-sided headaches, potentially with nausea or temporary visual disturbances. |
| Non-Visual | 4% | Tremors | Small, involuntary, rhythmic muscle contractions. |
| Non-Visual | 48% | Ear Ringing (Tinnitus) | Persistent perception of ringing or buzzing in the ears without external cause. |
| Non-Visual | 7% | Skin Sensitivity (Cutaneous Allodynia) | Pain or discomfort from light touch or normal skin contact. |
2. Methods
3. Treatment of VS/VSS
3.1. Discussion of the Condition with the Patient
3.2. Medications
3.3. Oculomotor-Based Vision Therapy
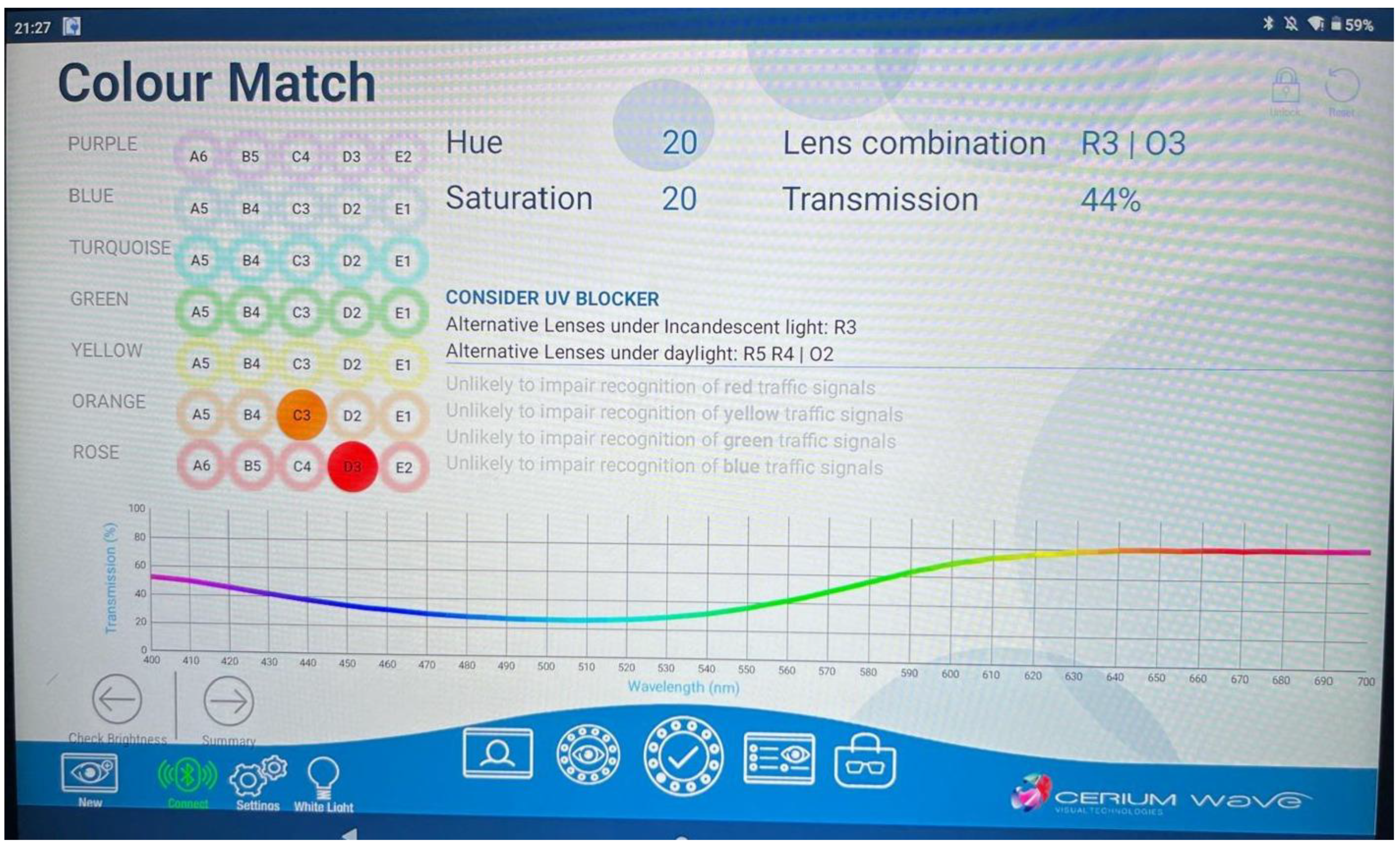
3.4. Chromatic Tints
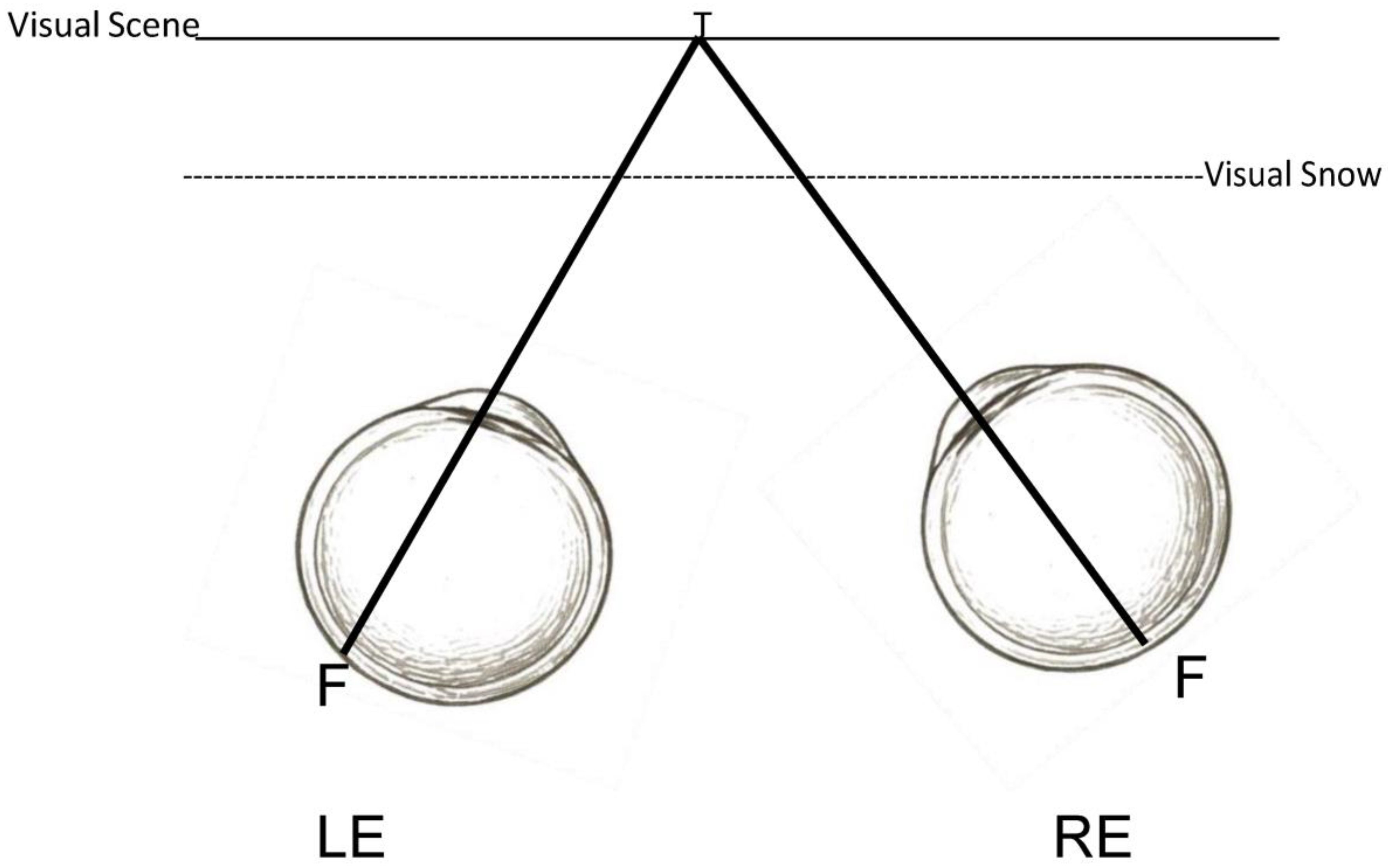
3.5. Adaptation to Visual Noise
3.6. Environmental Alterations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ciuffreda, K.J.; Tannen, B.; Tannen, N.; Rutner, R. Visual snow syndrome: Visual consequences, diagnosis and treatment. In Advances in Ophthalmology and Optometry; Yanoff, M., Ed.; Elsevier: Philadelphia, PA, USA, 2024; Volume 9, pp. 1–23. [Google Scholar]
- Eren, O.; Schankin, C.J. Insights into pathophysiology and treatment of visual snow syndrome: A systematic review. Prog. Brain Res. 2020, 255, 311–326. [Google Scholar]
- Carroll, F.D. Visual symptoms caused by digitalis. Trans. Am. Ophthalmol. Soc. 1944, 42, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.T.; Schatz, N.J.; Galetta, S.L.; Volpe, N.J.; Skobieranda, F.; Kosmorsky, G.S. Persistent positive visual phenomena in migraine. Neurology 1995, 45, 664–668. [Google Scholar] [CrossRef]
- Schankin, C.J.; Maniyar, F.H.; Digre, K.B.; Goadsby, P.J. ‘Visual snow’—A disorder distinct from persistent migraine aura. Brain 2014, 137, 1419–1428. [Google Scholar] [CrossRef]
- Schankin, C.J.; Goadsby, P.J. Visual snow—Persistent positive visual phenomenon distinct from migraine aura. Curr. Pain Headache Rep. 2015, 19, 23. [Google Scholar] [CrossRef]
- Browne, G.; Cassimatis, M.; Fyffe, A.; Orr, R. Visual snow syndrome: An important consideration in the adolescent with persisting headache and concussion symptoms. J. Sci. Med. Sport 2021, 24, S32–S33. [Google Scholar] [CrossRef]
- Mehta, D.G.; Garza, I.; Robertson, C.E. Two hundred and forty-eight cases of visual snow: A review of potential inciting events and contributing comorbidities. Cephalalgia 2021, 41, 1015–1026. [Google Scholar] [CrossRef]
- Scutelnic, A.; Slavova, N.; Klein, A.; Horvath, T.; de Beukelaer, S.A.L.; Arnold, M.; Jung, S.; Schankin, C.J. Symptomatic visual snow in acute ischemic stroke: A case series. Headache 2023, 63, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Metzler, A.I.; Robertson, C.E. Visual snow syndrome: Proposed criteria, clinical implications, and pathophysiology. Curr. Neurol. Neurosci. Rep. 2018, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Kondziella, D.; Olsen, M.H.; Dreier, J.P. Prevalence of visual snow syndrome in the UK. Eur. J. Neurol. 2020, 27, 764–772. [Google Scholar] [CrossRef]
- Graber, M.; Scutelnic, A.; Klein, A.; Puledda, F.; Goadsby, P.J.; Schankin, C.J. Natural course of visual snow syndrome: A long-term follow-up study. Brain Comms. 2022, 4, fcac230. [Google Scholar] [CrossRef]
- White, O.B.; Clough, M.; McKendrick, A.M.; Fielding, J. Visual snow: Visual misperception. J. Neuro-Ophthalmol. 2018, 38, 514–521. [Google Scholar] [CrossRef]
- Puledda, F.; Schankin, C.; Digre, K.; Goadsby, P.J. Visual snow syndrome: What we know so far. Curr. Opin. Neurol. 2018, 31, 52–58. [Google Scholar] [CrossRef]
- Puledda, F.; Schankin, C.; Goadsby, P.J. Visual snow syndrome: A clinical and phenotypical description of 1100 cases. Neurology 2020, 94, e564–e574. [Google Scholar] [CrossRef] [PubMed]
- Hepschke, J.L.; Seymour, R.A.; He, W.; Etchell, A.; Sowman, P.F.; Fraser, C.L. Cortical oscillatory dysrhythmias in visual snow syndrome: A magnetoencephalography study. Brain Commun. 2022, 4, fcab296. [Google Scholar] [CrossRef]
- Rusztyn, P.; Stańska, W.; Torbus, A.; Maciejewicz, P. Visual snow: A review on pathophysiology and treatment. J. Clin. Med. 2023, 12, 3868. [Google Scholar] [CrossRef] [PubMed]
- Puledda, F.; Vandenbussche, N.; Moreno-Ajona, D.; Eren, O.; Schankin, C.; Goadsby, P.J. Evaluation of treatment response and symptom progression in 400 patients with visual snow syndrome. Br. J. Ophthalmol. 2022, 106, 1318–1324. [Google Scholar] [CrossRef]
- Ghannam, A.B.; Pelak, V.S. Visual snow: A potential cortical hyperexcitability syndrome. Curr. Treat. Options Neurol. 2017, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- McKendrick, A.M.; Chan, Y.M.; Tien, M.; Millist, L.; Clough, M.; Mack, H.; Fielding, J.; White, O.B. Behavioral measures of cortical hyperexcitability assessed in people who experience visual snow. Neurology 2017, 88, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Tannen, B.; Brown, J.; Ciuffreda, K.J.; Tannen, N.M. Remediation of visual snow (VS) and related phenomena in a neuro-optometric practice: A retrospective analysis. Vis. Dev. Rehab. 2022, 8, 105–113. [Google Scholar]
- Han, M.E.; Ciuffreda, K.J.; Rutner, D. Historical, diagnostic, and chromatic treatment in visual snow syndrome: A retrospective analysis. Optom. Vis. Sci. 2023, 100, 328–333. [Google Scholar] [CrossRef]
- Gegenfurtner, K.R.; Kiper, D.C.; Beusmans, J.M.; Carandini, M.; Zaidi, Q.; Movshon, J.A. Chromatic properties of neurons in macaque MT. Vis. Neurosci. 1994, 11, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, R.M.; Waaijer, L.C.; Onderwater, G.L.; Ferrari, M.D.; Terwindt, G.M. Treatment effects and comorbid diseases in 58 patients with visual snow. Neurology 2019, 93, e398–e403. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Pontillo, G.; Kanber, B.; Prados, F.; Wingrove, J.; Yiannakas, M.; Davagnanam, I.; Gandini Wheeler-Kingshott, C.A.M.; Toosy, C.A.T. Visual snow syndrome improves with modulation of resting-state functional MRI connectivity after mindfulness-based cognitive therapy: An open-label feasibility study. J. Neuro-Ophthalmol. 2024, 44, 112–118. [Google Scholar] [CrossRef]
- Hang, C.; Leishangthem, L.; Yan, Y. Not all cases of visual snows are benign: Mimics of visual snow syndrome. Neuropsychiatr. Dis. Treat. 2021, 17, 3293–3300. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.S.; Lance, S.; Lallu, B.; Anderson, N.E. Visual snow: Not so benign. J. Clin. Neurosci. 2019, 64, 37–39. [Google Scholar] [CrossRef]
- Aeschlimann, S.A.; Klein, A.; Schankin, C.J. Visual snow syndrome: Recent advances in understanding the pathophysiology and potential treatment approaches. Curr. Opin. Neurol. 2024, 37, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.; Shidlofsky, C.; Mora, V. The efficacy of neuro-optometric visual rehabilitation therapy in patients with visual snow syndrome. Front. Neurol. 2022, 13, 999336. [Google Scholar] [CrossRef]
- Ciuffreda, K.J.; Ludlam, D.P.; Yadav, N.K.; Thiagarajan, P. Traumatic brain injury: Visual consequences, diagnosis, and treatment. In Advances in Ophthalmology and Optometry; Yanoff, M., Ed.; Elsevier: Philadelphia, PA, USA, 2016; Volume 1, pp. 307–333. [Google Scholar]
- Solly, E.J.; Clough, M.; McKendrick, A.M.; Foletta, P.; White, O.B.; Fielding, J. Ocular motor measures of visual processing changes in visual snow syndrome. Neurology 2020, 95, e1784–e1791. [Google Scholar] [CrossRef]
- Solly, E.J.; Clough, M.; McKendrick, A.M.; Foletta, P.; White, O.B.; Fielding, J. Eye movement characteristics provide an objective measure of visual processing changes in patients with visual snow syndrome. Sci. Rep. 2021, 11, 9607. [Google Scholar] [CrossRef]
- Foletta, P.J.; Clough, M.; McKendrick, A.M.; Solly, E.J.; White, O.B.; Fielding, J. Delayed onset of inhibition of return in visual snow syndrome. Front. Neurol. 2021, 12, 738599. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R. In Search of Memory; W.W. Norton and Company: New York, NY, USA, 2007; p. 284. [Google Scholar]
- Rutner, D.; Ciuffreda, K.J. A clinical diagnostic and treatment protocol for the patient with visual snow/visual snow syndrome and concurrent binocular dysfunction. Vis. Dev. Rehab. 2023, 9, 7–14. [Google Scholar]
- Ayesha, A.; Riehle, C.; Leishangthem, L. Diagnostic and management strategies of visual snow syndrome: Current perspectives. Eye Brain 2025, 17, 1–11. [Google Scholar] [CrossRef]
- Lauschke, J.L.; Plant, G.T.; Fraser, C.L. Visual snow: A thalamocortical dysrhythmia of the visual pathway? J. Clin. Neurosci. 2016, 28, 123–127. [Google Scholar] [CrossRef]
- Ciuffreda, K.J.; Han, M.E.; Tannen, B. Pediatric visual snow syndrome (VSS): A case series. Vis. Dev. Rehab. 2019, 5, 249–254. [Google Scholar] [CrossRef]
- Hepschke, J.L.; Martin, P.R.; Fraser, C.L. Short-wave sensitive (“blue”) cone activation is an aggravating factor for visual snow symptoms. Front. Neurol. 2021, 12, 697923. [Google Scholar] [CrossRef]
- Montoya, S.A.; Mulder, C.B.; Lee, M.S.; Schallmo, M.P.; Engel, S.A. Adapting to visual noise alleviates visual snow. Investig. Ophthal. Vis. Sci. 2023, 64, 23. [Google Scholar] [CrossRef]
- Anstis, S.; Verstraten, F.A.; Mather, G. The motion aftereffect. Trends Cogn. Sci. 1998, 2, 111–117. [Google Scholar] [CrossRef]
- Kenyon, R.V.; (University of Illinois, Chicago, IL, USA). Personal Communication, 2025.
- Amorim, R.; Molina-Moreno, V.; Peña-García, A. Proposal for sustainable dynamic lighting in sport facilities to decrease violence among spectators. Sustainability 2016, 8, 1298. [Google Scholar] [CrossRef]

| Treatment | Details |
|---|---|
| Discussion of the condition with the patient | Educate patients regarding the typically benign nature Refer to psychology and neurology |
| Medications |
|
| Oculomotor-based vision therapy |
|
| Chromatic tints |
|
| Adaptation to visual noise | Short treatment periods |
| Environmental alterations | Lighting Painting scheme |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciuffreda, K.J.; Rutner, D. Visual Snow Syndrome: Therapeutic Implications. J. Clin. Med. 2025, 14, 6070. https://doi.org/10.3390/jcm14176070
Ciuffreda KJ, Rutner D. Visual Snow Syndrome: Therapeutic Implications. Journal of Clinical Medicine. 2025; 14(17):6070. https://doi.org/10.3390/jcm14176070
Chicago/Turabian StyleCiuffreda, Kenneth J., and Daniella Rutner. 2025. "Visual Snow Syndrome: Therapeutic Implications" Journal of Clinical Medicine 14, no. 17: 6070. https://doi.org/10.3390/jcm14176070
APA StyleCiuffreda, K. J., & Rutner, D. (2025). Visual Snow Syndrome: Therapeutic Implications. Journal of Clinical Medicine, 14(17), 6070. https://doi.org/10.3390/jcm14176070






