Abstract
Solitary fibrous tumors (SFTs) of the spine are rare. SFTs, especially those classified as WHO grade II or III (previously termed hemangiopericytomas), are aggressive neoplasms with a high recurrence rate and metastatic potential. In the literature, descriptions of SFTs are limited to case reports and small case series. To our knowledge, 157 cases, including the current case, have been reported since Schirger’s 1958 report on spinal SFTs. This report describes two cases of WHO grade II and III SFTs in the spine and presents a review of the literature. In the first case, an extradural WHO grade II SFT recurred 6 years after the first surgery, and a second surgery was performed, including wide excision of the surrounding tissue. The patient has remained recurrence-free for 16 years since the second surgery. In the second case, an intradural extramedullary WHO grade III SFT was resected, including the dura mater, and the patient has remained recurrence-free for 3 years since the surgery. Few reports have described tumor recurrence and long-term outcomes after reoperation, as in the first case, or extensive resection including the dura, as in the second case. Furthermore, the literature review not only summarizes patients’ general and surgical information, but also indicates, based on multivariate analysis, that gross total resection (GTR) is an important factor in preventing recurrence and metastasis. This is the first study to comprehensively examine previous reports and identify risk factors for recurrence and metastasis. In addition, because recurrences have been reported long after surgery, we believe that even if GTR is performed surgically, it is important to conduct follow-ups to check for long-term recurrence.
1. Introduction
Solitary fibrous tumors (SFTs) and hemangiopericytomas (HPCs) were previously considered different types of tumors. As both tumors share inversions at 12q13 leading to STAT6 nuclear expression, the World Health Organization Classification of Tumors of the Central Nervous System (CNS WHO) assigned the combined term SFTs/HPCs in 2016 [1]. The term “hemangiopericytoma” has been revoked in the 2021 WHO Classification of CNS Tumors, with the tumor now being considered an SFT [2]. To create a single designation for tumors in the spectrum of low-grade SFTs and higher-grade lesions previously designated as HPCs and anaplastic HPCs, the CNS WHO assigns three grades for SFTs: grade I—highly collagenous, relatively low cellularity, spindle cell lesion previously diagnosed as an SFT; grade II—more cellular, less collagenous tumor with plump cells and “staghorn” vasculature that was previously diagnosed as an HPC; and grade III— termed an anaplastic HPC in the past, diagnosed on the basis of >5 mitoses per 10 high-power fields [1]. Although these tumors are understood to share a biologic basis, they remain behaviorally distinct; WHO grade I SFTs occurring in the CNS tend to be classified as benign [3], while WHO grade II or II are aggressive, known to metastasize systemically, and commonly recur [4,5,6].
SFTs are rare CNS tumors [7] that are commonly located in the brain, with occurrence in the spine being extremely rare [8]. This case report presents two targeted cases of WHO grade II and III SFTs in the spine (an extradural tumor with recurrence 6 years later and an intradural extramedullary tumor) and reviews the literature on the management of WHO grade II or III spinal SFTs. There are few reports of extradural tumor recurrence and long-term outcomes after reoperation, and intradural extramedullary tumors that have been resected, including the dura mater. Furthermore, the literature review summarizes patient demographics, pathology, and surgical outcomes, exploring factors contributing to recurrence. This is the first study to comprehensively examine previous reports and describe risk factors for recurrence and metastasis.
2. Case Reports
2.1. Case 1
History. A 40-year-old woman with a history of left leg pain underwent a diagnostic workup, including thoracolumbar computed tomography (CT) myelography, which revealed an intraspinal extradural tumor at T12–L1 (Figure 1). MRI was not performed, as this evaluation occurred 22 years ago.
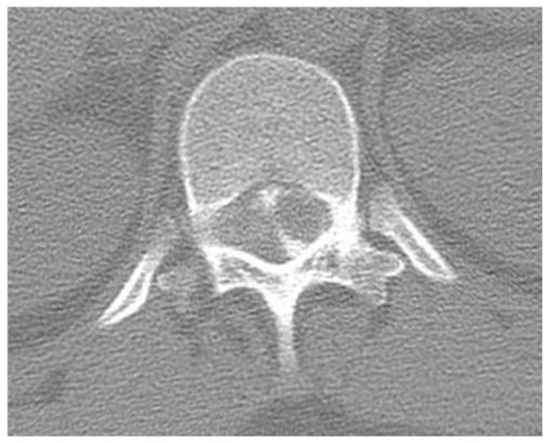
Figure 1.
Preoperative computed tomography myelography demonstrating an intraspinal extradural tumor at T12–L1.
Operation and Pathological Examination. The tumor was resected. Following T12 and L1 laminectomies, a brownish-red tumor covered by a thin capsule was observed on the dural tube. The tumor was well circumscribed, had a smooth margin with dural attachment, and was scarcely vascularized. The tumor did not adhere to the surrounding structures, including the T12 nerve root. Microscopic examination revealed tumor cell proliferation around the branched vessels. Oval- or spindle-shaped cells with a slightly acidic cytoplasm and oval nuclei were observed. No necrosis was observed. However, scattered mitotic figures were observed. Immunohistochemical staining was positive for STAT 6 and negative for CD 34. The Ki-67 labeling index was 2–3%. A pathological diagnosis of a WHO grade II SFT was considered (Figure 2).
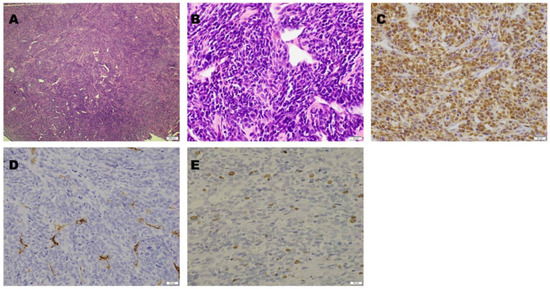
Figure 2.
Microscopic findings. Hematoxylin and eosin staining revealed tumor cell proliferation around the branched vessels ((A): magnification, 40×). The tumor cells were oval- or spindle-shaped with slightly acidic cytoplasm and oval nuclei ((B): magnification, 400×). On immunohistochemical staining, STAT6 was positive ((C): magnification, 400×), while CD34 was negative ((D): magnification, 400×). The Ki-67 labeling index was 2–3% ((E): magnification, 400×).
Postoperative Course. The patient was treated with teceleukin-based chemotherapy. Pain in the left leg recurred 6 years after the first surgery. Neurological deficits were not observed; however, magnetic resonance imaging (MRI) revealed tumor recurrence (Figure 3). Considering the aggressiveness, high recurrence rate, and need for a second surgery, gross total resection (GTR) including the neighboring structures, such as the T12 and L1 vertebrae, laminae, dura mater, and right T12 root, was performed (Figure 4). The pathological findings included WHO grade II SFT recurrence. No tumor recurrence or metastasis was observed during the 16-year follow-up period after the second surgery.
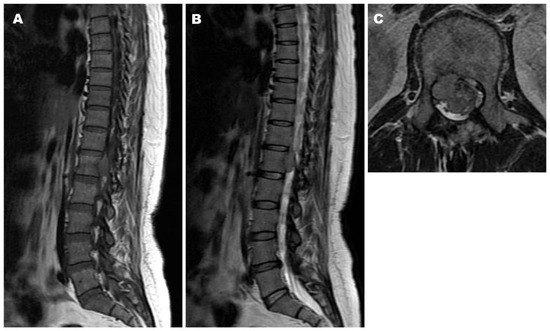
Figure 3.
Magnetic resonance imaging (MRI) six years after the first surgery demonstrating tumor recurrence at T12–L1, the same level as before. Iso intensity on both T1- and T2-weighted images ((A): Sagittal T1-weighted image, (B): Sagittal T2-weighted image, (C): Axial T2-weighted image).
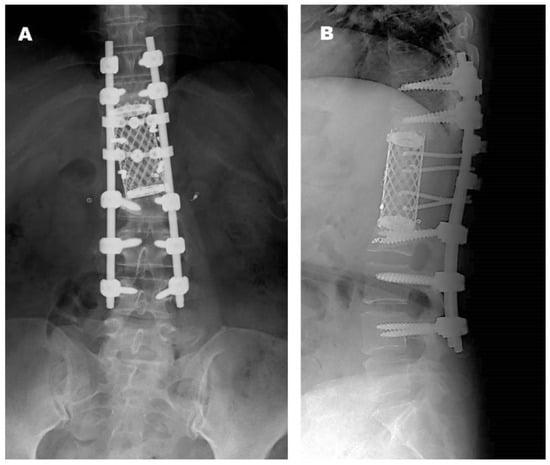
Figure 4.
Radiographs obtained at final follow-up after the second operation ((A): anteroposterior view, (B): lateral view).
2.2. Case 2
History. A 36-year-old woman reported back pain, numbness, and muscle weakness in both legs that developed gradually. MRI revealed a tumor with iso-signal intensity on both T1- and T2-weighted imaging posterior to the thecal sac at T3–4. Gadolinium-enhanced MRI revealed uniform enhancement of the tumor and a dural tail sign, which was highly suggestive of a meningioma (Figure 5).
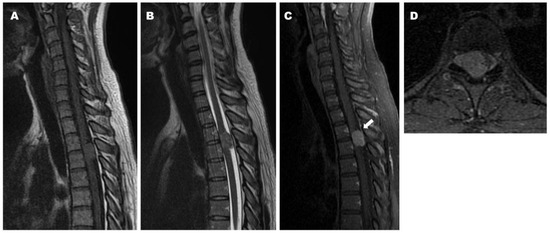
Figure 5.
Preoperative magnetic resonance imaging (MRI) demonstrating iso intensity of the tumor and cord compression on both T1- and T2-weighted images ((A): Sagittal T1-weighted image, (B): Sagittal T2-weighted image). Preoperative sagittal (C) and axial (D) T1-weighted MRI with contrast demonstrating homogeneous enhancement of the tumor and dural tail sign (arrow).
Operation and Pathological Examination. The tumor was resected. T3 and T4 laminectomies were performed to access the spinal canals. Dura mater incision revealed an oval, brownish-red tumor covered by a thin capsule in the subarachnoid space. Because the tumor strongly adhered to the right portion of the dura mater, we first resected the T3/4 right facet joint to secure an operative space, and the tumor and neighboring dura mater were subsequently resected en bloc. The dural defect was covered with artificial dura mater, and the resected T3/4 facet joint was reconstructed using a pedicle screw (Figure 6). Microscopic examination revealed tumor cell proliferation around the branched vessels. The sinus-like vessels demonstrated a “staghorn” pattern typical of HPCs. The tumor cells were spindle-shaped with oval nuclei and mitotic figures (>5 mitoses per 10 high-power fields). Immunohistochemical staining was positive for STAT 6 and negative for CD 34. The Ki67 labeling index was 6–7%. Thus, a pathological diagnosis of a WHO grade III SFT was considered (Figure 7).
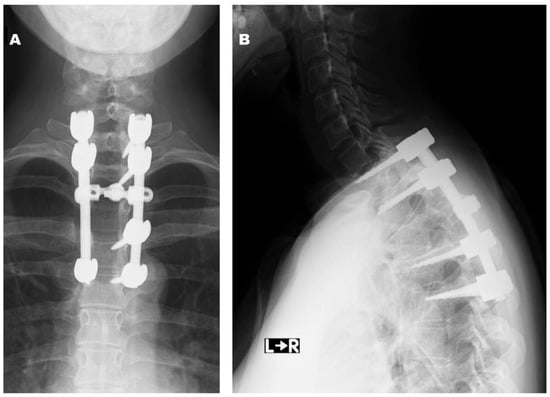
Figure 6.
Postoperative radiographs at final follow-up ((A): anteroposterior view, (B): lateral view).
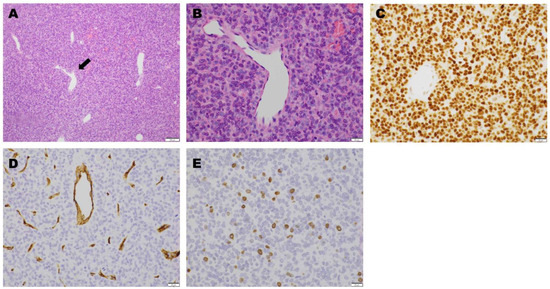
Figure 7.
Microscopic findings. Hematoxylin and eosin staining showed sinus-like large vascular branching in a “staghorn” (arrow) pattern ((A): magnification, 100×). There was a proliferation of oval- or spindle-shaped tumor cells around the vessels, with slightly acidic cytoplasm and oval nuclei with mitotic figures (>5 mitoses per 10 high-power field). ((B): magnification, 400×). On immunohistochemical staining, STAT6 was positive ((C): magnification, 400×), while CD34 was negative ((D): magnification, 400×). The Ki-67 labeling index was 6–7% ((E): magnification, 400×).
Postoperative Course. The patient’s neurological symptoms resolved postoperatively. No tumor recurrence or metastasis was observed during the 3-year follow-up period.
3. Review of the Literature
3.1. Literature Search
PubMed, Scopus, and Embase searches were performed for HPCs, HPCs/SFTs WHO grade II or III, and WHO grade II or III SFTs of the spine (Supplementary Figure S1). Additionally, the reference sections of each study were reviewed, to identify additional cases. The inclusion criteria were that the primary tumor was in the spine, and that reports included not only surgical cases but also cases treated with radiotherapy (RT), chemotherapy, and no treatment. Exclusion criteria included reports of distant metastasis to the spine from other sites and recurrence after tumor removal (those without detailed descriptions of the initial treatment). We identified 62 previously published papers with 170 cases [4,6,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68]. Fifteen reported cases [11,15,31,38,43,44,47,48,50,54,55,68] that focused on local recurrence or metastases to the spine were eliminated. A total of 157 reported cases of WHO grade II or III spinal SFTs were found, including our report and those previously diagnosed as spinal HPCs and WHO grade II or III HPCs/SFTs (Table 1).

Table 1.
Literature summary of spinal HPC, WHO grade II or III HPC/SFT, and WHO grade II or III SFT cases.
3.2. Summary of General and Surgical Information of Patients
3.2.1. Age and Sex
One hundred and twenty-eight patients reported their age at presentation. Most cases occurred in adults (85.2% of patients aged > 21 years). The patients’ ages ranged from 2 to 82 years (median: 40.8 years). One hundred and twenty-eight cases included information on the patient’s sex. A slight male predominance was observed among the reported cases (43.3% male vs. 38.2% female; Table 2).

Table 2.
Demographic and tumor characteristics.
3.2.2. Spinal Level and Compartment
All the cases included information on the spinal levels, except for two cases; the plurality of lesions occurred in the thoracic (40.1%), cervical (24.8%), and lumbar (15.3%) regions. The remaining tumors occurred in the sacrum or across the junctional levels. A total of 113 reported cases contained information on the spinal compartments involved. Based on the morphological data in the literature [6,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,32,33,34,35,36,37,39,40,41,42,45,46,47,49,50,51,52,53,54,56,57,58,59,60,61,62,63,64,65,66,67], spinal SFTs were divided into six types (Figure 8): Type 1, intracanal–intradural–extramedullary tumors (Case 2); Type 2, intracanal–intradural–intramedullary invasion tumors; Type 3, intracanal–extradural tumors (Case 1); Type 4, extracanal (vertebra, spinous process to lamina, intraforaminal, intraforaminal to paravertebra) tumors; Type 5, extradural to extracanal tumors; and Type 6, intradural to extracanal tumors. The plurality was Type 1 (21.7%) and Type 5 (21.0%), followed by Type 2 (8.9%) and Type 6 (7.7%).
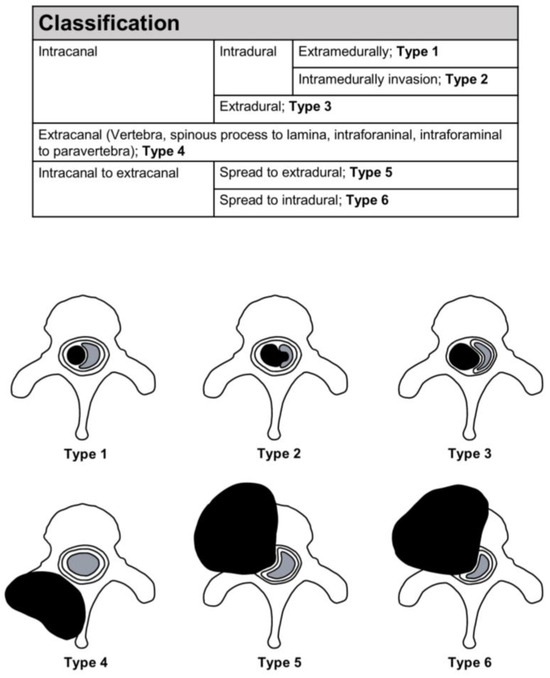
Figure 8.
Classification of tumors.
3.2.3. MRI Appearance and Differential Diagnosis
Ninety-three reported cases contained information on the MRI appearance. In all the cases, the tumors exhibited marked enhancement with intravenous contrast. Most lesions were hypointense or isointense on the T1-weighted images, and either isointense or hyperintense on the T2-weighted images. Some reports mentioned the preoperative diagnoses, which were most often meningioma or schwannoma [29,32,33,37,39,41,46,49,51,58,59,60,66,67]. One large sacral tumor was presumed to be either a chordoma or a giant cell tumor [39]. In cases of irregular bone invasion, Jia et al. [54] reported the radiological differential diagnoses to include giant cell tumors, malignant schwannomas, benign hemangiomas, and other metastatic tumors.
3.2.4. Treatment
A hundred and fifty-five of the reported cases contained information regarding the treatment. All the patients underwent surgery, except for three patients, two of whom received only radiation [12,40] and one of whom received radiation and chemotherapy [40]. One hundred and forty-seven reported cases contained detailed information on the surgery. GTR was performed in 58.0% of the patients, and subtotal resection (STR) or partial resection (PR) was performed in 35.7% (Table 3). A total of 14 patients (12 GTR, 1 STR, and 1 PR) underwent preoperative embolization of the tumor [18,25,30,36,47,49,54]. A total of 70 patients (36 GTR, 28 STR, 5 PR, and 1 surgery) underwent combined radiation [9,10,15,16,18,20,22,23,24,25,27,28,32,34,36,39,46,47,50,54,59,60,62,63], and 4 patients (3 GTR and 1 STR) underwent combined radiosurgery [46]. A total of 5 patients (3 GTR and 1 STR), including the patient in Case 1, underwent combination chemotherapy [23,46,50,63].
3.2.5. Tumor Origin
Few reports have indicated intraoperative assessments of the tumor origin [9,11,29,34,47,65]. Fifteen patients had tumors arising from the vertebral body [34], three from the nerve roots [9,29,65], and two from the spinal cord [11,47]. Fourteen patients, including the patient in Case 2, had tumors adherent to the dura [11,12,15,34,49,50], and five patients had tumors involving the nerve roots [20,42,57,62,64].
3.2.6. Histopathology
All cases indicated tumor histopathology except for one. The WHO grade was known for 76 patients. Thirty-nine patients (24.8%) had WHO grade II tumors, thirty-five patients (22.3%) had WHO grade III tumors, and two patients had borderline tumors between grades II and III (Table 3).
3.2.7. Clinical Outcome
One hundred and eight patients provided clinical outcome data. The follow-up period ranged from 1 month to 31 years. Seventeen patients had undergone a follow-up of <1 year, and eighty-one patients had undergone a follow-up of <5 years. Forty-six patients (29.3%), including the patient in Case 1, exhibited tumor recurrence or progression [9,10,18,19,20,23,30,34,40,46,50,53,54,63,66], and nine patients (5.7%) had metastasis [18,40,46,47,54]. Generally, outcomes depended largely on the histology and extent of resection. Thirty-three patients with WHO grade III tumors had reported the clinical outcome data. Nineteen patients (57.6%) had local recurrence [46,50,54,66], and four patients (12.1%) had distant metastasis [46,47] (including three patients who had both local recurrence and metastasis [46]). Thirty-nine patients with WHO grade II tumors had reported the clinical outcome data. Sixteen patients (41.0%) had local recurrence [46,53,54,66], with no distant metastasis. Furthermore, the outcomes seemed to depend largely on the extent of resection. Clinical outcome information was available for 14 patients with WHO grade III treated with STR or PR. Twelve patients (85.7%) had tumor recurrence or progression [46,50,54,66], and one of them had both local recurrence and metastasis [46]. Outcome information was available for 19 patients with WHO grade III treated with GTR. Seven patients (36.8%) exhibited tumor recurrence or progression [46,54], and three patients had metastasis [46,47] (including two patients who had both local recurrence and metastasis [46]). Outcome information was available for 11 patients with WHO grade II treated with STR or PR. Seven patients (63.6%) had tumor recurrence or progression [46,54,66], with no distant metastasis. The clinical outcome information was available for 28 patients with WHO grade II treated with GTR. Nine patients (32.1%), including the patient in our case report, had tumor recurrence or progression [46,53,63], with no distant metastasis.
3.3. Comparison Between Recurrence or Metastasis Cases and Disease-Free Cases
A statistical analysis was performed to determine the factors that influenced the outcome of either tumor recurrence or metastasis. Significance was set at p < 0.05. Statistical analyses were conducted using JMP Pro 17 Software (SAS Inc., Cary, NC, USA) for Windows. Table 3 shows the results of univariate analysis. The differences between the recurrence or metastasis cases and disease-free cases were analyzed using Pearson’s Chi-square test for nominal variables. Only the extent of resection was significantly associated with recurrence or metastasis (p < 0.0001). To identify the most significant risks for recurrence or metastasis, risk factor analysis was performed using multivariate logistic regression analysis (Table 4). A significant difference was noted in the extent of resection.

Table 3.
Differences between recurrence or metastasis and disease-free cases.
Table 3.
Differences between recurrence or metastasis and disease-free cases.
| Outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factors | All | Recurrence or Metastasis | Disease-Free | Unknown | ||||||
| Count | % | Count | % | Count | % | Count | % | p-Value | ||
| Age | 40.8 (yrs) | 39.6 (yrs) | 41.3 (yrs) | 0.61 | ||||||
| Sex | Male | 68 | 43.3 | 27 | 39.7 | 31 | 45.6 | 10 | 14.7 | 0.42 |
| Female | 60 | 38.2 | 19 | 31.7 | 30 | 50.0 | 11 | 18.3 | ||
| Unknown | 29 | 18.5 | 0 | 0.0 | 0 | 0.0 | 29 | 100.0 | ||
| Location | Occipitocervical or cervical | 43 | 27.4 | 16 | 37.2 | 12 | 27.9 | 15 | 34.8 | 0.19 |
| Cervicothoracic or thoracic | 66 | 42.0 | 15 | 22.7 | 32 | 48.5 | 19 | 28.8 | ||
| Thoracolumbar or lumbar | 32 | 20.4 | 12 | 37.5 | 12 | 37.5 | 8 | 25.0 | ||
| Lumbosacral or sacral | 14 | 8.9 | 2 | 14.3 | 4 | 28.6 | 8 | 57.1 | ||
| Unknown | 2 | 1.3 | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | ||
| Classification | Types 1–3 (intracanal) | 57 | 36.3 | 20 | 35.1 | 31 | 54.4 | 6 | 10.5 | 0.38 |
| Types 4–6 (expanding widely) | 56 | 35.7 | 23 | 41.1 | 25 | 44.6 | 8 | 14.3 | ||
| Unknown | 44 | 28.0 | 3 | 6.8 | 5 | 11.4 | 36 | 81.8 | ||
| Extent of resection | GTR | 91 | 58.0 | 20 | 22.0 | 48 | 52.7 | 23 | 25.3 | <0.0001 |
| STR or PR | 56 | 35.7 | 24 | 42.9 | 11 | 19.6 | 21 | 37.5 | ||
| Others | 10 | 6.3 | 2 | 20.0 | 2 | 20.0 | 6 | 60.0 | ||
| WHO grade | Grade II | 39 | 24.8 | 16 | 41.0 | 23 | 59.0 | 0 | 0.0 | 0.0967 |
| Grade III | 35 | 22.3 | 20 | 57.2 | 13 | 37.1 | 2 | 5.7 | ||
| Borderline between grade II and III | 2 | 1.3 | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | ||
| Unknown | 81 | 51.6 | 10 | 12.1 | 25 | 30.1 | 48 | 57.8 | ||
| Adjuvant radiotherapy (GTR) | GTR | 47 | 60.3 | 8 | 17.0 | 27 | 57.5 | 12 | 25.5 | 0.3 |
| GTR + RT | 31 | 39.7 | 10 | 32.2 | 19 | 61.3 | 2 | 6.5 | ||
| Adjuvant radiotherapy (STR or PR) | STR or PR | 25 | 45.5 | 7 | 28.0 | 6 | 24.0 | 12 | 48.0 | 0.21 |
| STR or PR + RT | 30 | 54.5 | 15 | 50.0 | 5 | 16.7 | 10 | 33.3 | ||

Table 4.
Multiple logistic regression analysis of recurrence or metastasis.
Table 4.
Multiple logistic regression analysis of recurrence or metastasis.
| Factors | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age | 0.99 | 0.95–1.03 | 0.57 |
| Sex (male) | 1.46 | 0.48–4.49 | 0.5 |
| Location | |||
| Occipitocervical or cervical | 3.39 | 0.89–12.90 | 0.07 |
| Cervicothoracic or thoracic | Reference | ||
| Thoracolumbar or lumbar | 1.8 | 0.40–8.11 | 0.44 |
| Lumbosacral or sacral | 0.53 | 0.02–16.40 | 0.71 |
| Classification (types 4–6) | 1.54 | 0.49–4.84 | 0.46 |
| Extent of resection (STR or PR) | 6.35 | 1.77–22.77 | 0.0045 |
| Adjuvant radiotherapy (without radiotherapy) | 0.68 | 0.22–2.17 | 0.52 |
| WHO grade III | 1.69 | 0.53–5.50 | 0.38 |
4. Discussion
SFTs are mesenchymal tumors that were first described in 1931 by Klemperer and Coleman as primary neoplasms arising from the pleura [69]. HPCs, which are derived from the pericytes surrounding the capillaries and post-capillary venules, were first described by Stout and Murray in 1942 [70]. Although both SFTs and HPCs can arise in any soft tissue of the human body, the CNS WHO assigned the term SFTs to such lesions in 2021, as both the tumors share inversions at 12q13 leading to STAT6 nuclear expression. WHO grade II or III SFTs are well-recognized aggressive neoplasms with a high rate of recurrence and propensity to metastasize [6].
The majority of spinal WHO grade II or III SFT cases have occurred in adults, with a slight male predominance. These tumors most often occur in the thoracic region but can also occur in a wide variety of locations, from the intradural to the vertebral regions.
SFTs did not show any specific imaging features. Intracanal tumors, such as Types 1, 2, and 3, were sometimes misdiagnosed preoperatively as benign tumors, such as schwannomas or meningiomas, owing to non-involvement of the surrounding bone. Residual SFTs from incomplete tumor removal can spread to the spinal cord and other tissues, causing tumor recurrence and regrowth [46,50]. Thus, distinguishing spinal SFTs from other types of tumors preoperatively is essential for improving clinical outcomes. Okubo et al. [66] reported that most patients with SFTs demonstrated isointensity in the spinal cord on both T1- and T2-weighted images compared to schwannomas, and no significant differences compared to meningiomas. Nearly all the patients with SFTs demonstrated highly uniform enhancement patterns, similar to those with meningiomas. Compared to meningiomas, SFTs lack the dural tail sign and intratumoral calcification, and have an irregular shape. However, the dural tail sign can appear when the SFTs arise in the dura mater [64], as in Case 2. The tumor often demonstrates marked enhancement with prominent flow voids on MRI owing to its hypervascularity [36]. However, cases that do not show such imaging features have been reported, which often mimic various tumors such as meningiomas and schwannomas on imaging [46,47].
Total resection is the gold standard of treatment for SFTs. Some studies demonstrated better recurrence-free and overall survival rates following GTR of SFTs in the brain [71,72]. Schiariti et al. [71] reported that patients who underwent GTR demonstrated longer mean overall survival (GTR 235 vs. STR 175 months; p = 0.47) and mean recurrence-free intervals (GTR 117 vs. STR 53 months; p = 0.0045). Soyuer et al. [72] reported 5-year local control rates in patients treated with GTR and STR of 84 and 38%, respectively (p = 0.0034); few studies found similar findings in spinal SFTs [50,54,73]. However, other studies did not find any clinical, overall, or recurrence-free survival benefits following complete resection of spinal SFTs [46,59,74]. Liu et al. [46] reported that total resection in high-risk patients may not be necessary for spinal SFTs, especially those that are difficult to completely remove, such as tumors that had grown out from the spinal canal, because total resection did not represent a significant survival or recurrence-free benefit. They described stereotactic radiosurgery for residual tumors that had a satisfactory tumor control effect. Li et al. [59] also proposed that subsequent adjuvant RT is more beneficial than total resection for patients at high risk of injury to the nerve root or dura mater. As in Case 2, when the tumor is tightly adhered to the dura mater in a location where it is relatively easy to remove, such as within the spinal canal, the possibility that the dura mater is the tumor origin cannot be ruled out, so removal of the dura mater as well should be considered.
RT for SFTs in the brain improves local control and overall survival [4,75,76]. However, whether postoperative RT should be performed for spinal SFTs remains unknown. Studies demonstrated no significant difference in the outcomes of patients with spinal SFTs who received adjuvant radiotherapy when compared to those of patients who did not receive RT [37,46,74], and this is consistent with our findings. Using precision radiotherapy, such as radiosurgery with high radiation doses, the morbidity associated with adjacent organ damage, especially spinal cord toxicity, is minimized [77]. Combs et al. [77] analyzed the use of high-precision RT in patients with SFTs, including two spinal cases. They demonstrated overall survival rates of 100% and 64% at 5 and 10 years, respectively, in patients treated with a combination of surgical and RT approaches. Furthermore, for recurrent SFTs of the CNS including spine cases undergoing radiosurgery, tumor control rates between 82% and 93% have been reported [74,78,79]. Ecker et al. [74] suggested radiosurgery to be a good alternative to repeated surgery in patients with recurrent SFTs. However, adjuvant chemotherapy has generally been proven to be ineffective [46,50,54,74].
The 5-year recurrence-free rate of intracranial SFTs was 46–89% in recent reports [71,74]. Liu et al. [46] reported 24 patients with intraspinal SFTs and demonstrated a 5-year recurrence-free survival rate of 29.4%, which is much lower than that of intracranial SFTs. Intraspinal SFTs can recur more often or earlier than intracranial SFTs. Several studies have shown that a higher pathological grade is associated with a greater local recurrence rate in intraspinal SFTs [37,46,54,73]. In contrast, our analysis did not identify pathological grade as a significant risk factor for recurrence or metastasis. One reason for this apparent discrepancy may be that pathological classification was not recorded in 81 cases (51.6%). However, consistent with our findings, total resection appears to significantly decrease the recurrence rate of spinal SFTs [54,73]. High-grade intraspinal SFTs may exhibit firm adherence to the dura mater, irregular invasion of the bone, and rich vascularization; thus, a small operating field in the spine may make complete removal of the tumor challenging. Recurrence often occurs many years after initial treatment [9,11], necessitating careful long-term follow-up of all patients with SFTs. In this review, distant metastases of spinal SFTs following initial treatment were observed in only nine cases (5.7%), a rate lower than that reported for intracranial SFTs, with distant metastases ranging from 14% to 50% [4,42,74]. Reports of survival rates for intraspinal SFTs are rare, but two available studies indicated a 76% 5-year survival rate [46] and an 85% 10-year survival rate [8].
Comparisons between intracranial and intraspinal SFTs are also uncommon. Boyett et al. [8] reported that intraspinal SFTs had a smaller tumor size, lower malignancy, and a lesser extent of resection compared to intracranial SFTs. They also found an association between spinal tumor location and improved survival in patients with SFTs of the CNS, consistent with the results from Giordan et al. [73]. As mentioned above, spinal tumors have a superior survival rate despite being more likely to recur. Local recurrence is expected if GTR cannot be performed on spinal tumors, but the malignancy is low. Hence, even such cases may not lead to fatal problems such as distant metastasis.
One of the limitations of this study is that the factors used in the multivariate analysis, particularly the WHO grades, contained a large number of missing data, which may have influenced the results. By continuing to increase the number of cases, missing data will be supplemented, and future research will involve creating treatment algorithms based on tumor location and malignancy.
5. Conclusions
Spinal SFTs of the spine are rare neoplasms. To date, no large case series has been performed. Spinal SFTs are less malignant than cranial SFTs, and patients have higher rates of long-term survival. However, spinal SFTs, especially WHO grade II or III, are aggressive neoplasms with a high recurrence rate. GTR is recommended to treat these lesions. When GTR is difficult, such as in recurrent cases, radiosurgery is one of the most important alternatives for treating spinal SFTs. Again, the reported incidence of local recurrence for this type of tumor is very high, and recurrence often occurs many years following the initial treatment. Therefore, careful long-term observation is necessary following surgery even if the tumor has been completely resected.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/d10.3390/jcm14176068/s1, Figure S1: Identification of studies via databases.
Author Contributions
Conceptualization, K.S. and K.K.; methodology, K.S. and K.K.; formal analysis, K.S.; investigation, K.S., Y.O. and S.T.; resources, K.S., Y.O., S.T. and Y.U.; data curation, K.S. and K.K.; writing—original draft preparation, K.S.; writing—review and editing, K.S., Y.U., K.K. and Y.K.; visualization, K.S., Y.O. and S.T.; supervision, K.K. and Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study. Case reports do not qualify as research according to ethical guidelines for medical and biological research involving human subjects given by the Bioethics and Safety Office, Life Sciences Division of the Ministry of Education, Culture, Sports, Science and Technology and therefore do not require ethical approval.
Informed Consent Statement
Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SFT | solitary fibrous tumor |
| HPC | hemangiopericytoma |
| CNS WHO | World Health Organization Classification of Tumors of the Central Nervous System |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| GTR | gross total resection |
| FU | Follow-up |
| yrs | years |
| mos | months |
| M | male |
| F | female |
| RT | radiotherapy |
| STR | subtotal resection |
| PR | partial resection |
| NR | not reported |
| mets | metastasis |
| Chemo | chemotherapy |
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Bisceglia, M.; Galliani, C.; Giannatempo, G.; Lauriola, W.; Bianco, M.; D’Angelo, V.; Pizzolitto, S.; Vita, G.; Pasquinelli, G.; Magro, G.; et al. Solitary Fibrous Tumor of the Central Nervous System: A 15-Year Literature Survey of 220 Cases (August 1996–July 2011). Adv. Anat. Pathol. 2011, 18, 356–392. [Google Scholar] [CrossRef] [PubMed]
- Mena, H.; Ribas, J.L.; Pezeshkpour, G.H.; Cowan, D.N.; Parisi, J.E. Hemangiopericytoma of the Central Nervous System: A Review of 94 Cases. Hum. Pathol. 1991, 22, 84–91. [Google Scholar] [CrossRef]
- Kinslow, C.J.; Rajpara, R.; Wu, C.C.; Bruce, S.S.; Canoll, P.D.; Wang, S.H.; Sonabend, A.M.; Sheth, S.A.; McKhann, G.M.; Sisti, M.B.; et al. Invasiveness Is Associated with Metastasis and Decreased Survival in Hemangiopericytoma of the Central Nervous Syste. J. Neurooncol. 2017, 133, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Ijiri, K.; Yuasa, S.; Yone, K.; Matsunaga, S.; Ryoki, Y.; Taniguchi, N.; Yonezawa, S.; Komiya, S. Primary Epidural Hemangiopericytoma in the Lumbar Spine: A Case Report. Spine 2002, 27, E189–E192. [Google Scholar] [CrossRef]
- Kinslow, C.J.; Bruce, S.S.; Rae, A.I.; Sheth, S.A.; McKhann, G.M.; Sisti, M.B.; Bruce, J.N.; Sonabend, A.M.; Wang, T.J.C. Solitary-Fibrous Tumor/Hemangiopericytoma of the Central Nervous System: A Population-Based Study. J. Neurooncol. 2018, 138, 173–182. [Google Scholar] [CrossRef]
- Boyett, D.; Kinslow, C.J.; Bruce, S.S.; Sonabend, A.M.; Rae, A.I.; McKhann, G.M.; Sisti, M.B.; Bruce, J.N.; Cheng, S.K.; Wang, T.J.C. Spinal Location Is Prognostic of Survival for Solitary-Fibrous Tumor/Hemangiopericytoma of the Central Nervous System. J. Neurooncol. 2019, 143, 457–464. [Google Scholar] [CrossRef]
- Schirger, A.; Uihlein, A.; Parker, H.L.; Kernohan, J.W. Hemangiopericytoma Recurring after 26 Years; Report of Case. Proc. Staff Meet. Mayo Clin. 1958, 33, 347–352. [Google Scholar]
- Kruse, F. Hemangiopericytoma of the Meniges (Angioblastic Meningioma of Cushing and Eisenhardt). Clinico-Pathologic Aspects and Follow-up Studies in 8 Cases. Neurology 1961, 11, 771–777. [Google Scholar] [CrossRef]
- Pitlyk, P.J.; Dockery, M.B.; Miller, R.H. Hemangiopericytoma of the Spinal Cord: Report of Three Cases. Neurology 1965, 15, 649–653. [Google Scholar] [CrossRef]
- Fathie, K. Hemangiopericytoma of the Thoracic Spine; Case Report. J. Neurosurg. 1970, 32, 371–374. [Google Scholar] [CrossRef]
- Gerner, R.E.; Moore, G.E.; Pickren, J.W. Hemangiopericytoma. Ann. Surg. 1974, 179, 128–132. [Google Scholar] [CrossRef]
- McMaster, M.J.; Soule, E.H.; Ivins, J.C. Hemangiopericytoma. A Clinicopathologic Study and Long-Term Followup of 60 Patients. Cancer 1975, 36, 2232–2244. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.J.; Fornasier, V.L.; Livingston, K.E. Hemangiopericytoma of the Spinal Canal. Report of Three Cases. J. Neurosurg. 1978, 49, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Cappabianca, P.; Maiuri, F.; Pettinato, G.; Di Prisco, B. Hemangiopericytoma of the Spinal Canal. Surg. Neurol. 1981, 15, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.B.; Grode, M.L.; Goodman, M.D. Hemangiopericytoma of the Cervical Spine: Report of an Unusual Case. Clin. Orthop. Relat. Res. 1980, 151, 201–204. [Google Scholar] [CrossRef]
- Muraszko, K.M.; Antunes, J.L.; Hilal, S.K.; Michelsen, W.J. Hemangiopericytomas of the Spine. Neurosurgery 1982, 10, 473–479. [Google Scholar] [CrossRef]
- Wold, L.E.; Unni, K.K.; Sim, F.H. Hemangiopericytoma of Bone. Am. J. Surg. Pathol. 1982, 6, 53–58. [Google Scholar] [CrossRef]
- Ciappetta, P.; Celli, P.; Palma, L.; Mariottini, A. Intraspinal Hemangiopericytomas. Report of Two Cases and Review of the Literature. Spine 1985, 10, 27–31. [Google Scholar] [CrossRef]
- Yagishita, A.; Hassoun, J.; Vincentelli, F.; Jiddane, M.; Salamon, G. Neuroradiological Study of Hemangiopericytomas. Neuroradiology 1985, 27, 420–425. [Google Scholar] [CrossRef]
- Bridges, L.R.; Roche, S.; Nashef, L.; Rose, F.C. Haemangiopericytic Meningioma of the Sacral Canal: A Case Report. J. Neurol. Neurosurg. Psychiatry 1988, 51, 288–290. [Google Scholar] [CrossRef]
- Tang, J.S.; Gold, R.H.; Mirra, J.M.; Eckardt, J. Hemangiopericytoma of Bone. Cancer 1988, 62, 848–859. [Google Scholar] [CrossRef]
- Salvati, M.; Ciappetta, P.; Artico, M.; Raco, A.; Fortuna, A. Intraspinal Hemangiopericytoma: Case Report and Review of the Literature. Neurosurg. Rev. 1991, 14, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Cizmeli, M.O.; Ligit, E.T.; Ulug, H.; Erdogan, A. A Giant Paraspinal Hemangiopericytoma and Its Preoperative Embolization. Neuroradiology 1992, 34, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Tu, Y.K.; Lin, S.M.; Shun, C.T. Primary Hemangiopericytoma in the Axis Bone: Case Report and Review of Literature. Neurosurgery 1996, 39, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Dufour, H.; Métellus, P.; Fuentes, S.; Murracciole, X.; Régis, J.; Figarella-Branger, D.; Grisoli, F. Meningeal Hemangiopericytoma: A Retrospective Study of 21 Patients with Special Review of Postoperative External Radiotherapy. Neurosurgery 2001, 48, 756–763. [Google Scholar] [CrossRef]
- Akhaddar, A.; Chakir, N.; Amarti, A.; Hassani, M.R.E.; Khamlichi, A.E.L.; Jiddane, M.; Boukhrissi, N. Thoracic Epidural Hemangiopericytoma. Case Report. J. Neurosurg. Sci. 2002, 46, 89–92. [Google Scholar]
- Betchen, S.; Schwartz, A.; Black, C.; Post, K.; Traynelis, V.C.; Benzel, E.C.; Antunes, J.L. Intradural Hemangiopericytoma of the Lumbar Spine: Case Report. Neurosurgery 2002, 50, 654–657. [Google Scholar] [CrossRef]
- Musacchio, M.; Mont’Alverne, F.; Belzile, F.; Belzile, V.; Lenz, V.; Riquelme, C.; Tournade, A. Posterior Cervical Haemangiopericytoma with Intracranial and Skull Base Extension. Diagnostic and Therapeutic Challenge of a Rare Hypervascular Neoplasm. J. Neuroradiol. 2003, 30, 180–187. [Google Scholar]
- Chang, C.-C.; Chang, Y.-Y.; Lui, C.-C.; Huang, C.-C.; Liu, J.-S. Meningeal Hemangiopericytoma with Delayed Multiple Distant Metastases. J. Chin. Med. Assoc. 2004, 67, 527–532. [Google Scholar]
- Mohammadianpanah, M.; Torabinejad, S.; Bagheri, M.H.; Omidvari, S.; Mosalaei, A.; Ahmadloo, N. Primary Epidural Malignant Hemangiopericytoma of Thoracic Spinal Column Causing Cord Compression: Case Report. Sao Paulo Med. J. 2004, 122, 220–222. [Google Scholar] [CrossRef]
- Kashiwazaki, D.; Hida, K.; Yano, S.; Seki, T.; Iwasaki, Y. Subpial Hemangiopericytoma with Marked Extramedullary Growth: Case Report. Neurosurgery 2007, 61, E1336–E1337. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J. Clinical and Pathological Characteristics of Primary Intraspinal Hemangiopericytoma and Choice of Treatment. Chin. Med. J. 2007, 120, 115–119. [Google Scholar] [CrossRef]
- Endo, H.; Murakami, K.; Kumade, T.; Watanabe, M.; Ozawa, H.; Tominaga, T. Hemangiopericytoma of the Lumbar Spine: A Case Report. Spinal Surg. 2008, 22, 33–37. [Google Scholar] [CrossRef][Green Version]
- Fitzpatrick, D.; Mahajan, J.; Lewkowitz, M.; Black, K.; Setton, A.; Woldenberg, R. Intradural Hemangiopericytoma of the Lumbar Spine: A Rare. Am. J. Neuroradiol. 2009, 30, 152–154. [Google Scholar] [CrossRef]
- Chou, C.W.; Hsu, S.P.C.; Lin, S.C.; Chen, M.H.; Shih, Y.H.; Lee, L.S.; Lin, C.F. Primary Intradural Hemangiopericytoma with Intramedullary Invasion. J. Chin. Med. Assoc. 2009, 72, 536–541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cole, C.D.; Schmidt, M.H. Hemangiopericytomas of the Spine: Case Report and Review of the Literature. Rare Tumors 2009, 1, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Zentar, A.; Sall, I.; Ali, A.A.; Bouchentouf, S.M.; Quamous, M.; Chahdi, H.; Hajjouji, A.; Fahssi, M.; El Kaoui, H.; Al Bouzidi, A.; et al. Sacral Hemangiopericytoma Involving the Retrorectal Space: Report of a Case. Surg. Today 2009, 39, 344–348. [Google Scholar] [CrossRef]
- Verbeke, S.L.J.; Fletcher, C.D.M.; Alberghini, M.; Daugaard, S.; Flanagan, A.M.; Parratt, T.; Kroon, H.M.; Hogendoorn, P.C.W.; Bovée, J.V.M.G. A Reappraisal of Hemangiopericytoma of Bone; Analysis of Cases Reclassified as Synovial Sarcoma and Solitary Fibrous Tumor of Bone. Am. J. Surg. Pathol. 2010, 34, 777–783. [Google Scholar] [CrossRef]
- Ackerman, P.D.; Khaldi, A.; Shea, J.F. Intradural Hemangiopericytoma of the Thoracic Spine: A Case Report. Spine J. 2011, 11, e9–e14. [Google Scholar] [CrossRef]
- Moscovici, S.; Ramirez-Denoriega, F.; Fellig, Y.; Rosenthal, G.; Cohen, J.E.; Itshayek, E. Intradural Extramedullary Hemangiopericytoma of the Thoracic Spine Infiltrating a Nerve Root: A Case Report and Literature Review. Spine 2011, 36, E1534–E1539. [Google Scholar] [CrossRef]
- Torigoe, K.; Akai, T.; Iida, T.; Shiraga, S.; Sasagawa, Y.; Tachibana, O.; Iizuka, H. Hemangiopericytoma on the Intradural Thoracic Spinal Cord: A Case Report. No Shinkei Geka. Neurol. Surg. 2012, 40, 351–357. [Google Scholar]
- Nakashima, H.; Imagama, S.; Sakai, Y.; Nakamura, H.; Katayama, Y.; Ito, Z.; Wakao, N.; Matsuyama, Y.; Ishiguro, N. Dumbbell-Type Hemangiopericytoma in the Cervical Spine: A Case Report and Review. J. Orthop. Sci. 2013, 18, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Drazin, D.; Shweikeh, F.; Bannykh, S.; Johnson, J. Hemangiopericytoma Invading the Craniovertebral Junction: First Reported Case and Review of the Literature. J. Craniovertebr. Junction Spine 2013, 4, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.G.; Yang, A.C.; Chen, N.; Yang, J.; Qiu, X.G.; Zhang, J.G. Hemangiopericytomas in the Spine: Clinical Features, Classification, Treatment, and Long-Term Follow-up in 26 Patients. Neurosurgery 2013, 72, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi, A.; Drazin, D.; Gates, M.; Shirzadi, N.; Banykh, S.; Fan, X.; Hunt, L.; Baron, E.M.; King, W.A.; Kim, T.T.; et al. Surgical Management of Primary Spinal Hemangiopericytomas: An Institutional Case Series and Review of the Literature. Eur. Spine J. 2013, 22 (Suppl. S3), 450–459. [Google Scholar] [CrossRef]
- Kaur, J.; Pandit, S.; Sharma, M.C.; Julka, P.K.; Rath, G.K. Intradural Extra Medullary Hemangiopericytoma of Dorsal Spine. Childs Nerv. Syst. 2015, 31, 173–175. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, J.; Zhou, D. Hemangiopericytoma of the Cervicothoracic Spine: A Case Report and Literature Review. Turk. Neurosurg. 2014, 24, 948–953. [Google Scholar] [CrossRef]
- Das, A.; Singh, P.K.; Suri, V.; Sable, M.N.; Sharma, B.S. Spinal Hemangiopericytoma: An Institutional Experience and Review of Literature. Eur. Spine J. 2015, 24, 606–613. [Google Scholar] [CrossRef]
- Türk, C.Ç.; Kara, N.N.; Süren, D.; Özdöl, Ç.; Gediz, T.; Yildiz, S. Distinctive Characteristic Features of Intramedullary Hemangiopericytomas. Asian Spine J. 2015, 9, 522–528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chew, L.S.; Han, X.J.; Tan, K.K.; Bundele, M.M. Hemangiopericytoma of the Thoracic Spine: A Case Report. J. Surg. Case Rep. 2017, 2017, rjx121. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Xiao, D.; He, Y.; Yin, H.; Gong, G.; Long, X.; Liao, W.; Li, X.; Sun, L.; Zhang, Y.; et al. Spinal Solitary Fibrous Tumor/Hemangiopericytoma: A Clinicopathologic and Radiologic Analysis of Eleven Cases. World Neurosurg. 2017, 104, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhou, Z.; Zhang, D.; Yang, J.; Liu, C.; Wang, T.; Wu, Z.; Yang, C.; Wei, H.; Zhao, J.; et al. Surgical Management of Spinal Solitary Fibrous Tumor/Hemangiopericytoma: A Case Series of 20 Patients. Eur. Spine J. 2018, 27, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Onoki, T.; Kanno, H.; Aizawa, T.; Hashimoto, K.; Itoi, E.; Ozawa, H. Recurrent Primary Osseous Hemangiopericytoma in the Thoracic Spine: A Case Report and Literature Review. Eur. Spine J. 2018, 27 (Suppl. S3), 386–392. [Google Scholar] [CrossRef]
- Shukla, P.; Gulwani, H.V.; Kaur, S.; Shanmugasundaram, D. Reappraisal of Morphological and Immunohistochemical Spectrum of Intracranial and Spinal Solitary Fibrous Tumors/Hemangiopericytomas with Impact on Long-Term Follow-Up. Indian J. Cancer 2018, 55, 214–221. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Lu, Y.; Lu, C.; Xu, T.; Yan, Y.; Chen, J. Remarkable Recovery in a Patient with Intradural Extramedullary Haemangiopericytoma: A Case Report and Literature Review. Folia Neuropathol. 2018, 56, 151–157. [Google Scholar] [CrossRef]
- Fujita, T.; Nakashima, H.; Sakai, Y.; Takatsu, T. Primary Intradural Extramedullary Solitary FibrousTumor/Hemangiopericytoma of the Thoracic Spine: Case Report. Clin. Surg. 2019, 4, 2515. [Google Scholar]
- Li, Z.; Deng, Y.; Li, Z.; Wang, T.; Gao, J.; Zhou, W.; Li, Y.; Wang, Y. Primary Epidural Hemangiopericytoma of the Thoracic Spine: Case Report and Literature Review. J. Clin. Neurosci. 2019, 60, 142–147. [Google Scholar] [CrossRef]
- Louis, D.K.; Serge, I.B.G.; André, T.; Yao, K.S.; Nassim, B.; Michel, L. A Rare Case of a Thoracic Spinal Hemangiopericytoma. Open J. Mod. Neurosurg. 2019, 9, 1–6. [Google Scholar] [CrossRef][Green Version]
- Paeng, S. Intradural Extramedullary Thoracic Spinal Cord Hemangiopericytoma: A Case Report and Review of Literature. Neurol. Asia 2019, 24, 179–183. [Google Scholar][Green Version]
- Fiorenza, V.; Ascanio, F.; Ferlito, F.; Lo Duca, B.; Librizzi, D. Primary Intra and Extradural Solitary Fibrous Tumor/Hemangiopericytoma of Thoracic Spine with Paravertebral Intrathoracic Spread: Case Report and Review of the Literature. Interdiscip. Neurosurg. 2020, 21, 100746. [Google Scholar] [CrossRef]
- Singla, R.; Singh, P.; Khanna, G.; Suri, V.; Agarwal, D.; Chandra, P.; Kale, S.; Mahapatra, A. An Institutional Review of 10 Cases of Spinal Hemangiopericytoma/Solitary Fibrous Tumor. Neurol. India 2020, 68, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Nishii, T.; Nagashima, Y.; Nishimura, Y.; Ito, H.; Oyama, T.; Matsuo, M.; Sakakibara, A.; Shimada, S.; Saito, R. Two Cases of Solitary Fibrous Tumor/Hemangiopericytoma with Different Clinical Features According to the World Health Organization Classification: Case Report and Review of the Literature. J. Spine Surg. 2021, 7, 532–539. [Google Scholar] [CrossRef]
- Olmsted, Z.T.; Tabor, J.; Doron, O.; Hosseini, H.; Schneider, D.; Green, R.; Wahl, S.J.; Scuibba, D.M.; D’Amico, R.S. Intradural Extramedullary Solitary Fibrous Tumor of the Thoracic Spinal Cord. Cureus 2021, 13, e18613. [Google Scholar] [CrossRef]
- Okubo, T.; Nagoshi, N.; Tsuji, O.; Tachibana, A.; Kono, H.; Suzuki, S.; Okada, E.; Fujita, N.; Yagi, M.; Matsumoto, M.; et al. Imaging Characteristics and Surgical Outcomes in Patients With Intraspinal Solitary Fibrous Tumor/Hemangiopericytoma: A Retrospective Cohort Study. Glob. Spine J. 2023, 13, 276–283. [Google Scholar] [CrossRef]
- Kumar, R.; Vaid, V.K.; Kumar, V.; Kalra, S.K. Hemangiopericytoma of Thoracic Spine: A Rare Bony Tumor. Childs Nerv. Syst. 2007, 23, 1215–1219. [Google Scholar] [CrossRef]
- Radley, M.G.; McDonald, J.V. Meningeal Hemangiopericytoma of the Posterior Fossa and Thoracic Spinal Epidural Space. Neurosurgery 1992, 30, 446–452. [Google Scholar] [CrossRef]
- Klemperer, P.; Coleman, B.R. Primary Neoplasms of the Pleura. A Report of Five Cases. Am. J. Ind. Med. 1992, 22, 1–31. [Google Scholar] [CrossRef]
- Stout, A.P.; Murray, M.R. HEMANGIOPERICYTOMA: A VASCULAR TUMOR FEATURING ZIMMERMANN’S PERICYTES. Ann. Surg. 1942, 116, 26–33. [Google Scholar] [CrossRef]
- Schiariti, M.; Goetz, P.; El-Maghraby, H.; Tailor, J.; Kitchen, N. Hemangiopericytoma: Long-Term Outcome Revisited. J. Neurosurg. 2011, 114, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Soyuer, S.; Chang, E.L.; Selek, U.; McCutcheon, I.E.; Maor, M.H. Intracranial Meningeal Hemangiopericytoma: The Role of Radiotherapy: Report of 29 Cases and Review of the Literature. Cancer 2004, 100, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Giordan, E.; Marton, E.; Wennberg, A.M.; Guerriero, A.; Canova, G. A Review of Solitary Fibrous Tumor/Hemangiopericytoma Tumor and a Comparison of Risk Factors for Recurrence, Metastases, and Death among Patients with Spinal and Intracranial Tumors. Neurosurg. Rev. 2021, 44, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Ecker, R.D.; Marsh, W.R.; Pollock, B.E.; Kurtkaya-Yapicier, Ö.; McClelland, R.; Scheithauer, B.W.; Buckner, J.C. Hemangiopericytoma in the Central Nervous System: Treatment, Pathological Features, and Long-Term Follow up in 38 Patients. J. Neurosurg. 2003, 98, 1182–1187. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, H.W.; Kim, Y.S.; Kim, C.J.; Hwang, S.K.; Paek, S.H.; Kim, D.G.; Kwun, B.D. Meningeal Hemangiopericytomas: Long-Term Outcome and Biological Behavior. Surg. Neurol. 2003, 59, 47–53. [Google Scholar] [CrossRef]
- Guthrie, B.L.; Ebersold, M.J.; Scheithauer, B.W.; Shaw, E.G. Meningeal Hemangiopericytoma: Histopathological Features, Treatment, and Long-Term Follow-up of 44 Cases. Neurosurgery 1989, 25, 514–522. [Google Scholar] [CrossRef]
- Combs, S.E.; Thilmann, C.; Debus, J.; Schulz-Ertner, D. Precision Radiotherapy for Hemangiopericytomas of the Central Nervous System. Cancer 2005, 104, 2457–2465. [Google Scholar] [CrossRef]
- Payne, B.R.; Prasad, D.; Steiner, M.; Steiner, L. Gamma Surgery for Hemangiopericytomas. Acta Neurochir. 2000, 142, 527–537. [Google Scholar] [CrossRef]
- Coffey, R.J.; Cascino, T.L.; Shaw, E.G. Radiosurgical Treatment of Recurrent Hemangiopericytomas of the Meninges: Preliminary Results. J. Neurosurg. 1993, 78, 903–908. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).