Comparison of Open Versus Minimally Invasive Repair of Colovesical Fistula: A Case Report and Propensity-Matched National Database Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Report Identification
2.2. National Inpatient Sample Data Extraction
2.3. Propensity Score Matching and Statistical Analysis
2.4. Ethical Considerations
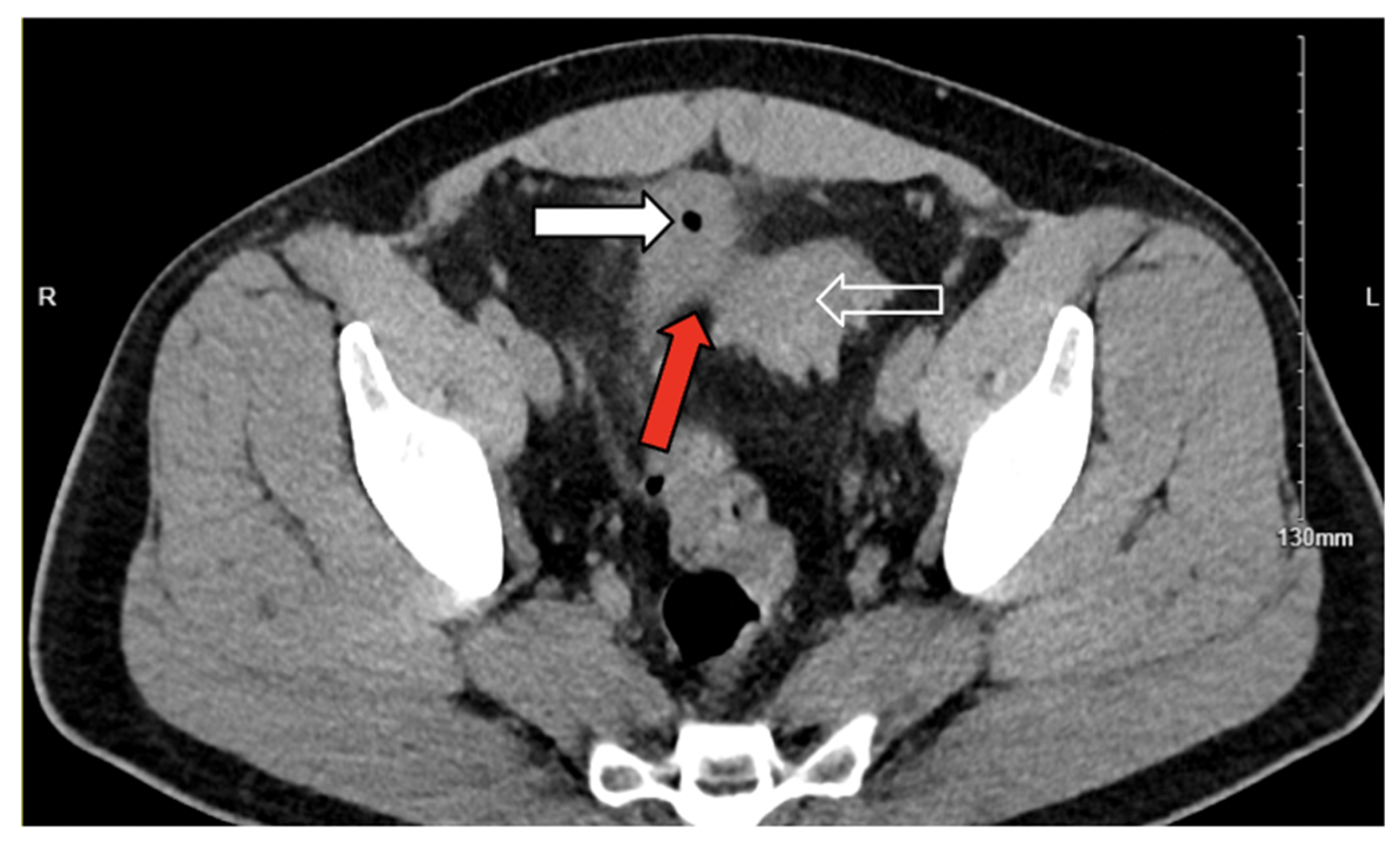
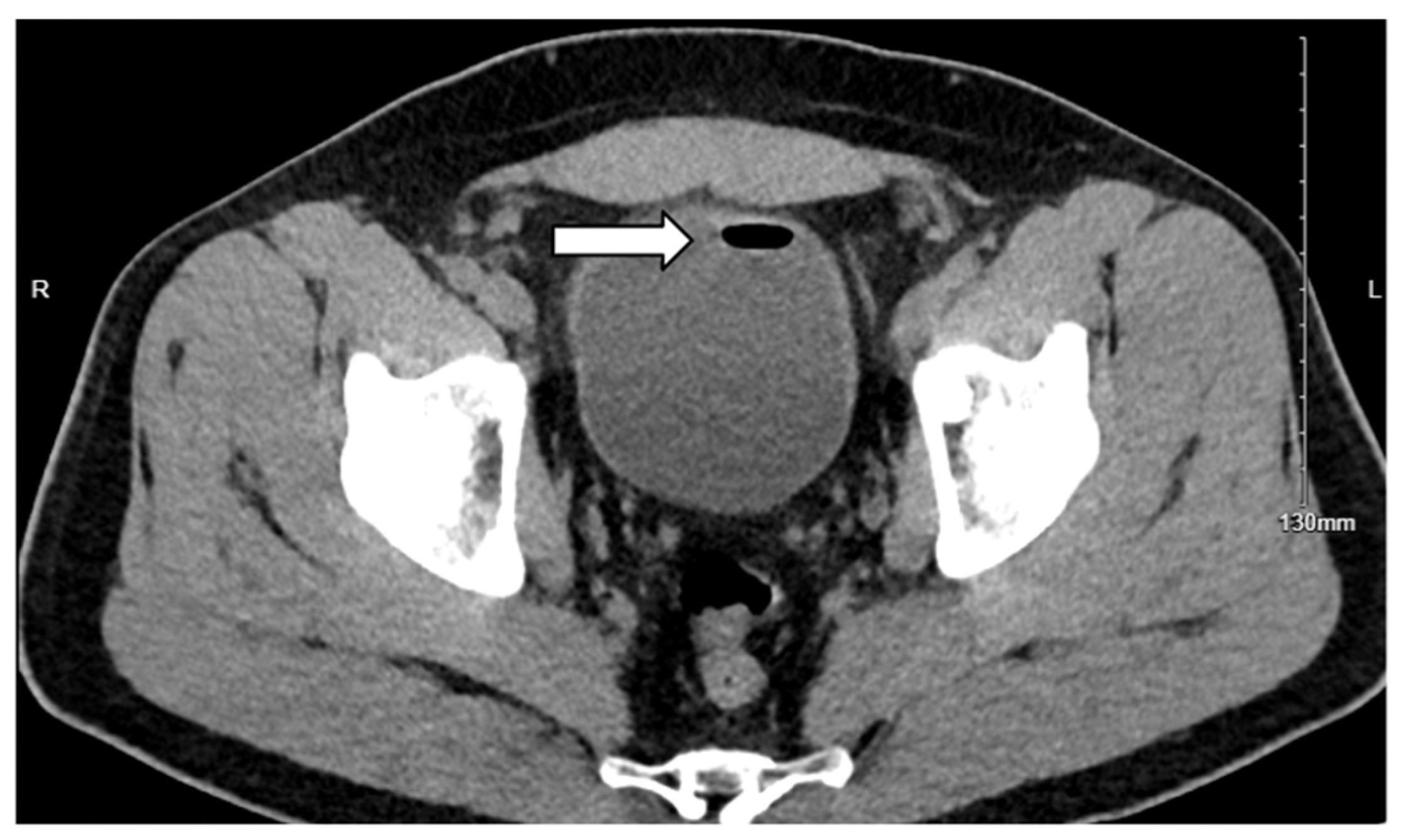
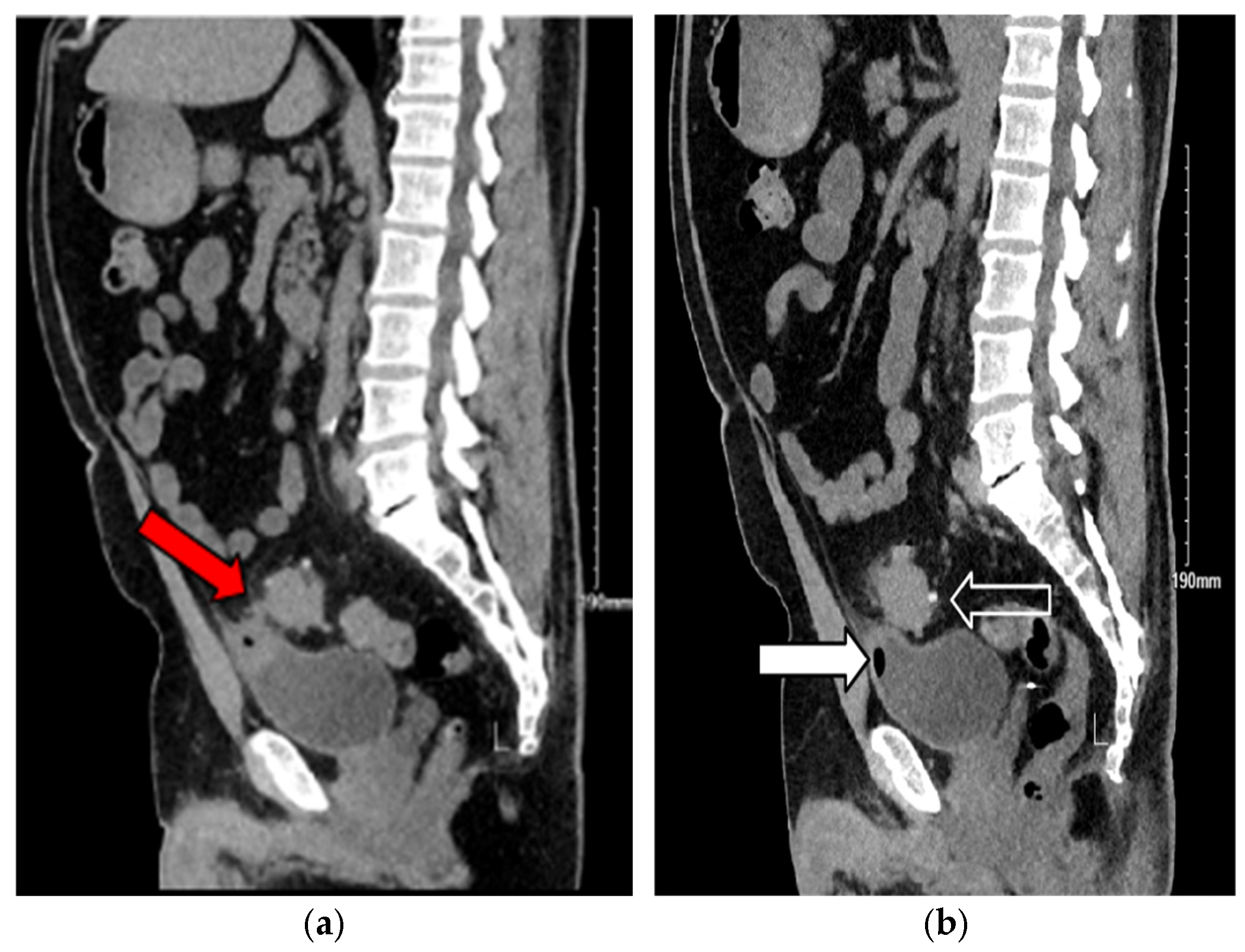
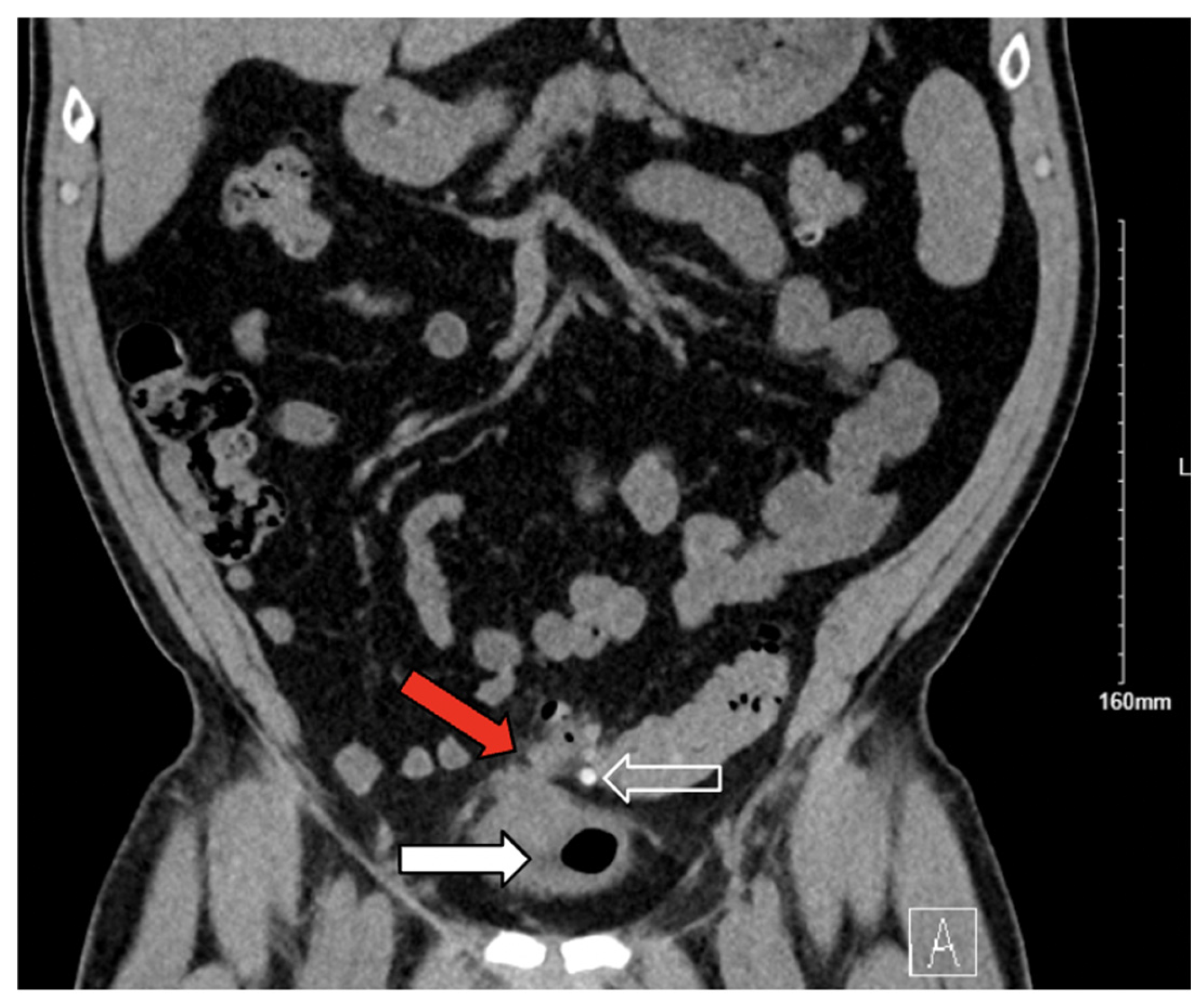
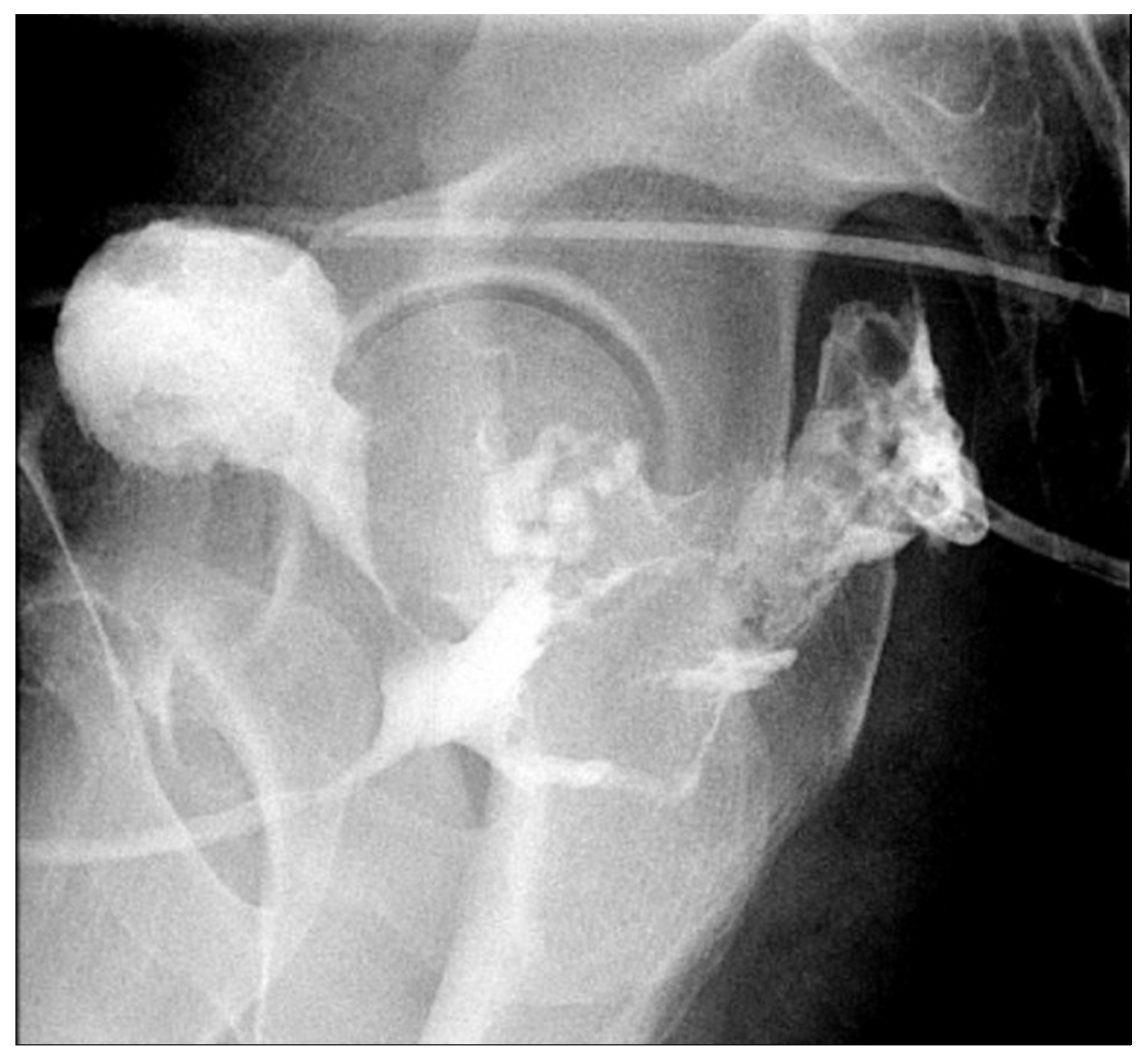
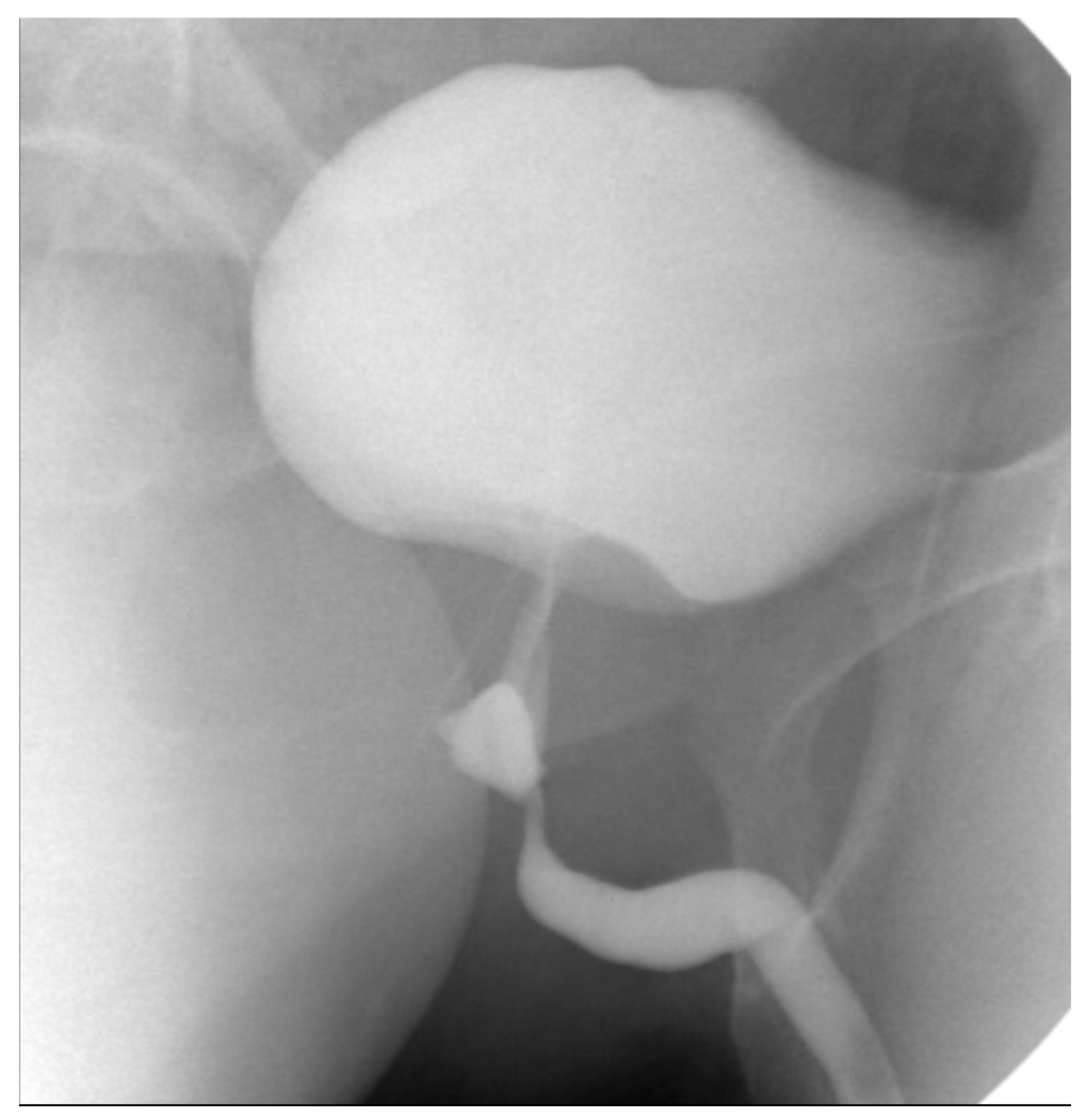
3. Case Presentation
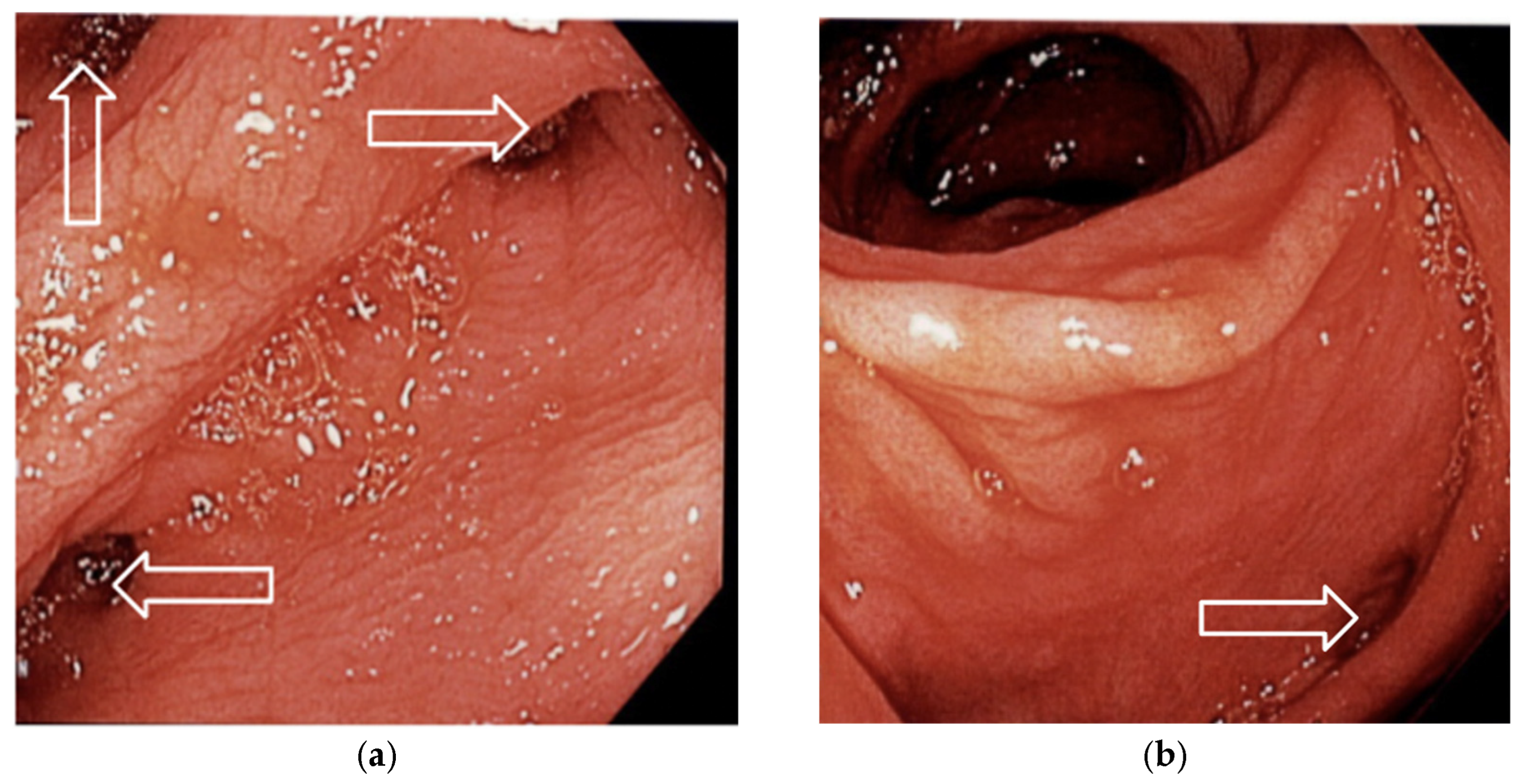
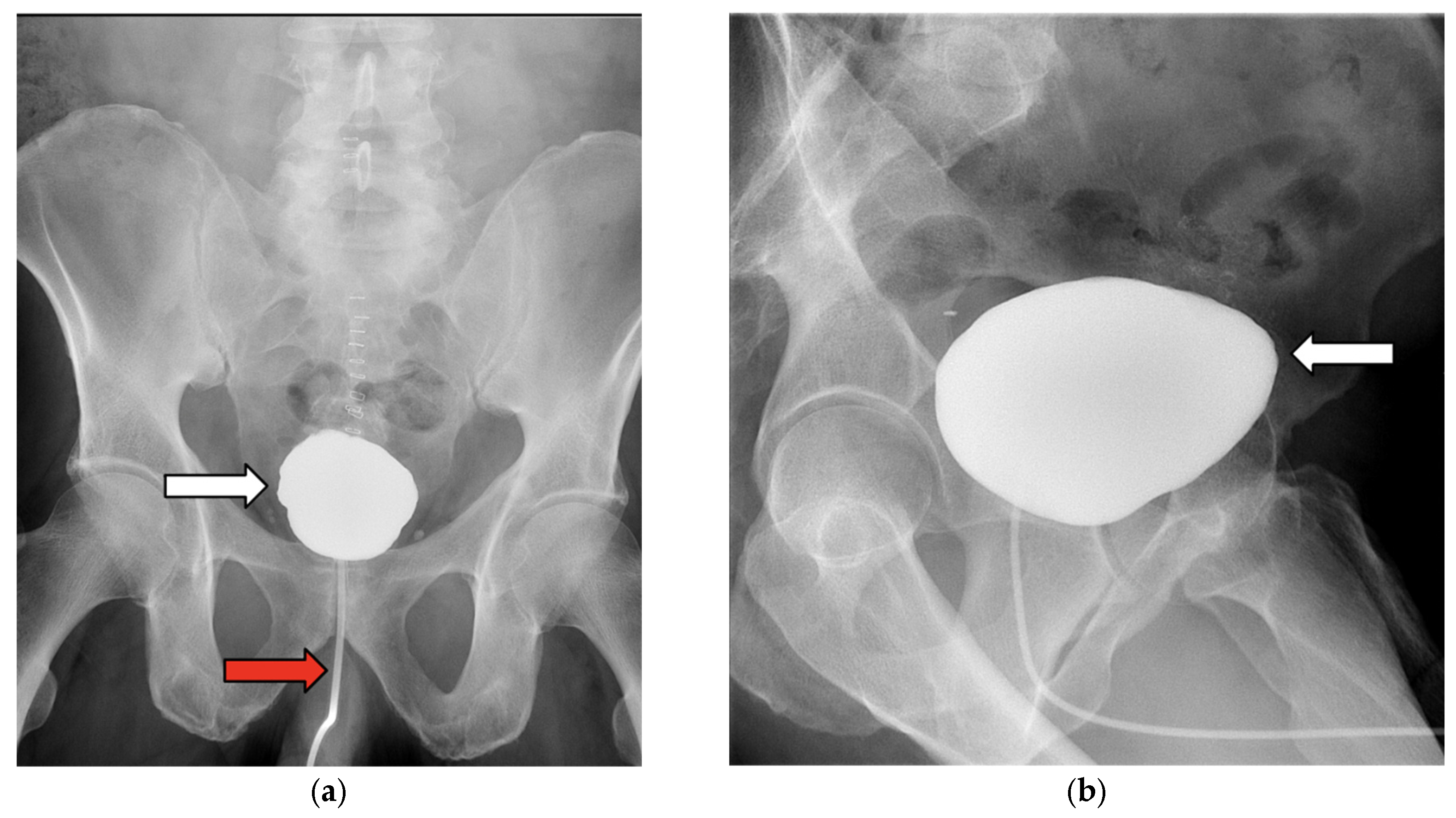
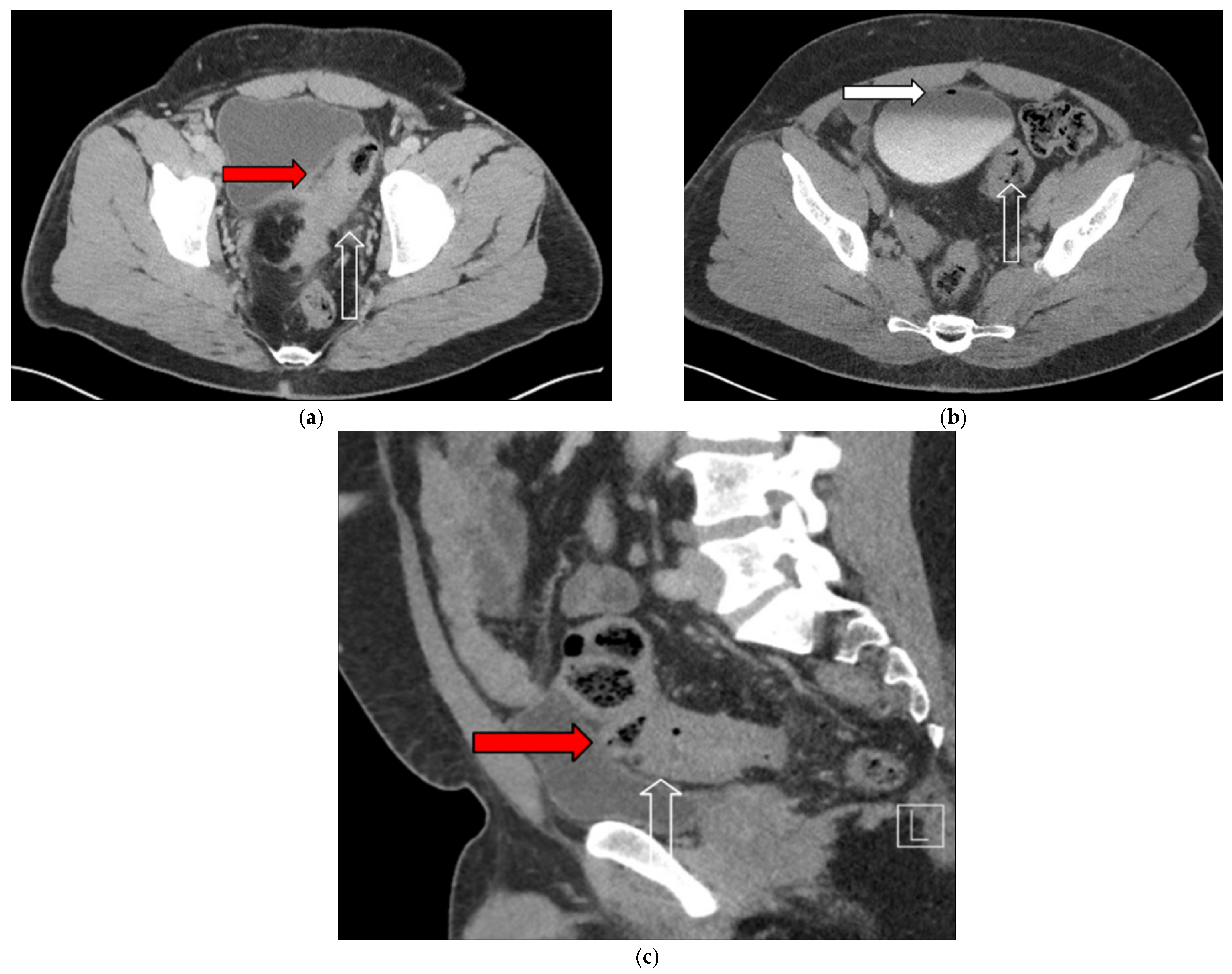
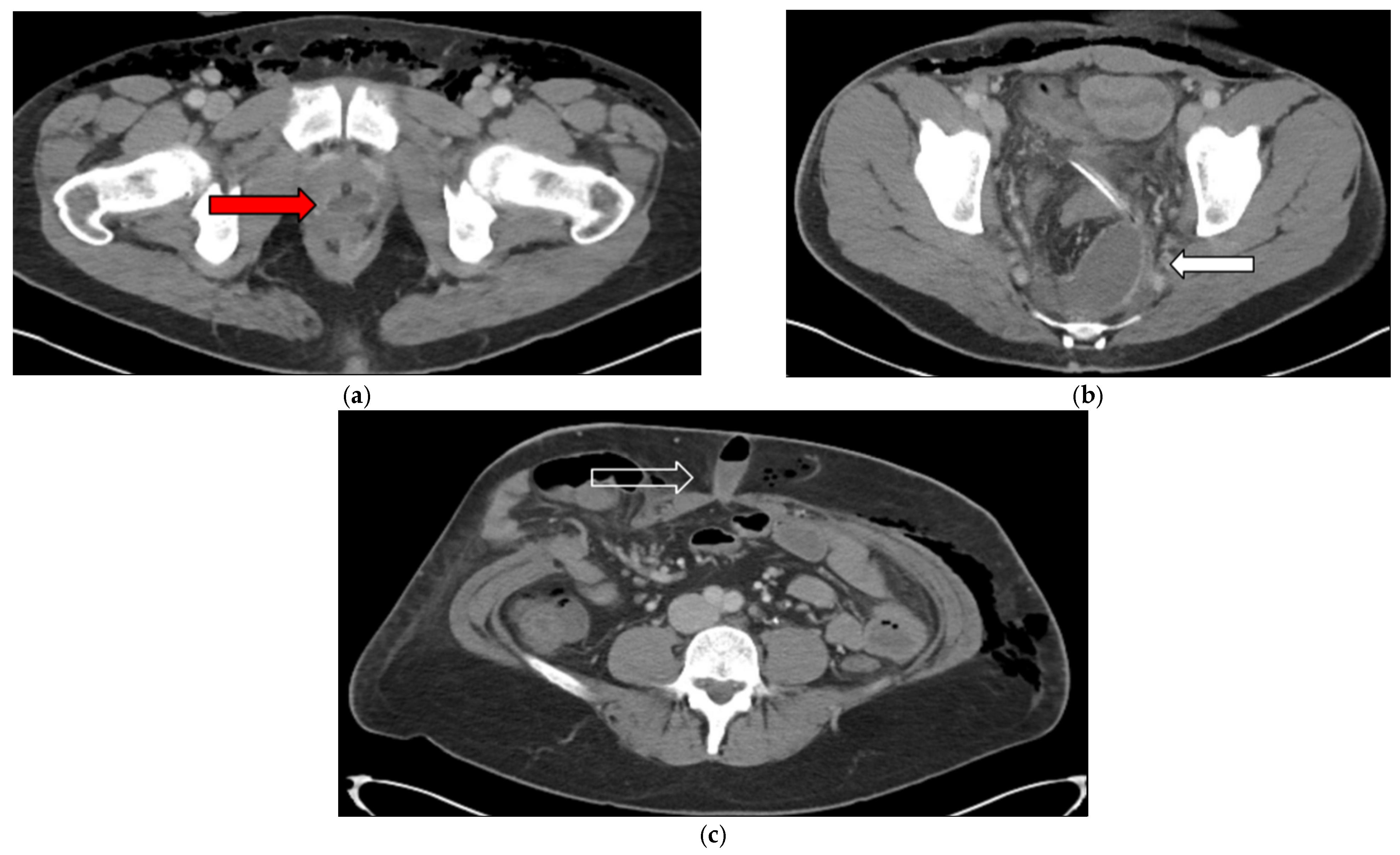
3.1. Case 1
3.2. Case 2
4. Results
4.1. Patient Selection and Matching
4.2. Postoperative Outcomes
4.3. Length of Stay and Hospital Charges
4.4. Hospital Characteristics
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| EVF | Enterovesical fistula |
| HPF | High-power field |
| IRB | Institutional Review Board |
| MIS | Minimally invasive surgery |
| NIS | National Inpatient Sample |
| CI | Confidence Interval |
| OR | Odds ratio |
| POD | Postoperative day |
| RBC | Red blood cell |
| SD | Standard deviation |
| SPSS | Statistical Package for the Social Sciences |
| UTI | Urinary tract infection |
| WBC | White blood cells |
Appendix A
| Predictors of MIS | Open Surgery (n = 1378) | MIS (n = 1387) | Adjusted OR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Age at Admission, years (mean ± SD) | 62.92 ± 14.03 | 63.02 ± 13.66 | 1.003 | 0.996–1.010 | 0.439 |
| Median Age | 65 | 65 | |||
| Admission on Weekend | |||||
| Yes, no. (%) | 55 (4) | 62 (4.5) | 1.178 | 0.792–1.752 | 0.419 |
| No, no. (%) | 1323 (96) | 1316 (95.5) | - | - | |
| Elective Admission | |||||
| Elective, no. (%) | 1089 (79.0) | 1089 (79.0) | 0.969 | 0.787–1.193 | 0.768 |
| Nonelective, no. (%) | 289 (21.0) | 289 (21.0) | - | - | |
| Sex | |||||
| Male, no. (%) | 846 (61.4) | 824 (59.8) | |||
| Female, no. (%) | 532 (38.6) | 554 (40.2) | 1.079 | 0.918–1.268 | 0.357 |
| Race | |||||
| White, no. (%) | 1033 (75.0) | 1045 (75.8) | - | - | |
| Black, no. (%) | 111 (8.1) | 102 (7.4) | 0.882 | 0.656–1.187 | 0.408 |
| Hispanic, no. (%) | 164 (11.9) | 170 (12.3) | 1.025 | 0.800–1.314 | 0.846 |
| Asian/PI, no. (%) | 16 (1.2) | 18 (1.3) | 1.155 | 0.583–2.290 | 0.68 |
| Native American, no. (%) | 12 (0.9) | NR | 0.601 | 0.233–1.548 | 0.292 |
| Other, no. (%) | 42 (3) | 36 (2.6) | 0.815 | 0.514–1.293 | 0.386 |
| Transfer Status | 0.569 | ||||
| Not transferred, no. (%) | 1347 (97.8) | 1354 (98.3) | - | - | |
| From acute care, no. (%) | 15 (1.1) | 13 (0.9) | 0.829 | 0.388–1.774 | 0.629 |
| From another care facility, no. (%) | 16 (1.2) | 11 (0.8) | 0.684 | 0.313–1.493 | 0.34 |
| Primary Insurance | 0.725 | ||||
| Government, no. (%) | 805 (58.4) | 781 (56.7) | - | - | |
| Private, no. (%) | 520 (37.7) | 537 (39.0) | 1.092 | 0.903–1.320 | 0.363 |
| Self-pay, no. (%) | 30 (2.2) | 34 (2.5) | 1.19 | 0.703–2.014 | 0.518 |
| Other, no. (%) | 23 (1.7) | 26 (1.2) | 1.227 | 0.687–2.190 | 0.49 |
| Patient Location | 0.351 | ||||
| Nonmetro, no. (%) | 52 (3.8) | 73(5.3) | - | - | |
| Central counties of metro > 1 M, no. (%) | 396 (28.7) | 399 (29.0) | 1.025 | 0.833–1.260 | 0.817 |
| Fringe counties of metro > 1 M, no. (%) | 380 (27.6) | 397 (28.8) | 0.919 | 0.736–1.147 | 0.457 |
| Metro 250 K–999 K, no. (%) | 307 (22.3) | 290 (21) | 0.894 | 0.665–1.204 | 0.461 |
| Metro < 250 K, no. (%) | 107 (7.8) | 151 (11.0) | 0.971 | 0.668–1.410 | 0.876 |
| Micropolitan, no. (%) | 106 (7.7) | 98 (7.1) | 1.432 | 0.945–2.171 | 0.09 |
| ZIP Code Income Quartile | 0.816 | ||||
| 0–25%—no. (%) | 266 (19.3) | 281 (20.4) | 0.909 | 0.721–1.148 | 0.424 |
| 26–50%—no. (%) | 346 (25.1) | 330 (23.9) | 0.912 | 0.722–1.153 | 0.441 |
| 51–75%—no. (%) | 390 (28.3) | 374 (27.1) | 0.964 | 0.749–1.239 | 0.772 |
| 76–100%—no. (%) | 376 (27.3) | 393 (28.5) | - | - | |
| Hospital Type | 0.887 | ||||
| Urban teaching, no. (%) | 1027 (74.5) | 1032 (74.9) | - | - | |
| Rural, no. (%) | 71 (5.2) | 69 (5.0) | 1.065 | 0.681–1.667 | 0.783 |
| Urban nonteaching, no. (%) | 280 (20.3) | 277 (20.1) | 0.995 | 0.718–1.672 | 0.673 |
| Hospital Ownership | |||||
| Private, no. (%) | 1248 (90.6) | 1248 (90.6) | - | - | |
| Government, no. (%) | 130 (9.4) | 130 (9.4) | 0.986 | 0.758–1.282 | 0.916 |
| Intestinal and Bladder Malignancy | |||||
| No, no. (%) | 1340 (97.2) | 1340 (97.2) | - | - | |
| Yes, no. (%) | 38 (2.8) | 38 (2.8) | 1.094 | 0.683–1.752 | 0.708 |
| Elixhauser Score | |||||
| Mean ± SD (Median) | 4.42 ± 7.61 (1) | 4.05 ± 7.63 (0) | 0.992 | 0.981–1.003 | 0.135 |
Appendix B
| Variable | ICD-10 Codes |
|---|---|
| Enterovesical Fistula | 0DQ80ZZ 0DQ83ZZ 0DQ84ZZ 0DQ87ZZ 0DQ88ZZ 0DQB0ZZ 0DQB3ZZ 0DQB4ZZ 0DQB7ZZ 0DQB8ZZ 0DQE0ZZ 0DQE3ZZ 0DQE4ZZ 0DQE7ZZ 0DQE8ZZ 0DQN0ZZ 0DQN3ZZ 0DQN4ZZ 0DQN7ZZ 0DQN8ZZ 0DQP0ZZ 0DQP3ZZ 0DQP4ZZ 0DQP7ZZ 0DQP8ZZ 0TQB0ZZ 0TQB3ZZ 0TQB4ZZ 0TQB7ZZ 0TQB8ZZ |
References
- Granieri, S.; Sessa, F.; Bonomi, A.; Paleino, S.; Bruno, F.; Chierici, A.; Sciannamea, I.M.; Germini, A.; Campi, R.; Talso, M.; et al. Indications and outcomes of enterovesical and colovesical fistulas: Systematic review of the literature and meta-analysis of prevalence. BMC Surg. 2021, 21, 265. [Google Scholar] [CrossRef] [PubMed]
- Stefanou, C.K.; Gkogkos, S.; Flindris, S.; Paxinos, A.K.; Tsiantis, T.; Oikonomou, P.; Tepelenis, K.; Stefanou, S.K. Colovesical fistula due to sigmoid diverticulitis. Case Rep. Surg. 2023, 2023, 8835222. [Google Scholar] [CrossRef] [PubMed]
- Froiio, C.; Bernardi, D.; Asti, E.; Bonavina, G.; Conti, A.; Carmignani, L.; Bonavina, L. Burden of colovesical fistula and changing treatment pathways: A systematic literature review. Surg. Laparosc. Endosc. Percutan. Tech. 2022, 32, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Oner, M.; Abbas, M.A. Current management of colovesical fistula. Turk. J. Color. Dis. 2022, 32, 229–237. [Google Scholar] [CrossRef]
- Smeenk, R.M.; Plaisier, P.W.; van der Hoeven, J.A.B.; Hesp, W.L.E.M. Outcome of surgery for colovesical and colovaginal fistulas of diverticular origin in 40 patients. J. Gastrointest. Surg. 2012, 16, 1559–1565. [Google Scholar] [CrossRef]
- Sassun, R.; Sileo, A.; Ng, J.C.; Mari, G.; Behm, K.T.; Shawki, S.F.; Larson, D.W. Diverticular disease complicated by colovesical and colovaginal fistulas: Not so complex robotically. Surg. Endosc. 2025, 39, 3941–3946. [Google Scholar] [CrossRef]
- Fletcher, J.G.; Fidler, J.L.; Bruining, D.H.; Huprich, J.E. New concepts in intestinal imaging for inflammatory bowel diseases. Gastroenterology 2011, 140, 1795–1806.e7. [Google Scholar] [CrossRef]
- Jarrett, T.W.; Vaughan, E.D. Accuracy of computerized tomography in the diagnosis of colovesical fistula secondary to diverticular disease. J. Urol. 1995, 153, 44–46. [Google Scholar] [CrossRef]
- Tenny, B.C.; O’Neill, M. Diagnostic Cystoscopy: The Cystoscopist Reference; Springer: Cham, Switzerland, 2022; p. 147. ISBN 978-3-031-10668-2. [Google Scholar]
- Healthcare Cost and Utilization Project (HCUP). NIS Description of Data Elements: HOSP_BEDSIZE; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2025. Available online: https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nisnote.jsp (accessed on 21 July 2025).
- Pallant, J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using IBM SPSS; Taylor & Francis: Hoboken, NJ, USA; McGraw-Hill Education: New York, NY, USA, 2020. [Google Scholar]
- Boedec, K.L. Sensitivity and specificity of normality tests and consequences on reference interval accuracy at small sample size: A computer-simulation study. Vet. Clin. Pathol. 2016, 45, 648–656. [Google Scholar] [CrossRef]
- Williford, E.; Haley, V.; McNutt, L.A.; Lazarium, V. Dealing with highly skewed hospital length of stay distributions: The use of Gamma mixture models to study delivery hospitalizations. PLoS ONE 2020, 15, e0231825. [Google Scholar] [CrossRef]
- Tursi, A.; Scarpignato, C.; Strate, L.L.; Lanas, A.; Kruis, W.; Lahat, A.; Danese, S. Colonic diverticular disease. Nat. Rev. Dis. Primers 2020, 6, 20. [Google Scholar] [CrossRef]
- Underhill, J.; Pinzon, M.C.M.; Ritz, E.; Grunvald, M.; Jochum, S.; Becerra, A.; Bhama, A.; Govekar, H.; Saclarides, T.; Hayden, D. Defining diverticular fistula through inpatient admissions: A population study. Surg. Endosc. 2023, 37, 645–652. [Google Scholar] [CrossRef] [PubMed]
- El-Haddad, H.M.; Kassem, M.I.; Sabry, A.A.; Abouelfotouh, A. Surgical protocol and outcome for sigmoidovesical fistula secondary to diverticular disease of the left colon: A retrospective cohort study. Int. J. Surg. 2018, 56, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Garcea, G.; Majid, I.; Sutton, C.D.; Pattenden, C.J.; Thomas, W.M. Diagnosis and management of colovesical fistulae; six-year experience of 90 consecutive cases. Color. Dis. 2006, 8, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Golabek, T.; Szymanska, A.; Szopinski, T.; Bukowczan, J.; Furmanek, M.; Powroznik, J.; Chlosta, P. Enterovesical fistulae: Aetiology, imaging, and management. Gastroenterol. Res. Pract. 2013, 2013, 617967. [Google Scholar] [CrossRef]
- Melchior, S.; Cudovic, D.; Jones, J.; Thomas, C.; Gillitzer, R.; Thüroff, J. Diagnosis and surgical management of colovesical fistulas due to sigmoid diverticulitis. J. Urol. 2009, 182, 978–982. [Google Scholar] [CrossRef]
- Kovalcik, P.J.; Veidenheimer, M.C.; Corman, M.L. Colovesical fistula. Dis. Colon. Rectum 1976, 19, 425–427. [Google Scholar] [CrossRef]
- Xu, R.; Vaughan, A.; Fagan, M.; Schumacher, D.P.; Wekullo, V.; Gehrke, B. Colovesical fistula in men with chronic urinary tract infection: A diagnostic challenge. Cleve. Clin. J. Med. 2023, 90, 165–171. [Google Scholar] [CrossRef]
- Brown, R.F.; Lopez, K.; Smith, C.B.; Charles, A. Diverticulitis: A review. JAMA 2025. [Google Scholar] [CrossRef]
- Najjar, S.F.; Jamal, M.K.; Savas, J.F.; Miller, T.A. The spectrum of colovesical fistula and diagnostic paradigm. Am. J. Surg. 2004, 188, 617–621. [Google Scholar] [CrossRef]
- Kuhlman, J.E.; Fishman, E.K. CT evaluation of enterovaginal and vesicovaginal fistulas. J. Comput. Assist. Tomogr. 1990, 14, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Holroyd, D.J.; Banerjee, S.; Beavan, M.; Prentice, R.; Vijay, V.; Warren, S.J. Colovaginal and colovesical fistulae: The diagnostic paradigm. Tech. Coloproctol. 2012, 16, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Scozzari, G.; Arezzo, A.; Morino, M. Enterovesical fistulas: Diagnosis and management. Tech. Coloproctol. 2010, 14, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.O.; Armenakas, N.A.; Scharf, S.C.; Panagopoulos, G.; Fracchia, J.A. The poppy seed test for colovesical fistula: Big bang, little bucks! J. Urol. 2008, 179, 1425–1427. [Google Scholar] [CrossRef] [PubMed]
- Movassaghi, M.; Moran, G.; Chung, R.; Sugahara, K.N.; McKiernan, J.; Healy, K.A. The poppy seed test for enterovesical fistula. ACS Case Rev. Surg. 2021, 3, 53–57. [Google Scholar]
- Aydinli, H.H.; Benlice, C.; Ozuner, G.; Gorgun, E.; Abbas, M.A. Risk factors associated with postoperative morbidity in over 500 colovesical fistula patients undergoing colorectal surgery: A retrospective cohort study from ACS-NSQIP database. Int. J. Color. Dis. 2017, 32, 469–474. [Google Scholar] [CrossRef]
- Pogorelić, Z.; Stričević, L.; Elezović Baloević, S.; Todorić, J.; Budimir, D. Safety and effectiveness of triclosan-coated polydioxanone (PDS Plus) versus uncoated polydioxanone (PDS II) sutures for prevention of surgical site infection after hypospadias repair in children: A 10-year single center experience with 550 hypospadias. Biomedicines 2024, 12, 583. [Google Scholar] [CrossRef]
- Keady, C.; Hechtl, D.; Joyce, M. When the bowel meets the bladder: Optimal management of colorectal pathology with urological involvement. World J. Gastrointest. Surg. 2020, 12, 208–225. [Google Scholar] [CrossRef]
- Radwan, R.; Saeed, Z.M.; Phull, J.S.; Williams, G.L.; Carter, A.C.; Stephenson, B.M. How safe is it to manage diverticular colovesical fistulation non-operatively? Color. Dis. 2013, 15, 448–450. [Google Scholar] [CrossRef]
- Solkar, M.H.; Forshaw, M.J.; Sankararajah, D.; Stewart, M.; Parker, M.C. Colovesical fistula–Is a surgical approach always justified? Color. Dis. 2005, 7, 467–471. [Google Scholar] [CrossRef]
- DeLong, C.G.; Scow, J.S.; Morrell, D.J.; Knoedler, J.J.; Alli, V.V.; Winder, J.S.; Pauli, E.M. Endoscopic management of colovesical and colovaginal fistulas with over-the-scope clips: A single-institution case series. Color. Dis. 2022, 24, 314–321. [Google Scholar] [CrossRef]
- Steiner, C.A.; Elixhauser, A.; Schnaier, J. The healthcare cost and utilization project: An overview. Eff. Clin. Pract. 2002, 5, 143–151. [Google Scholar] [PubMed]
- Khera, R.; Angraal, S.; Couch, T.; Welsch, J.W.; Nallamothu, B.K.; Girotra, S.; Chan, P.S.; Krumholz, H.M. Adherence to methodological standards in research using the National Inpatient Sample. JAMA 2017, 318, 2011–2018. [Google Scholar] [CrossRef]
- Gilshtein, H.; Yellinek, S.; Maenza, J.; Wexner, S.D. Surgical management of colovesical fistulas. Tech. Coloproctol. 2020, 24, 851–854. [Google Scholar] [CrossRef]
- Maciel, V.; Lujan, H.J.; Plasencia, G.; Zeichen, M.; Mata, W.; Jorge, I.; Lee, D.; Viamonte III, M.; Hartmann, R.F. Diverticular disease complicated with colovesical fistula: Laparoscopic versus robotic management. Int. Surg. 2014, 99, 203–210. [Google Scholar] [CrossRef]
- Martinolich, J.; Croasdale, D.R.; Bhakta, A.S.; Ata, A.; Chismark, A.D.; Valerian, B.T.; Canete, J.J.; Lee, E.C. Laparoscopic surgery for diverticular fistulas: Outcomes of 111 consecutive cases at a single institution. J. Gastrointest. Surg. 2019, 23, 1015–1021. [Google Scholar] [CrossRef]
- Menenakos, E.; Hahnloser, D.; Nassiopoulos, K.; Chanson, C.; Sinclair, V.; Petropoulos, P. Laparoscopic surgery for fistulas that complicate diverticular disease. Langenbecks Arch. Surg. 2003, 388, 189–193. [Google Scholar] [CrossRef]
- Liau, M.Y.Q.; Toh, E.Q.; Muhamed, S.; Selvakumar, S.V.; Shelat, V.G. Can propensity score matching replace randomized controlled trials? World J. Methodol. 2024, 14, 90590. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.M.; Rigby, E.; Mamidanna, R.; Bottle, A.; Aylin, P.; Ziprin, P.; Faiz, O.D. Systematic review of discharge coding accuracy. J. Public Health 2012, 34, 138–148. [Google Scholar] [CrossRef]
- Pan, J.; Lee, S.; Cheligeer, C.; Li, B.; Wu, G.; Eastwood, C.A.; Xu, Y.; Quan, H. Assessing the validity of ICD-10 administrative data in coding comorbidities. BMJ Health Care Inform. 2025, 32, e101381. [Google Scholar] [CrossRef]
- Jolley, R.J.; Sawka, K.J.; Yergens, D.W.; Quan, H.; Jetté, N.; Doig, C.J. Validity of administrative data in recording sepsis: A systematic review. Crit. Care 2015, 19, 139. [Google Scholar] [CrossRef] [PubMed]
| Postoperative Outcomes | Open Surgery (n = 1378) | MIS (n = 1378) | Adjusted OR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Hospital mortality, no. (%) | NR | NR | 1.087 | 0.164–7.207 | 0.931 |
| Wound complications, no. (%) | 19 (1.4) | 14 (1.0) | 0.799 | 0.380–1.680 | 0.554 |
| Postoperative pneumonia, no. (%) | 19 (1.4) | 21 (1.5) | 1.249 | 0.627–2.487 | 0.528 |
| Postoperative sepsis/shock, no. (%) | 98 (7.1) | 77 (5.6) | 0.74 | 0.509–1.075 | 0.114 |
| Postoperative hemorrhagic shock, no. (%) | 14 (1.0) | 15 (1.1) | 1.065 | 0.500–2.268 | 0.87 |
| Length of stay, mean (SD), days | 7.49 (7.3) | 6.89 (6.9) | 1.11 | 1.06–1.15 | <0.001 * |
| Total hospital charges, mean (USD) | 129,102 | 124,672 | 0.995 | 0.95–1.04 | 0.82 |
| Hospital bed size, no. (%) | 0.026 | ||||
| Large | 681 (49.4) | 657 (47.7) | - | - | |
| Medium | 398 (28.9) | 461 (33.5) | 1.108 | 0.906–1.357 | 0.318 |
| Small | 299 (21.7) | 260 (18.9) | 1.325 | 1.068–1.644 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkert, A.; Nigam, A.; Stover, D.; Meshram, P.; Naaz, R.; Onongaya, C.; Nguyen, S.H.-T.; Sauve, J.; Gaertner, W.; Harmon Jr., J.V. Comparison of Open Versus Minimally Invasive Repair of Colovesical Fistula: A Case Report and Propensity-Matched National Database Analysis. J. Clin. Med. 2025, 14, 6065. https://doi.org/10.3390/jcm14176065
Volkert A, Nigam A, Stover D, Meshram P, Naaz R, Onongaya C, Nguyen SH-T, Sauve J, Gaertner W, Harmon Jr. JV. Comparison of Open Versus Minimally Invasive Repair of Colovesical Fistula: A Case Report and Propensity-Matched National Database Analysis. Journal of Clinical Medicine. 2025; 14(17):6065. https://doi.org/10.3390/jcm14176065
Chicago/Turabian StyleVolkert, Alexis, Anmol Nigam, David Stover, Pravin Meshram, Rubeena Naaz, Chidiebere Onongaya, Sean Huu-Tien Nguyen, Jordan Sauve, Wolfgang Gaertner, and James V. Harmon Jr. 2025. "Comparison of Open Versus Minimally Invasive Repair of Colovesical Fistula: A Case Report and Propensity-Matched National Database Analysis" Journal of Clinical Medicine 14, no. 17: 6065. https://doi.org/10.3390/jcm14176065
APA StyleVolkert, A., Nigam, A., Stover, D., Meshram, P., Naaz, R., Onongaya, C., Nguyen, S. H.-T., Sauve, J., Gaertner, W., & Harmon Jr., J. V. (2025). Comparison of Open Versus Minimally Invasive Repair of Colovesical Fistula: A Case Report and Propensity-Matched National Database Analysis. Journal of Clinical Medicine, 14(17), 6065. https://doi.org/10.3390/jcm14176065






