Treatment Landscape of Metabolic-Dysfunction-Associated Steatotic Liver Disease
Abstract
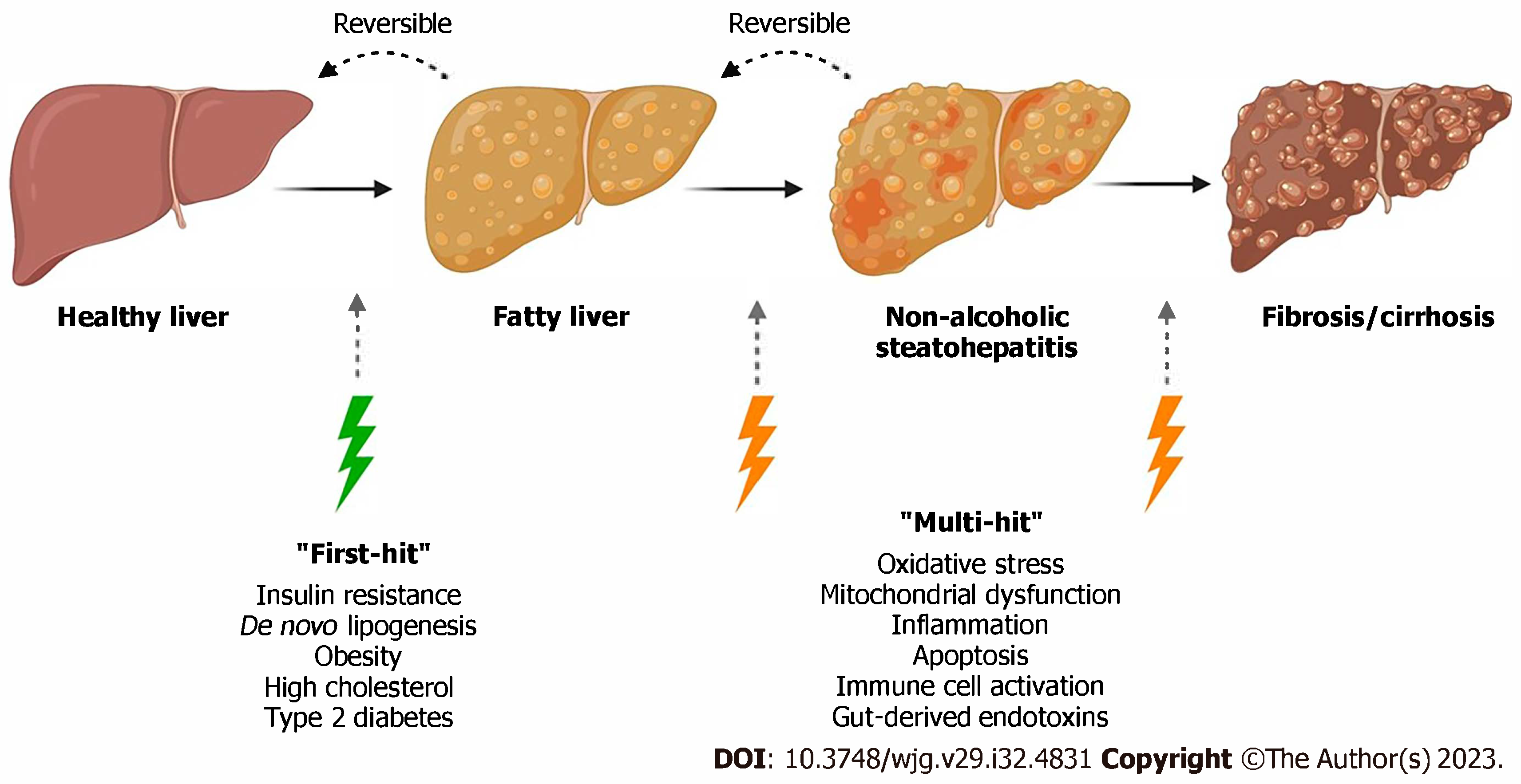
1. Introduction
2. Materials and Methods
3. Results
3.1. Background
3.2. Benefits of Lifestyle Modifications
3.3. Diabetic Drugs Targeting MASH
3.3.1. Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RA)
3.3.2. Pioglitazone
3.3.3. Sodium-Glucose Co-Transporter-2 Inhibitors (SGLT-2 Inhibitors)
3.3.4. Vitamin E
4. Novel Therapeutics for MASLD
4.1. Resmetirom
4.2. Lanifibranor
4.3. Fibroblast Growth Factor (FGF) Analogs
5. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Le, P.; Tatar, M.; Dasarathy, S.; Alkhouri, N.; Herman, W.H.; Taksler, G.B.; Deshpande, A.; Ye, W.; Adekunle, O.A.; McCullough, A.; et al. Estimated Burden of Metabolic Dysfunction–Associated Steatotic Liver Disease in US Adults, 2020 to 2050. JAMA Netw. Open. 2025, 8, e2454707. [Google Scholar] [CrossRef]
- Abdelhameed, F.; Mustafa, A.; Kite, C.; Lagojda, L.; Dallaway, A.; Than, N.N.; Kassi, E.; Kyrou, I.; Randeva, H.S. Gut Microbiota and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Emerging Pathogenic Mechanisms and Therapeutic Implications. Livers 2025, 5, 11. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 2023, 79, 1179–1191. [Google Scholar] [CrossRef]
- Kudaravalli, P.; John, S. Nonalcoholic Fatty Liver. In StatPearls [Internet]; [Updated 7 April 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541033/ (accessed on 21 May 2025).
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Petagine, L.; Zariwala, M.G.; Patel, V.B. Non-alcoholic fatty liver disease: Immunological mechanisms and current treatments. World J. Gastroenterol. 2023, 29, 4831–4850. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Rosina, F.; Gambino, R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia 2012, 55, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Long, M.T.; Corey, K.E.; Sallis, R.E.; Allen, A.M.; Armstrong, M.J.; Conroy, D.E.; Cuthbertson, D.J.; Duarte-Rojo, A.; Hallsworth, K.; et al. American College of Sports Medicine (ACSM) International Multidisciplinary Roundtable report on physical activity and nonalcoholic fatty liver disease. Hepatol. Commun. 2023, 7, e0108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verrastro, O.; Panunzi, S.; Gissey-Castragneto, L.; de Gaetano, A.; Lembo, E.; Capristo, E.; Guidone, C.; Angelini, G.; Pennestrì, F.; Sessa, L.; et al. Bariatric-metabolic surgery vs. lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): A multi-centre, open-label, randomized trial. Lancet 2023, 401, 1786–1797. [Google Scholar] [CrossRef]
- Krishnan, A.; Hadi, Y.; Alqahtani, S.A.; Woreta, T.A.; Fang, W.; Abunnaja, S.; Szoka, N.; Tabone, L.E.; Thakkar, S.; Singh, S. Cardiovascular Outcomes and Mortality After Bariatric Surgery in Patients With Nonalcoholic Fatty Liver Disease and Obesity. JAMA Netw. Open. 2023, 6, e237188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koullias, E.; Papavdi, M.; Koskinas, J.; Deutsch, M.; Thanopoulou, A. Targeting Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Available and Future Pharmaceutical Options. Cureus 2025, 17, e76716. [Google Scholar] [CrossRef] [PubMed]
- Boer, G.A.; Hay, D.L.; Tups, A. Obesity pharmacotherapy: Incretin action in the central nervous system. Trends Pharmacol. Sci. 2023, 44, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Newsome, P.N.; Kliers, I.; Østergaard, L.H.; Long, M.T.; Kjær, M.S.; Cali, A.M.; Bugianesi, E.; Rinella, M.E.; Roden, M.; et al. Phase 3 Trial of Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis. N. Engl. J. Med. 2025, 392, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Aronne, L.J.; Horn, D.B.; le Roux, C.W.; Ho, W.; Falcon, B.L.; Valderas, E.G.; Das, S.; Lee, C.J.; Glass, L.C.; Senyucel, C.; et al. SURMOUNT-5 Trial Investigators. Tirzepatide as Compared with Semaglutide for the Treatment of Obesity. N. Engl. J. Med. 2025; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Nahra, R.; Wang, T.; Gadde, K.M.; Oscarsson, J.; Stumvoll, M.; Jermutus, L.; Hirshberg, B.; Ambery, P. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults With Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care. 2021, 44, 1433–1442, Erratum in Diabetes Care 2022, 45, 3112. https://doi.org/10.2337/dc22-er12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boehringer Ingelheim. Boehringer Receives U.S. FDA Breakthrough Therapy Designation and Initiates Two Phase III Trials in MASH for Survodutide. Available online: https://www.boehringer-ingelheim.com/human-health/metabolic-diseases/survodutide-us-fda-breakthrough-therapy-phase-3-trials-mash (accessed on 3 July 2025).
- Sanyal, A.J.; Bedossa, P.; Fraessdorf, M.; Neff, G.W.; Lawitz, E.; Bugianesi, E.; Anstee, Q.M.; Hussain, S.A.; Newsome, P.N.; Ratziu, V.; et al. 1404-0043 Trial Investigators. A Phase 2 Randomized Trial of Survodutide in MASH and Fibrosis. N. Engl. J. Med. 2024, 391, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sohal, A.; Batta, A. GLP-1, GIP/GLP-1, and GCGR/GLP-1 receptor agonists: Novel therapeutic agents for metabolic dysfunction-associated steatohepatitis. World J. Gastroenterol. 2024, 30, 5205–5211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aldhaleei, W.A.; Abegaz, T.M.; Bhagavathula, A.S. Glucagon-like Peptide-1 Receptor Agonists Associated Gastrointestinal Adverse Events: A Cross-Sectional Analysis of the National Institutes of Health All of Us Cohort. Pharmaceuticals 2024, 17, 199. [Google Scholar] [CrossRef]
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chopra, S.; Lai, M. Management of Metabolic Dysfunction Associated Steatotic Liver Disease (Nonalcoholic Fatty Liver Disease) in Adults. UpToDate. Updated 9 February 2025. Available online: https://www.uptodate.com/contents/management-of-metabolic-dysfunction-associated-steatotic-liver-disease-nonalcoholic-fatty-liver-disease-in-adults? (accessed on 3 July 2025).
- Belfort, R.; Harrison, S.A.; Brown, K.; Darland, C.; Finch, J.; Hardies, J.; Balas, B.; Gastaldelli, A.; Tio, F.; Pulcini, J.; et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 2006, 355, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulou, E.; Thymis, J.; Lampsas, S.; Pavlidis, G.; Katogiannis, K.; Vlachomitros, D.; Katsanaki, E.; Kostelli, G.; Pililis, S.; Pliouta, L.; et al. The Triad of Risk: Linking MASLD, Cardiovascular Disease and Type 2 Diabetes; From Pathophysiology to Treatment. J. Clin. Med. 2025, 14, 428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ong Lopez, A.M.C.; Pajimna, J.A.T. Efficacy of sodium glucose cotransporter 2 inhibitors on hepatic fibrosis and steatosis in non-alcoholic fatty liver disease: An updated systematic review and meta-analysis. Sci. Rep. 2024, 14, 2122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perumpail, B.J.; Li, A.A.; John, N.; Sallam, S.; Shah, N.D.; Kwong, W.; Cholankeril, G.; Kim, D.; Ahmed, A. The Role of Vitamin E in the Treatment of NAFLD. Diseases 2018, 6, 86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, Y.; Ni, W.; Zheng, M.; Sheng, H.; Wang, J.; Xie, S.; Yang, Y.; Chi, X.; Chen, J.; He, F.; et al. Chinese NAFLD Clinical Research Network (CNAFLD CRN). Vitamin E (300 mg) in the treatment of MASH: A multi-center, randomized, double-blind, placebo-controlled study. Cell Rep. Med. 2025, 6, 101939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abera, M.; Suresh, S.B.; Malireddi, A.; Boddeti, S.; Noor, K.; Ansar, M.; Malasevskaia, I. Vitamin E and Non-alcoholic Fatty Liver Disease: Investigating the Evidence Through a Systematic Review. Cureus 2024, 16, e72596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harrison, S.A.; Taub, R.; Neff, G.W.; Lucas, K.J.; Labriola, D.; Moussa, S.E.; Alkhouri, N.; Bashir, M.R. Resmetirom for nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2023, 29, 2919–2928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inventiva. Inventiva Receives FDA Breakthrough Therapy Designation for Lead Drug Candidate Lanifibranor in NASH. Sofinnova Partners. 12 October 2020. Available online: https://sofinnovapartners.com/news/inventiva-receives-fda-breakthrough-therapy-designation-for-lead-drug-candidate-lanifibranor-in-nash?utm_source=chatgpt.com (accessed on 3 July 2025).
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Inventiva. Inventiva Announces Completion of Enrollment in the Phase 3 NATiV3 Clinical Trial of Lanifibranor in Patients with MASH and Advanced Fibrosis; BioSpace (Daix, France): 2025. Available online: https://www.biospace.com/press-releases/inventiva-announces-completion-of-enrollment-in-the-phase-3-nativ3-clinical-trial-of-lanifibranor-in-patients-with-mash-and-advanced-fibrosis (accessed on 3 July 2025).
- Ocker, M. Fibroblast growth factor signaling in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: Paving the way to hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 279–290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdelmalek, M.F.; Sanyal, A.J.; Nakajima, A.; Neuschwander-Tetri, B.A.; Goodman, Z.D.; Lawitz, E.J.; Harrison, S.A.; Jacobson, I.M.; Imajo, K.; Gunn, N.; et al. Pegbelfermin in Patients With Nonalcoholic Steatohepatitis and Compensated Cirrhosis (FALCON 2): A Randomized Phase 2b Study. Clin. Gastroenterol. Hepatol. 2024, 22, 113–123.e9. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Rinella, M.E.; Chalasani, N.P.; Neff, G.W.; Lucas, K.J.; Rodriguez, M.E.; Rudraraju, M.; Patil, R.; Behling, C.; Burch, M.; et al. Efruxifermin in Compensated Liver Cirrhosis Caused by MASH. N. Engl. J. Med. 2025; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Akero Therapeutics. Clinical Trials—Efruxifermin for MASH. Akero Therapeutics Website. 2025. Available online: https://akerotx.com/clinical-trials/ (accessed on 22 June 2025).
- Akero Therapeutics. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety and Efficacy of Efruxifermin in Subjects with Non-Cirrhotic NASH/MASH and fibrosis (ClinicalTrials.gov Identifier: NCT06215716). 2025. Available online: https://clinicaltrials.gov (accessed on 22 June 2025).
- Marey, M.M.; Belal, M.; Awad, A.A.; Rabea, E.M.; Hassan, M.A.; Abbas, A.W.; Nashwan, A.J. Efficacy and safety of aldafermin in non-alcoholic steatohepatitis: A systematic review and meta-analysis of randomized controlled trials. Clin. Res. Hepatol. Gastroenterol. 2024, 48, 102357. [Google Scholar] [CrossRef] [PubMed]
| Drug Class | Liver Fibrosis | Hepatic Steatosis | Body Weight | Cardiorenal Effects | MASH Resolution |
|---|---|---|---|---|---|
| GLP-1 Receptor Agonists (e.g., Semaglutide, Tirzepatide, Cotadutide) | Strong improvement in fibrosis markers and histology | Strong reduction in liver fat content | Consistent and clinically meaningful weight loss | Significant cardiovascular and potential renal benefits | High rates of steatohepatitis resolution in trials |
| SGLT-2 Inhibitors (e.g., Empagliflozin, Dapagliflozin) | Modest improvement (evidence low-to-moderate certainty) | Mild reduction in steatosis | Moderate weight loss | Strong and well-established cardiorenal protection | Limited evidence for MASH resolution |
| DPP-IV Inhibitors (e.g., Sitagliptin, Saxagliptin) | No significant effect | No meaningful impact | Weight-neutral | Generally safe with modest metabolic benefit | No evidence for MASH resolution |
| Pioglitazone | Moderate improvement in fibrosis | Moderate reduction in steatosis | Often associated with weight gain | Some cardiorenal benefit but risk of heart failure | Moderate rates of MASH resolution |
| Metformin | No proven direct effect | Mild indirect improvement via insulin sensitization | Weight-neutral to mild loss | No established cardiorenal benefit | No clear evidence for MASH resolution |
| Lanifibranor | Moderate to strong improvement in fibrosis (dose-dependent) | Moderate to strong reduction in steatosis and SAF score | Mild weight loss or neutral effect | Favorable lipid and inflammation profile | Moderate to strong resolution rates without worsening fibrosis in higher-dose arm |
| FGF Analogs (e.g., Efruxifermin, Aldafermin) | Moderate improvement in fibrosis (stronger in some FGF19 analog studies) | Moderate to strong liver fat reduction on imaging | Mild weight loss or neutral | Potential metabolic benefits; limited direct CV outcome data | Moderate resolution rates, variable by agent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, P. Treatment Landscape of Metabolic-Dysfunction-Associated Steatotic Liver Disease. J. Clin. Med. 2025, 14, 6060. https://doi.org/10.3390/jcm14176060
Patel P. Treatment Landscape of Metabolic-Dysfunction-Associated Steatotic Liver Disease. Journal of Clinical Medicine. 2025; 14(17):6060. https://doi.org/10.3390/jcm14176060
Chicago/Turabian StylePatel, Pranav. 2025. "Treatment Landscape of Metabolic-Dysfunction-Associated Steatotic Liver Disease" Journal of Clinical Medicine 14, no. 17: 6060. https://doi.org/10.3390/jcm14176060
APA StylePatel, P. (2025). Treatment Landscape of Metabolic-Dysfunction-Associated Steatotic Liver Disease. Journal of Clinical Medicine, 14(17), 6060. https://doi.org/10.3390/jcm14176060







