The Lymphocyte-to-Monocyte Ratio (LMR) as a Novel Biomarker for Cervical Cancer Risk Stratification in Conization Patients
Abstract
1. Introduction
2. Materials and Methods
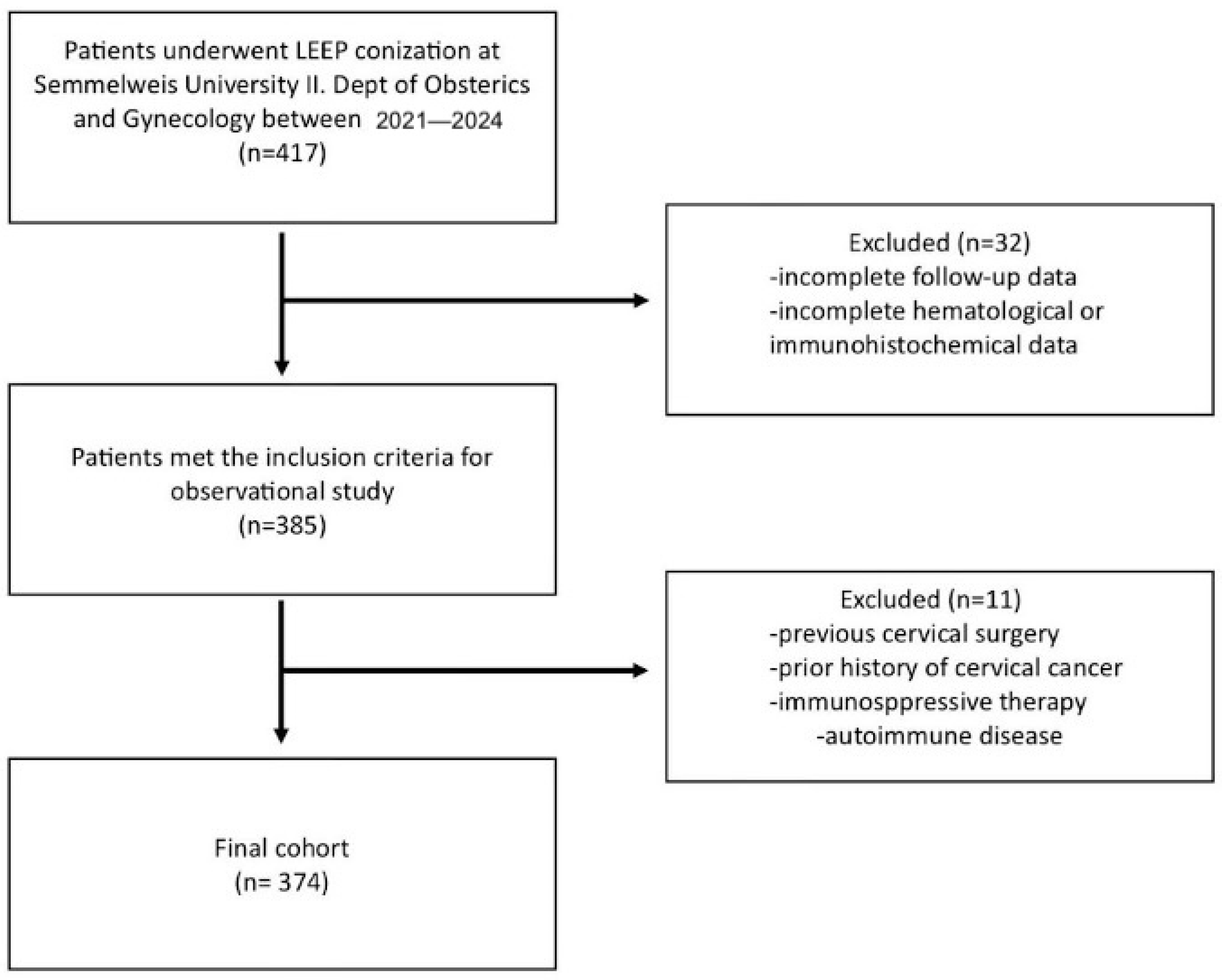
2.1. Patients
2.2. Characteristics
2.3. Data Management
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
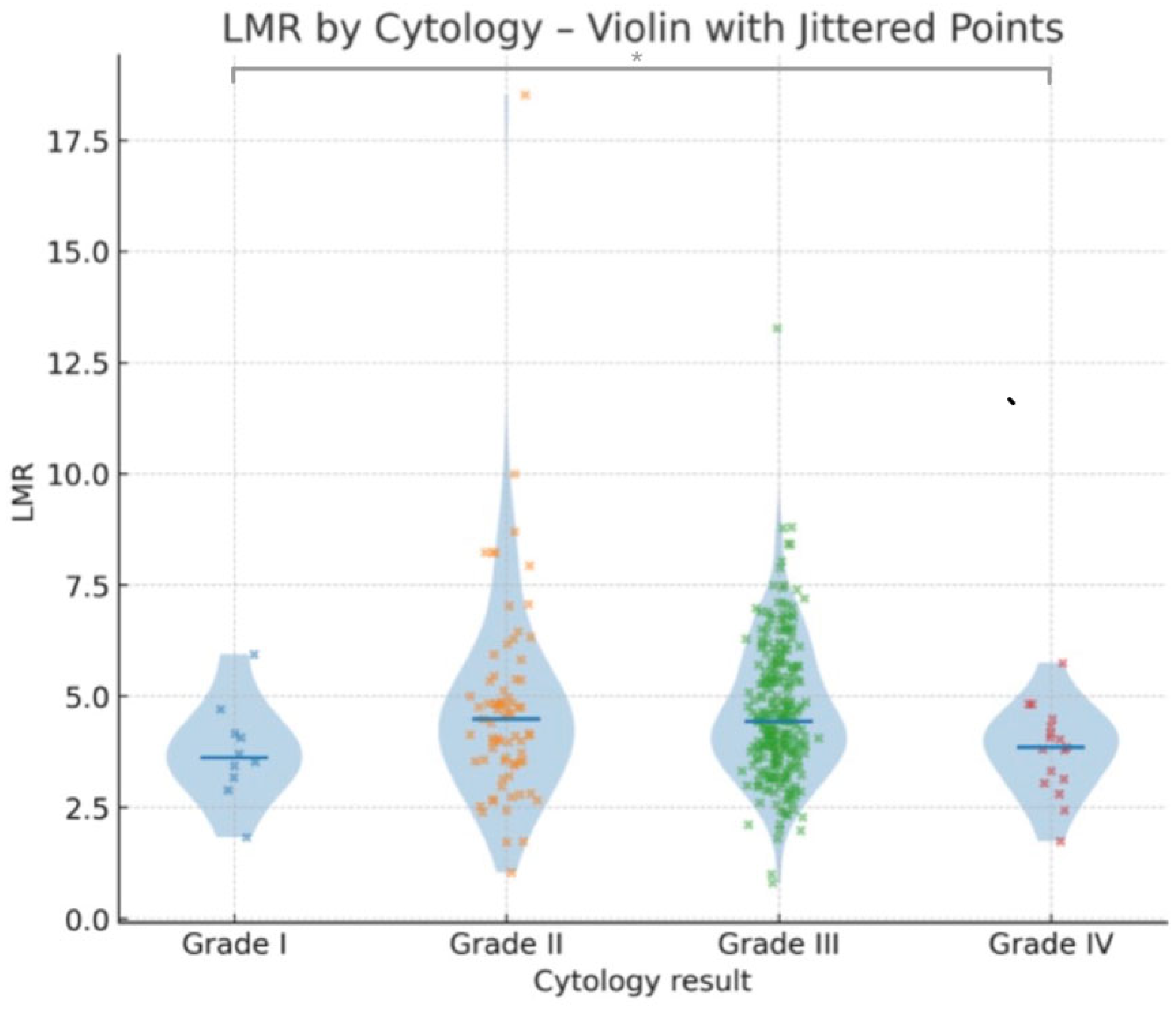
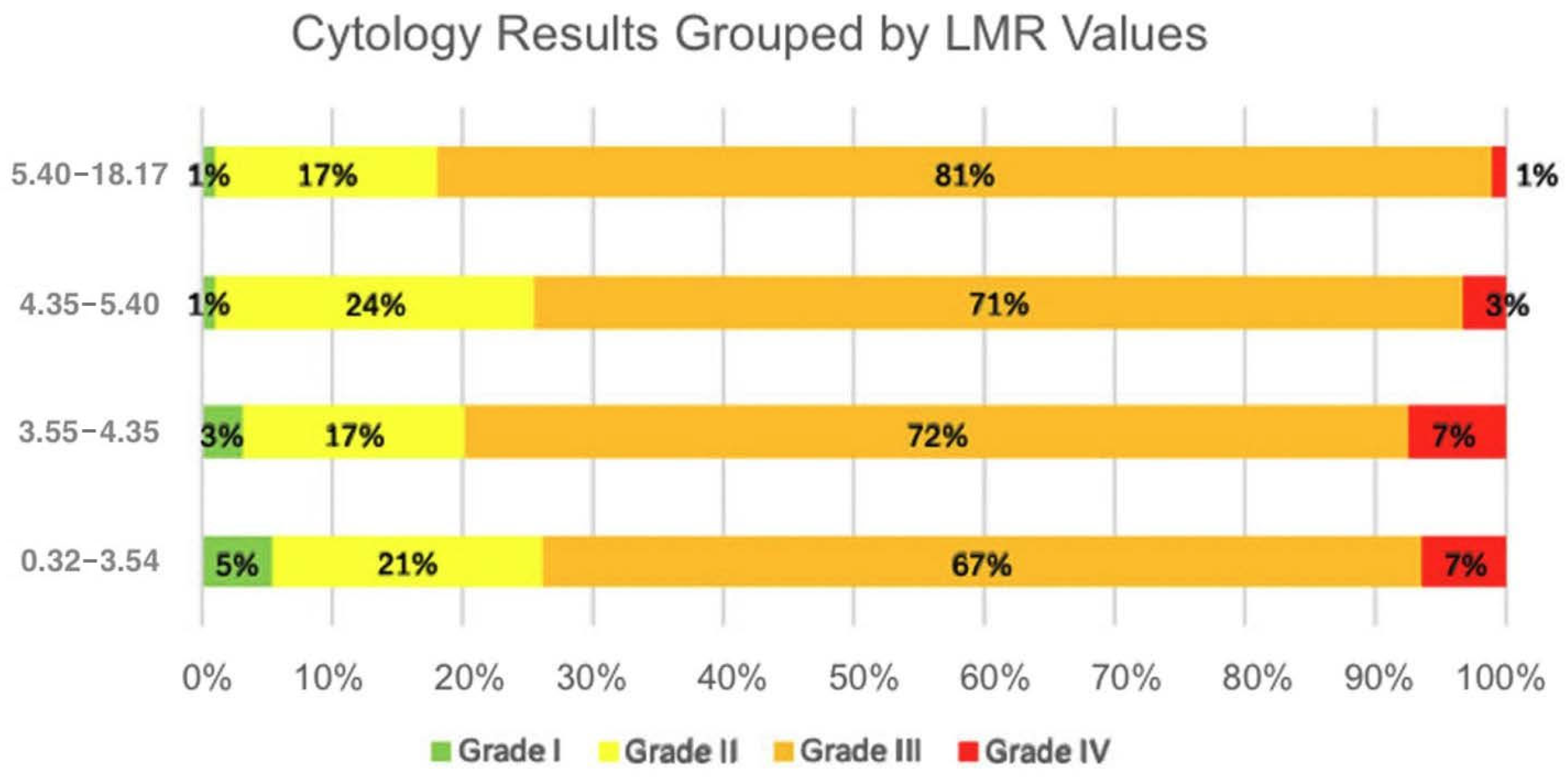
3.2. Relationship Between Laboratory Parameters and Cytology Results
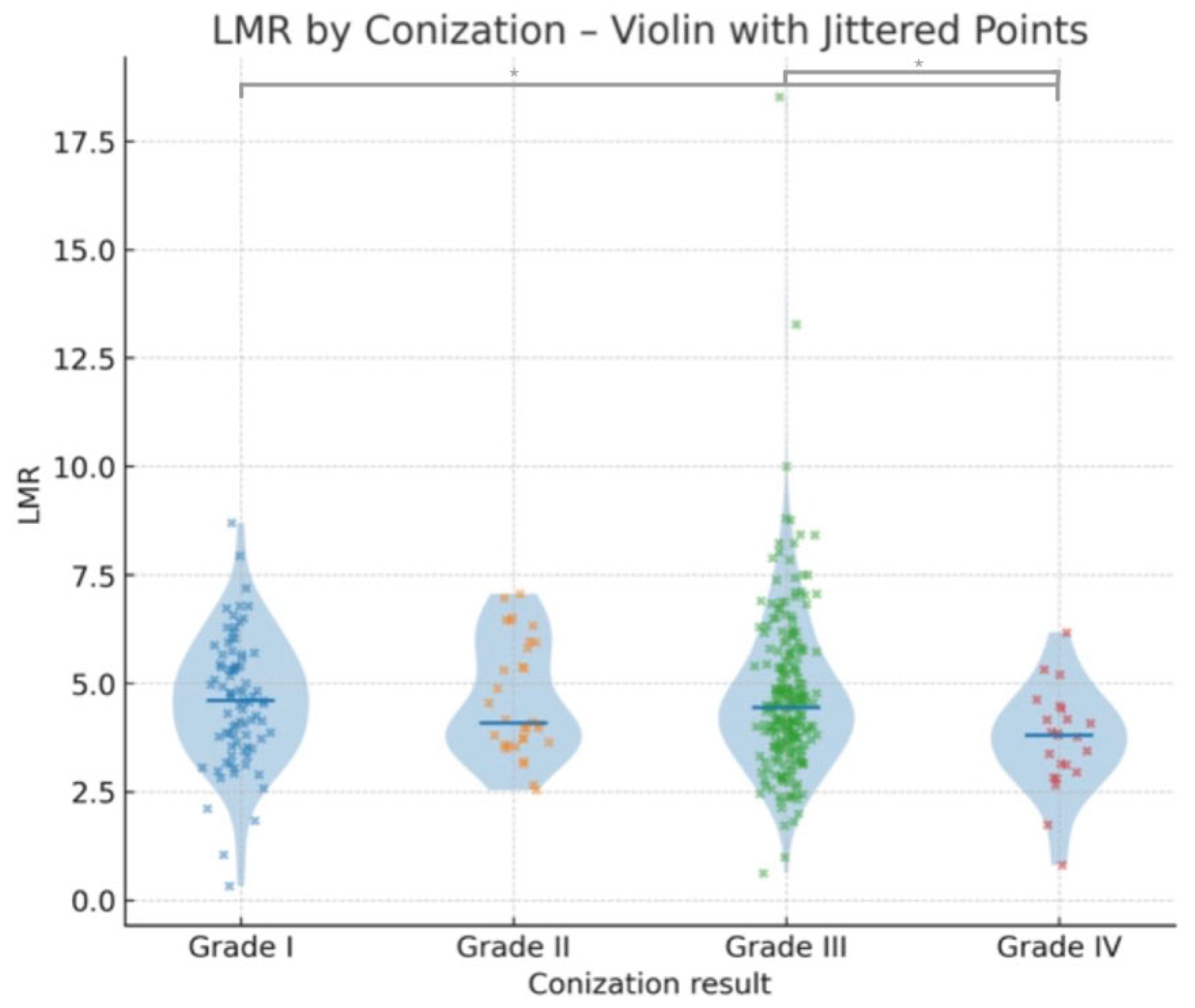
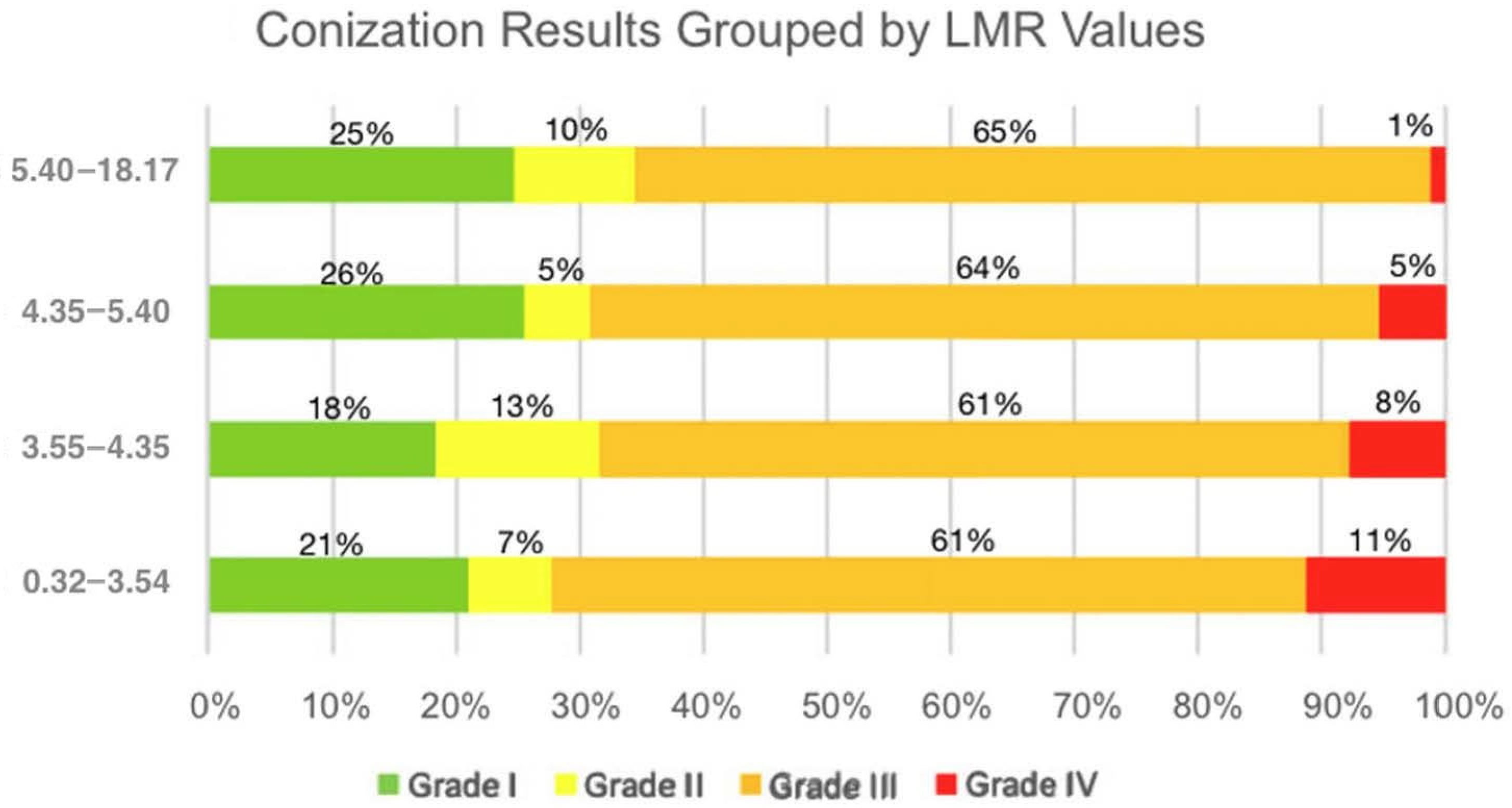
3.3. Relationship Between Laboratory Parameters and Conization (Histological) Results
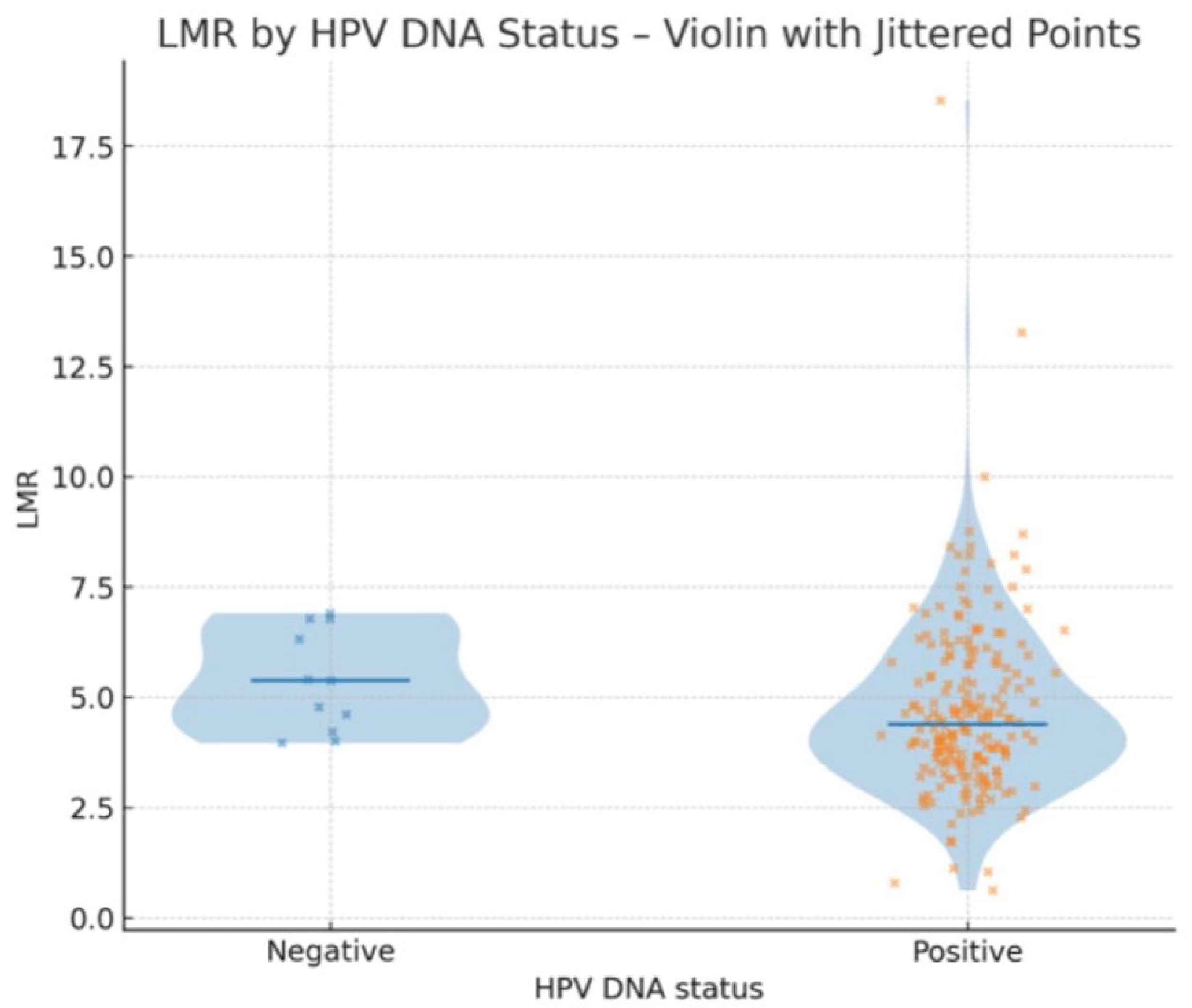
3.4. Relationship Between Laboratory Parameters and HPV DNA Positivity
3.5. Relationship Between Laboratory Parameters and Age
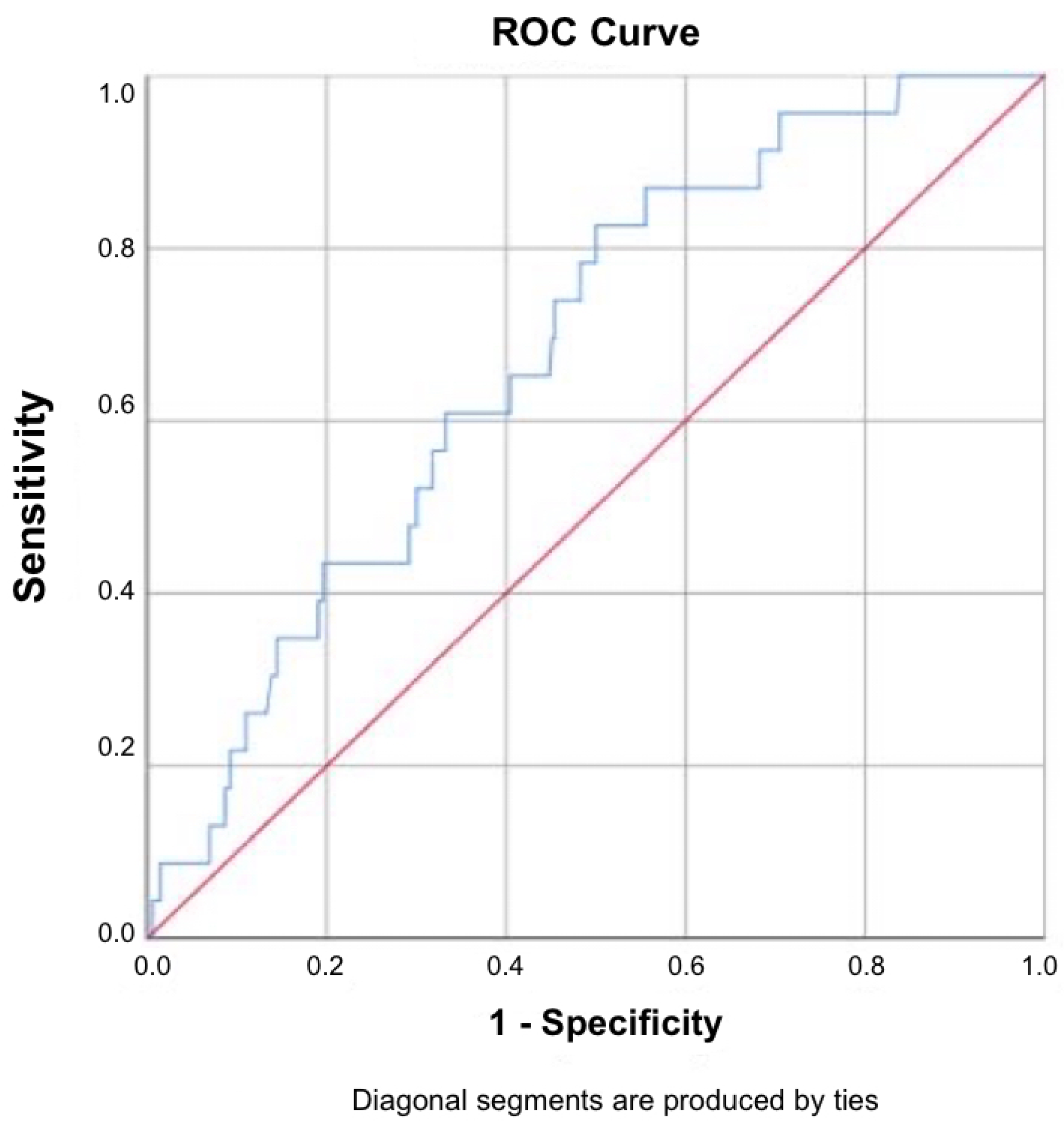
3.6. Diagnostic Performance
3.7. Statistical Analysis and Data Visualization
4. Discussion
4.1. Strengths and Limitations
4.2. Implications for Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGC | atypical glandular cell |
| AIS | adenocarcinoma in situ |
| ASC-H | atypical squamous cells, cannot exclude HSIL |
| ASC-US | atypical squamous cells, undetermined significance |
| AUC | area under the curve |
| BMI | body mass index |
| CI | confidence interval |
| CIN | cervical intraepithelial neoplasia |
| DFS | disease-free survival |
| HPV | human papillomavirus |
| HSIL | high-grade squamous intraepithelial lesion |
| LEEP | Loop Electrosurgical Excision Procedure |
| LMICs | low- and middle-income countries |
| LMR | lymphocyte-to-monocyte ratio |
| LSIL | low-grade squamous intraepithelial lesion |
| NLR | neutrophil-to-lymphocyte ratio |
| NPV | negative predictive value |
| OR | odds ratio |
| OS | overall survival |
| PLR | platelet-to-lymphocyte ratio |
| PPV | positive predictive value |
| RFS | recurrence-free survival |
| ROC | receiver operating characteristic |
| TAMs | tumor-associated macrophages |
| WHO | World Health Organization |
References
- Vu, M.; Yu, J.; Awolude, O.A.; Chuang, L. Cervical cancer worldwide. Curr. Probl. Cancer 2018, 42, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.M.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle-income countries. Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef]
- Tóth, Z.; Lőczi, L.; Sebők, B.; Merkely, P.; Keszthelyi, E.; Lintner, B.; Ács, N.; Keszthelyi, A.; Várbíró, S.; Tóth, R.; et al. Neutrophil/Lymphocyte Ratio (NLR) as a Predictive Marker for p16 Positivity and Cervical Cancer Progression: Insights from the SCOPE Study. Cancers 2025, 17, 921. [Google Scholar] [CrossRef]
- Tsikouras, P.; Zervoudis, S.; Manav, B.; Tomara, E.; Iatrakis, G.; Romanidis, C.; Bothou, A.; Galazios, G. Cervical cancer: Screening, diagnosis and staging. J. Buon 2016, 21, 320–325. [Google Scholar] [PubMed]
- Bedell, S.L.; Goldstein, L.S.; Goldstein, A.R.; Goldstein, A.T. Cervical Cancer Screening: Past, Present, and Future. Sex. Med. Rev. 2020, 8, 28–37. [Google Scholar] [CrossRef]
- Kusakabe, M.; Taguchi, A.; Sone, K.; Mori, M.; Osuga, Y. Carcinogenesis and management of human papillomavirus-associated cervical cancer. Int. J. Clin. Oncol. 2023, 28, 965–974. [Google Scholar] [CrossRef]
- Vida, B.; Keszthelyi, E.; Tóth, Z.; Lőczi, L.; Sebők, B.; Merkely, P.; Lintner, B.; Bánhidy, F.; Keszthelyi, A.; Várbíró, S.; et al. The Neutrophil-to-Lymphocyte Ratio (NLR) as a Potential Predictor in Conization Outcomes for Cervical Cancer. Cancers 2025, 17, 1856. [Google Scholar] [CrossRef]
- Khan, M.J.; Smith-McCune, K.K. Treatment of cervical precancers: Back to basics. Obstet. Gynecol. 2014, 123, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Shimoi, T.; Nishikawa, T.; Kawachi, A.; Okuma, H.S.; Tokura, M.; Yazaki, S.; Mizoguchi, C.; Arakaki, M.; Saito, A.; et al. Lymphocyte-to-monocyte ratio as a prognostic and potential tumor microenvironment indicator in advanced soft tissue sarcoma treated with first-line doxorubicin therapy. Sci. Rep. 2023, 13, 10734. [Google Scholar] [CrossRef] [PubMed]
- Minici, R.; Venturini, M.; Guzzardi, G.; Fontana, F.; Coppola, A.; Piacentino, F.; Torre, F.; Spinetta, M.; Maglio, P.; Guerriero, P.; et al. Prognostic Role of Lymphocyte-to-Monocyte Ratio (LMR) in Patients with Intermediate-Stage Hepatocellular Carcinoma (HCC) Undergoing Chemoembolizations (DEM-TACE or cTACE) of the Liver: Exploring the Link Between Tumor Microenvironment and Interventional Radiology. Diseases 2024, 12, 137. [Google Scholar]
- Huang, H.; Liu, Q.; Zhu, L.; Zhang, Y.; Lu, X.; Wu, Y.; Liu, L. Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Patients with Cervical Cancer. Sci. Rep. 2019, 9, 3284. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Jeong, M.J.; Cha, J.; Lee, J.S.; Yoo, J.G.; Song, M.J.; Kim, J.H.; Lee, S.J.; Lee, H.N.; Yoon, J.H.; et al. Preoperative neutrophil-to-lymphocyte, platelet-to-lymphocyte and monocyte-to-lymphocyte ratio as a prognostic factor in non-endometrioid endometrial cancer. Int. J. Med. Sci. 2021, 18, 3712–3717. [Google Scholar] [CrossRef] [PubMed]
- Kalas, N.; Szabó, V.; Vida, B.; Tóth, Z.; Lőczi, L.; Sebők, B.; Merkely, P.; Lintner, B.; Ács, N.; Keszthelyi, A.; et al. The Platelet-to-Lymphocyte Ratio (PLR) as a Non-Invasive Biomarker for Cervical Malignancy in Conization Patients. Life 2025, 15, 971. [Google Scholar] [CrossRef]
- Li, S.W.; Yuan, W.; Zhao, B.; He, Z.K.; Guo, X.; Xia, W.X.; Xu, L.H. Positive effect of HPV status on prognostic value of blood lymphocyte-to-monocyte ratio in advanced cervical carcinoma. Cancer Cell Int. 2016, 16, 54. [Google Scholar] [CrossRef]
- Bing, R.S.; Tsui, W.L.; Ding, D.C. The Association Between Diabetes Mellitus, High Monocyte/Lymphocyte Ratio, and Survival in Endometrial Cancer: A Retrospective Cohort Study. Diagnostics 2022, 13, 44. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, N.; Zhou, Y.; Chen, J.; Wei, Q.; Han, M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm. Sin. B 2020, 10, 2156–2170. [Google Scholar] [CrossRef]
- Andre, F.; Dieci, M.V.; Dubsky, P.; Sotiriou, C.; Curigliano, G.; Denkert, C.; Loi, S. Molecular pathways: Involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin. Cancer Res. 2013, 19, 28–33. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, J.; Zhong, Y.; Mai, Y.; Huang, D.; Wei, W.; Huang, J.; Zhao, P.; Lin, F.; Jin, J. Predictive value of the monocyte-to-lymphocyte ratio in the diagnosis of prostate cancer. Medicine 2021, 100, e27244. [Google Scholar] [CrossRef]
- Gu, L.; Li, H.; Chen, L.; Ma, X.; Li, X.; Gao, Y.; Zhang, Y.; Xie, Y.; Zhang, X. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: Evidence from a systematic review and meta-analysis. Oncotarget 2016, 7, 31926–31942. [Google Scholar] [CrossRef]
- Hirahara, N.; Matsubara, T.; Kaji, S.; Hayashi, H.; Sasaki, Y.; Kawakami, K.; Hyakudomi, R.; Yamamoto, T.; Tajima, Y. Novel inflammation-combined prognostic index to predict survival outcomes in patients with gastric cancer. Oncotarget 2023, 14, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, D.; Song, H.; Qiu, B.; Tian, D.; Li, Z.; Ji, Y.; Wang, J. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: A systematic review and meta-analysis. BMJ Open 2021, 11, e048324. [Google Scholar] [CrossRef]
- Misiewicz, A.; Dymicka-Piekarska, V. Fashionable, but What is Their Real Clinical Usefulness? NLR, LMR, and PLR as a Promising Indicator in Colorectal Cancer Prognosis: A Systematic Review. J. Inflamm. Res. 2023, 16, 69–81. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef]
- Chen, L.; Kong, X.; Yan, C.; Fang, Y.; Wang, J. The Research Progress on the Prognostic Value of the Common Hematological Parameters in Peripheral Venous Blood in Breast Cancer. Onco Targets Ther. 2020, 13, 1397–1412. [Google Scholar] [CrossRef]
- Ni, X.J.; Zhang, X.L.; Ou-Yang, Q.W.; Qian, G.W.; Wang, L.; Chen, S.; Jiang, Y.Z.; Zuo, W.J.; Wu, J.; Hu, X.; et al. An elevated peripheral blood lymphocyte-to-monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS ONE 2014, 9, e111886. [Google Scholar] [CrossRef]
- Li, P.; Li, H.; Ding, S.; Zhou, J. NLR, PLR, LMR and MWR as diagnostic and prognostic markers for laryngeal carcinoma. Am. J. Transl. Res. 2022, 14, 3017–3027. [Google Scholar]
- Woodley, N.; Rogers, A.D.G.; Turnbull, K.; Slim, M.A.M.; Ton, T.; Montgomery, J.; Douglas, C. Prognostic scores in laryngeal cancer. Eur. Arch. Otorhinolaryngol. 2022, 279, 3705–3715. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Y.; Wang, H.; Qin, R.; Han, Z.; Li, R. Global cervical cancer elimination: Quantifying the status, progress, and gaps. BMC Med. 2025, 23, 67. [Google Scholar] [CrossRef]
- Huang, L.; Hu, Z.; Luo, R.; Li, H.; Yang, Z.; Qin, X.; Mo, Z. Predictive Values of the Selected Inflammatory Indexes in Colon Cancer. Cancer Control 2022, 29, 10732748221091333. [Google Scholar] [CrossRef]
- Yapar, A.; Tokgöz, M.A.; Yapar, D.; Atalay, İ.B.; Ulucaköy, C.; Güngör, B. Diagnostic and prognostic role of neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and lymphocyte/monocyte ratio in patients with osteosarcoma. Jt. Dis. Relat. Surg. 2021, 32, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Balbaloglu, H.; Tasdoven, I.; Karadeniz Cakmak, G. Can inflammatory indices predict sentinel lymph node status in patients with early-stage breast cancer? Medicine 2023, 102, e34808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, W.; Wu, J.; Tian, J.; Yan, W.; Yuan, Y.; Yao, Y.; Shang, A.; Quan, W. Immune cell-lipoprotein imbalance as a marker for early diagnosis of non-small cell lung cancer metastasis. Front. Oncol. 2022, 12, 942964. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Chan, D.L.; Diakos, C.I.; Engel, A.; Pavlakis, N.; Gill, A.; Clarke, S.J. The Lymphocyte-to-Monocyte Ratio is a Superior Predictor of Overall Survival in Comparison to Established Biomarkers of Resectable Colorectal Cancer. Ann. Surg. 2017, 265, 539–546. [Google Scholar] [CrossRef]
- Huszno, J.; Kołosza, Z.; Mrochem-Kwarciak, J.; Telka, E.; Jochymek, B.; Miszczyk, L. Role of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, lymphocyte-monocyte ratio and platelets in prognosis of patients with prostate cancer. Oncol. Lett. 2022, 24, 305. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, L. The predictive value of serum inflammatory markers for the severity of cervical lesions. BMC Cancer 2024, 24, 780. [Google Scholar] [CrossRef]
- Xu, M.; Wu, Q.; Cai, L.; Sun, X.; Xie, X.; Sun, P. Systemic Inflammatory Score predicts Overall Survival in patients with Cervical Cancer. J. Cancer 2021, 12, 3671–3677. [Google Scholar] [CrossRef]
- Trinh, H.; Dzul, S.P.; Hyder, J.; Jang, H.; Kim, S.; Flowers, J.; Vaishampayan, N.; Chen, J.; Winer, I.; Miller, S. Prognostic value of changes in neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR) for patients with cervical cancer undergoing definitive chemoradiotherapy (dCRT). Clin. Chim. Acta 2020, 510, 711–716. [Google Scholar] [CrossRef]
| Characteristics (n = 374) | N (Range or %) |
|---|---|
| Total | 374 |
| Median age (years) | 40 (23–78) |
| Median BMI | 22.85 (14.64–46.48) |
| Median LMR | 4.35 (0.32–18.52) |
| Cytology results | 370 |
| Grade I (negative) | 10 (2.70%) |
| Grade II (LSIL, ASC-US) | 73 (19.73%) |
| Grade III (HSIL, ASC-H, AGC, AIS) | 270 (72.97%) |
| Grade IV (cancer) | 17 (4.59%) |
| N/A | 4 |
| Conization results | 369 |
| Grade I (negative) | 83 (22.49%) |
| Grade II (LSIL, ASC-US) | 32 (8.67%) |
| Grade III (HSIL, ASC-H, AGC, AIS) | 231 (62.60%) |
| Grade IV (cancer) | 23 (6.23%) |
| N/A | 5 |
| HPV status | 223 |
| HPV positive | 212 (95.07%) |
| HPV negative | 11 (4.93%) |
| Grade I (Negative) | Grade II (LSIL, ASC-US) | Grade III (HSIL, ASC-H, AGC, AIS) | Grade IV (Cancer) | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LMR | Cytol. | Coniz. | Cytol. | Coniz. | Cytol. | Coniz. | Cytol | Coniz. | Cytol. | Coniz. | |
| N | 10 | 83 | 73 | 32 | 270 | 231 | 17 | 23 | 370 | 369 | |
| Lymphocyte (%) | Median | 25.70 | 32.80 | 33.30 | 32.95 | 31.25 | 31.50 | 27.40 | 25.30 | 31.45 | 31.40 |
| Mean | 28.14 | 32.06 | 33.11 | 34.02 | 31.76 | 31.83 | 27.95 | 25.10 | 31.76 | 31.65 | |
| Minimum | 14.30 | 1.52 | 8.20 | 21.10 | 2.00 | 2.00 | 13.40 | 7.30 | 2.00 | 1.52 | |
| Maximum | 47.50 | 47.50 | 53.70 | 53.00 | 60.60 | 60.60 | 39.30 | 39.30 | 60.60 | 60.60 | |
| Monocyte (%) | Median | 7.20 | 7.10 | 7.50 | 7.25 | 7.00 | 7.00 | 7.70 | 7.10 | 7.00 | 7.00 |
| Mean | 7.49 | 7.26 | 7.64 | 7.64 | 7.19 | 7.25 | 7.65 | 7.04 | 7.31 | 7.27 | |
| Minimum | 5.50 | 4.46 | 2.90 | 4.20 | 0.34 | 0.34 | 5.20 | 4.80 | 0.34 | 0.34 | |
| Maximum | 10.20 | 16.10 | 16.10 | 11.40 | 14.90 | 14.90 | 12.90 | 10.20 | 16.10 | 16.10 | |
| LMR (L/M) | Median | 3.62 | 4.60 | 4.49 | 4.08 | 4.44 | 4.44 | 3.85 | 3.81 | 4.34 | 4.41 |
| Mean | 3.75 | 4.61 | 4.76 | 4.62 | 4.64 | 4.70 | 3.79 | 3.69 | 4.60 | 4.61 | |
| Minimum | 1.83 | 0.32 | 1.04 | 2.54 | 0.80 | 0.62 | 1.74 | 0.80 | 0.80 | 0.32 | |
| Maximum | 5.94 | 8.70 | 18.52 | 7.06 | 13.28 | 18.52 | 5.75 | 6.18 | 18.52 | 18.52 | |
| Comparison Between Groups | Mann–Whitney U | Z-Score | Bonferroni Corrected p | |||
|---|---|---|---|---|---|---|
| Cytol. | Coniz. | Cytol. | Coniz. | Cytol. | Coniz. | |
| Grade I vs. Grade II | 1191.5 | 1220.5 | −0.09 | −0.082 | 1.00 | 1.00 |
| Grade I vs. Grade III | 8773.0 | 9205.0 | −0.40 | −0.261 | 1.00 | 1.00 |
| Grade I vs. Grade IV | 1189.0 | 561.0 | −3.01 | −2.770 | 0.02 | 0.03 |
| Grade II vs. Grade III | 3483.0 | 3663.0 | −0.03 | −0.063 | 1.00 | 1.00 |
| Grade II vs. Grade IV | 470.0 | 281.0 | −2.33 | −1.729 | 0.12 | 0.56 |
| Grade III vs. Grade IV | 1826.5 | 1856.5 | −1.49 | −2.495 | 0.82 | 0.047 |
| Predictor | B | S.E. | Wald | df | Sig. (p) | Exp(B) | 95% CI for Exp(B) |
|---|---|---|---|---|---|---|---|
| LMR | −0.48 | 0.18 | 7.36 | 1 | 0.01 | 0.62 | [0.44–0.87] |
| Age | 0.06 | 0.02 | 8.37 | 1 | 0.004 | 1.06 | [1.02–1.10] |
| Constant | −3.29 | 1.13 | 8.43 | 1 | 0.004 | 0.04 | [0.004–0.37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, V.; Várbíró, S.; Kalas, N.; Vida, B.; Tóth, Z.; Lőczi, L.; Sebők, B.; Merkely, P.; Lintner, B.; Ács, N.; et al. The Lymphocyte-to-Monocyte Ratio (LMR) as a Novel Biomarker for Cervical Cancer Risk Stratification in Conization Patients. J. Clin. Med. 2025, 14, 6057. https://doi.org/10.3390/jcm14176057
Szabó V, Várbíró S, Kalas N, Vida B, Tóth Z, Lőczi L, Sebők B, Merkely P, Lintner B, Ács N, et al. The Lymphocyte-to-Monocyte Ratio (LMR) as a Novel Biomarker for Cervical Cancer Risk Stratification in Conization Patients. Journal of Clinical Medicine. 2025; 14(17):6057. https://doi.org/10.3390/jcm14176057
Chicago/Turabian StyleSzabó, Verita, Szabolcs Várbíró, Noémi Kalas, Balázs Vida, Zsófia Tóth, Lotti Lőczi, Barbara Sebők, Petra Merkely, Balázs Lintner, Nándor Ács, and et al. 2025. "The Lymphocyte-to-Monocyte Ratio (LMR) as a Novel Biomarker for Cervical Cancer Risk Stratification in Conization Patients" Journal of Clinical Medicine 14, no. 17: 6057. https://doi.org/10.3390/jcm14176057
APA StyleSzabó, V., Várbíró, S., Kalas, N., Vida, B., Tóth, Z., Lőczi, L., Sebők, B., Merkely, P., Lintner, B., Ács, N., Keszthelyi, A., Keszthelyi, M., & Tóth, R. (2025). The Lymphocyte-to-Monocyte Ratio (LMR) as a Novel Biomarker for Cervical Cancer Risk Stratification in Conization Patients. Journal of Clinical Medicine, 14(17), 6057. https://doi.org/10.3390/jcm14176057








