Combined Cardiac Arrhythmias Leading to Electrical Chaos Developed in the Convalescent Phase of SARS-CoV-2 Infection: A Case Report and Literature Review
Abstract
1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| ASD | Atrial septal defect |
| AV | Atrioventricular |
| Bpm | Beats per minute |
| CK | Creatine kinase |
| CMR | Cardiac magnetic resonance imaging |
| ECG | Electrocardiogram |
| IgA | Immunoglobulin A |
| JR | Junctional rhythm |
| LDH | Lactate dehydrogenase |
| LVEF | Left ventricular ejection fraction |
| NT-proBNP | N-terminal pro-b-type natriuretic peptide |
| VT | Ventricular tachycardia |
References
- Fairweather, D.; Beetler, D.J.; Di Florio, D.N.; Musigk, N.; Heidecker, B.; Cooper, L.T. COVID-19, Myocarditis and Pericarditis. Circ. Res. 2023, 132, 1302–1319. [Google Scholar] [CrossRef]
- Saha, S.A.; Russo, A.M.; Chung, M.K.; Deering, T.F.; Lakkireddy, D.; Gopinathannair, R. COVID-19 and Cardiac Arrhythmias: A Contemporary Review. Curr. Treat. Options Cardiovasc. Med. 2022, 24, 87–107. [Google Scholar] [CrossRef]
- Gopinathannair, R.; Olshansky, B.; Chung, M.K.; Gordon, S.; Joglar, J.A.; Marcus, G.M.; Mar, P.L.; Russo, A.M.; Srivatsa, U.N.; Wan, E.Y.; et al. Cardiac Arrhythmias and Autonomic Dysfunction Associated With COVID-19: A Scientific Statement from the American Heart Association. Circulation 2024, 150, 21. [Google Scholar] [CrossRef]
- Tsampasian, V.; Bäck, M.; Bernardi, M.; Cavarretta, E.; Dębski, M.; Gati, S.; Hansen, D.; Kränkel, N.; Koskinas, K.C.; Niebauer, J.; et al. Cardiovascular Disease as Part of Long COVID: A Systematic Review. Eur. J. Prev. Cardiol. 2025, 32, 485–498. [Google Scholar] [CrossRef]
- Brendel, J.M.; Klingel, K.; Gräni, C.; Blankstein, R.; Kübler, J.; Hagen, F.; Gawaz, M.; Nikolaou, K.; Krumm, P.; Greulich, S. Multiparametric Cardiac Magnetic Resonance Imaging to Discriminate Endomyocardial Biopsy-Proven Chronic Myocarditis from Healed Myocarditis. JACC Cardiovasc. Imaging 2024, 17, 1182–1195. [Google Scholar] [CrossRef]
- Marques, K.C.; Quaresma, J.A.S.; Falcão, L.F.M. Cardiovascular Autonomic Dysfunction in “Long COVID”: Pathophysiology, Heart Rate Variability, and Inflammatory Markers. Front. Cardiovasc. Med. 2023, 10, 1256512. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Lee, K.; Yoo, S.-J. The Impact of Long COVID on Heart Rate Variability: A Cross-Sectional Study. BMC Infect. Dis. 2025, 25, 261. [Google Scholar] [CrossRef] [PubMed]
- Parotto, M.; Gyöngyösi, M.; Howe, K.; Myatra, S.N.; Ranzani, O.; Shankar-Hari, M.; Herridge, M.S. Post-Acute Sequelae of COVID-19: Understanding and Addressing the Burden of Multisystem Manifestations. Lancet Respir. Med. 2023, 11, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A Clinical Case Definition of Post-COVID-19 Condition by a Delphi Consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Long, B.; Brady, W.J.; Bridwell, R.E.; Ramzy, M.; Montrief, T.; Singh, M.; Gottlieb, M. Electrocardiographic Manifestations of COVID-19. Am. J. Emerg. Med. 2021, 41, 96–103. [Google Scholar] [CrossRef]
- Cimino, G.; Pascariello, G.; Bernardi, N.; Calvi, E.; Arabia, G.; Salghetti, F.; Bontempi, L.; Vizzardi, E.; Metra, M.; Curnis, A. Sinus Node Dysfunction in a Young Patient With COVID-19. JACC Case Rep. 2020, 2, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.D.; Boursiquot, B.C.; Moore, C.J.; Gopinathannair, R.; Waase, M.P.; Rubin, G.A.; Wan, E.Y. Autonomic Dysfunction Post–Acute COVID-19 Infection. Heart Case Rep. 2022, 8, 143–146. [Google Scholar] [CrossRef]
- Gyöngyösi, M.; Alcaide, P.; Asselbergs, F.W.; Brundel, B.J.J.M.; Camici, G.G.; Martins, P.d.C.; Ferdinandy, P.; Fontana, M.; Girao, H.; Gnecchi, M.; et al. Long COVID and the Cardiovascular System-Elucidating Causes and Cellular Mechanisms in Order to Develop Targeted Diagnostic and Therapeutic Strategies: A Joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc. Res. 2023, 119, 336–356. [Google Scholar] [CrossRef]
- Cole, A.P.; Perry, D.; Hobbs, J.R. Abnormalities of Immunoglobulins in Infants with Congenital Heart Disease. Acta Paediatr. 1977, 66, 421–424. [Google Scholar] [CrossRef]
- Singampalli, K.L.; Jui, E.; Shani, K.; Ning, Y.; Connell, J.P.; Birla, R.K.; Bollyky, P.L.; Caldarone, C.A.; Keswani, S.G.; Grande-Allen, K.J. Congenital Heart Disease: An Immunological Perspective. Front. Cardiovasc. Med. 2021, 8, 701375. [Google Scholar] [CrossRef]
- Han, E.; Müller-Zlabinger, K.; Hasimbegovic, E.; Poschenreithner, L.; Kastner, N.; Maleiner, B.; Hamzaraj, K.; Spannbauer, A.; Riesenhuber, M.; Vavrikova, A.; et al. Circulating Autoantibodies Against Vasoactive Biomarkers Related to Orthostatic Intolerance in Long COVID Patients Compared to No-Long-COVID Populations: A Case-Control Study. Biomolecules 2025, 15, 300. [Google Scholar] [CrossRef]
- Aranyó, J.; Bazan, V.; Lladós, G.; Dominguez, M.J.; Bisbal, F.; Massanella, M.; Sarrias, A.; Adeliño, R.; Riverola, A.; Paredes, R.; et al. Inappropriate Sinus Tachycardia in Post-COVID-19 Syndrome. Sci. Rep. 2022, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Dmytrenko, O.; Lavine, K.J. Cardiovascular Tropism and Sequelae of SARS-CoV-2 Infection. Viruses 2022, 14, 1137. [Google Scholar] [CrossRef]
- Tsai, E.J.; Čiháková, D.; Tucker, N.R. Cell-Specific Mechanisms in the Heart of COVID-19 Patients. Circ. Res. 2023, 132, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Navaratnarajah, C.K.; Pease, D.R.; Halfmann, P.J.; Taye, B.; Barkhymer, A.; Howell, K.G.; Charlesworth, J.E.; Christensen, T.A.; Kawaoka, Y.; Cattaneo, R.; et al. Highly Efficient SARS-CoV-2 Infection of Human Cardiomyocytes: Spike Protein-Mediated Cell Fusion and Its Inhibition. J. Virol. 2021, 95, e01368-21. [Google Scholar] [CrossRef]
- Sakai, Y.; Imai, S.; Sato, Y.; Yagi, H.; Kushiro, T. Clinical and Electrophysiological Characteristics of Binodal Disease. Circ. J. 2006, 70, 1580–1584. [Google Scholar] [CrossRef][Green Version]
- Phillips, C.M.; Smyth, J.W. Viral Infection and Connexin Dysfunction in the Heart. Curr. Cardiol. Rep. 2025, 27, 76. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, N.; Mariani, M.V.; Iannetti, G.; Maffei, L.; Coluccio, A.; Laviola, D.; Palombi, M.; Trivigno, S.; Spadafora, L.; Chourda, E.; et al. Atrial Cardiomyopathy: New Pathophysiological and Clinical Aspects. Minerva Cardiol. Angiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Corradi, D.; Dobrev, D.; Aguinaga, L.; Cabrera, J.-A.; Chugh, S.S.; De Groot, J.R.; Soulat-Dufour, L.; Fenelon, G.; Hatem, S.N.; et al. Atrial Cardiomyopathy Revisited—Evolution of a Concept: A Clinical Consensus Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS). Europace 2024, 26, euae204. [Google Scholar] [CrossRef] [PubMed]


| Time | Event or Finding | Consequence |
|---|---|---|
| Pre-COVID infection | Electrocardiogram (ECG): normofrequent junctional escape rhythm after surgical closure of the atrial septal defect | Asymptomatic, adequate chronotropic response to stress, no bradycardia or syncope: Patient’s choice to avoid electrophysiology investigation and pacemaker implantation. Regular follow-ups instead. |
| Baseline | COVID-19 infection | - |
| 2 months later | 24 h ECG at primary care: misinterpreted combined arrhythmia | - |
| At 4 months | Cardiac magnetic resonance imaging (CMR) suggested subacute myocarditis with edema and slightly reduced left ventricular ejection fraction Laboratory investigation at primary care: cardiac enzymes and inflammatory parameters in normal range (numeric values not reported) | - |
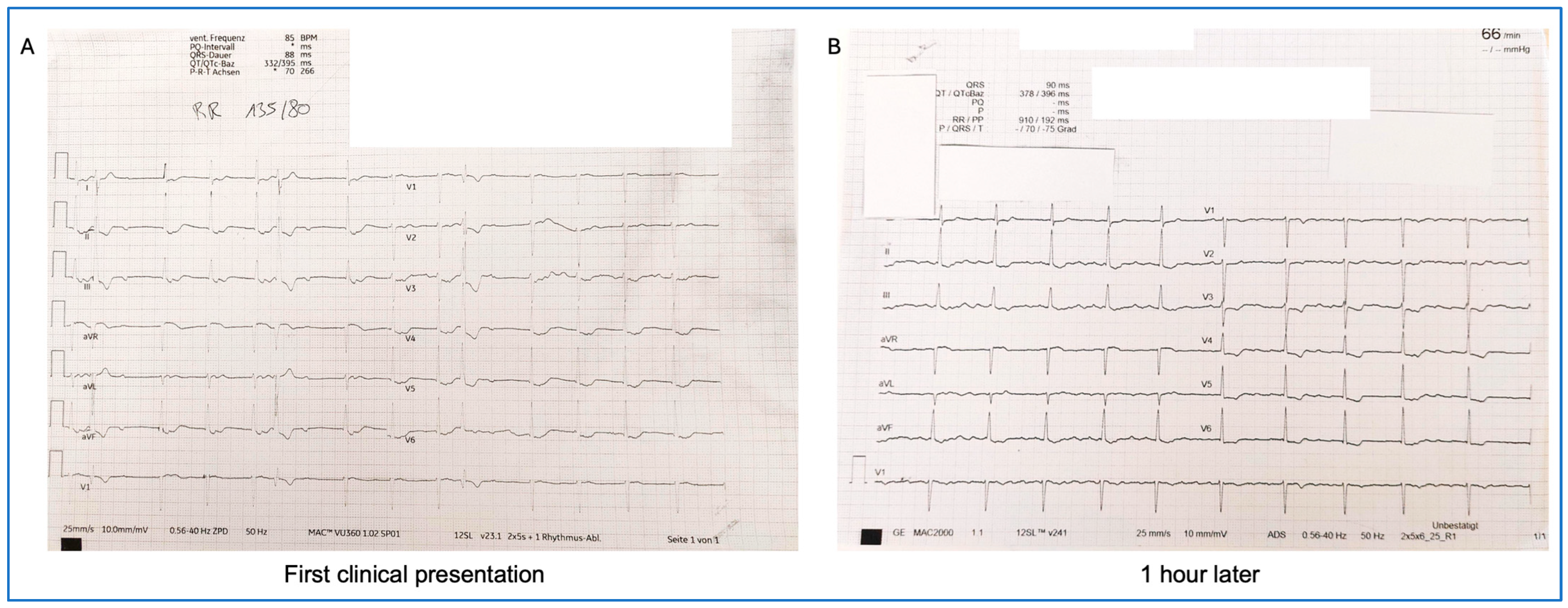
| At 6 months (first clinical presentation at the outpatient long COVID cardiac care unit) | Laboratory testing:
ECG: chaotic cardiac rhythm with accelerated junctional rhythm with retrograde P waves, 85 bpm One hour later, de novo atrial flutter | Transthoracic echocardiography (TTE): normal left ventricular function Overnight admission with 24 h ECG monitoring Initiation of 60 mg edoxaban once daily (CHADS-VASc-Score 1) |
| Next day | Transesophageal echocardiography (TEE): no intracardiac thrombi detected | Electric cardioversion without success, continued anticoagulation and frequent follow-ups in heart rhythm outpatient care |
| At 8 months | Repeated CMR: recovered left ventricular ejection fraction (LVEF 64%), no signs of cardiac edema or ventricular late enhancement Resting ECG: alternating junctional escape rhythm and atrial fibrillation Exercise testing: alternating junctional escape rhythm and atrial fibrillation, polymorphic ventricular extrasystoles, ventricular couplets and triplets, but no complaints during testing Laboratory testing:
| Continuation of 60 mg edoxaban once daily Considered electrophysiology investigation, but the patient was asymptomatic Close follow-up in heart rhythm outpatient care |
| At 11 months | Improving symptoms and no complaints, no dizziness | No indication for ablation of atrial fibrillation due to oligosymptomatic and ventricular normofrequency and possible risk of necessary pacemaker implantation after ablation |
| 1-year follow-up | ECG: alternating atrial fibrillation with accelerated junctional rhythm Laboratory testing:
| Continue anticoagulation, frequent clinical and heart rhythm clinic follow-ups |
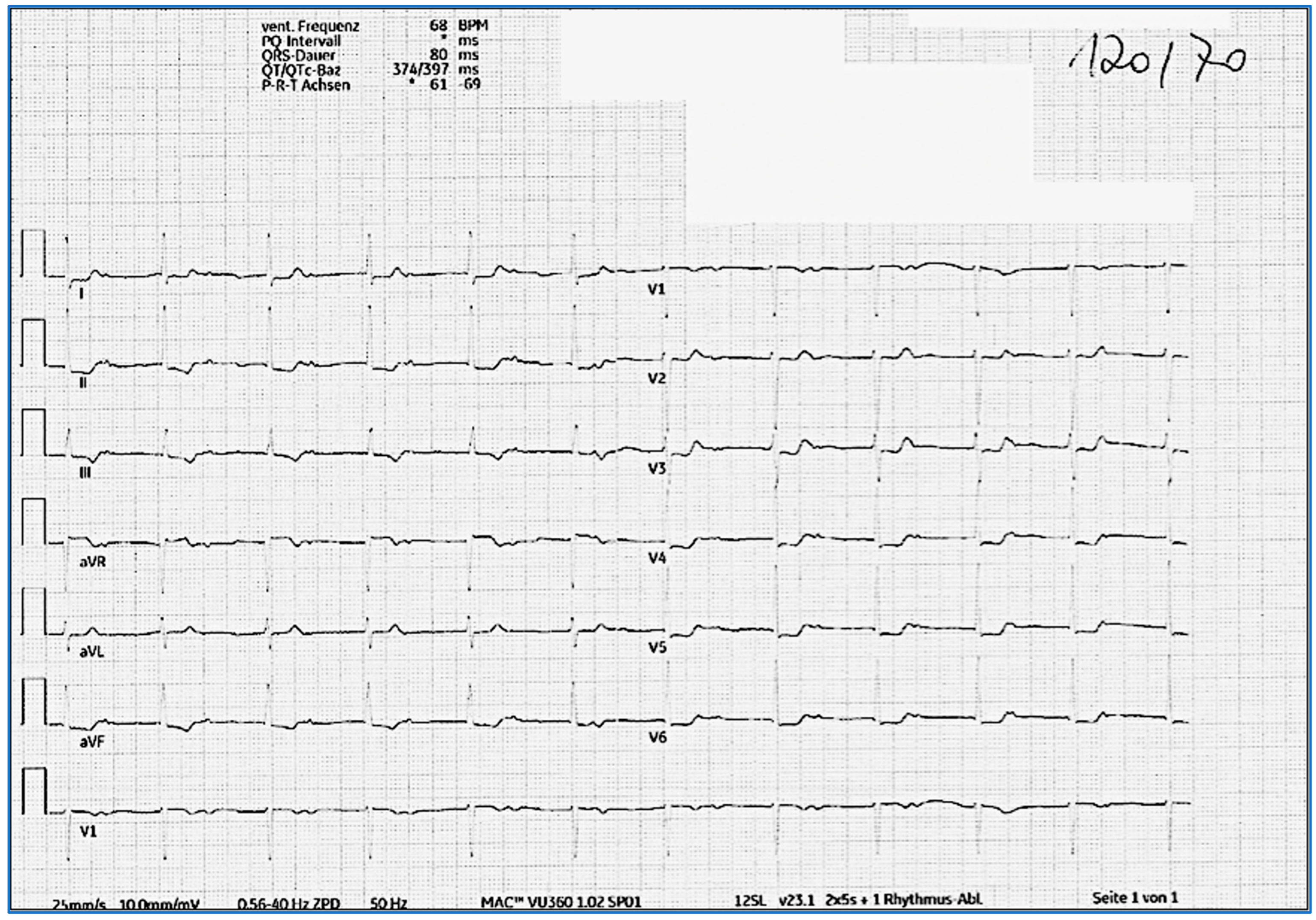
| 2-year follow-up | ECG: normofrequent atrial rhythm with prolonged PQ time (>500 ms) with antegrade conduction. P wave morphology is different compared to that of pre-COVID ECG 24 h ECG: no atrial fibrillation episodes | Decision to stop anticoagulation; continue regular yearly clinical, laboratory, and rhythmic follow-ups |
| 3-year follow-up | ECG: normofrequent atrial rhythm with prolonged PQ time (>500 ms) with antegrade conduction Laboratory testing:
| Continue with regular clinical, laboratory, and rhythmic follow-ups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, E.; Hasimbegovic, E.; Schönbauer, R.; Beitzke, D.; Gyöngyösi, M. Combined Cardiac Arrhythmias Leading to Electrical Chaos Developed in the Convalescent Phase of SARS-CoV-2 Infection: A Case Report and Literature Review. J. Clin. Med. 2025, 14, 6053. https://doi.org/10.3390/jcm14176053
Han E, Hasimbegovic E, Schönbauer R, Beitzke D, Gyöngyösi M. Combined Cardiac Arrhythmias Leading to Electrical Chaos Developed in the Convalescent Phase of SARS-CoV-2 Infection: A Case Report and Literature Review. Journal of Clinical Medicine. 2025; 14(17):6053. https://doi.org/10.3390/jcm14176053
Chicago/Turabian StyleHan, Emilie, Ena Hasimbegovic, Robert Schönbauer, Dietrich Beitzke, and Mariann Gyöngyösi. 2025. "Combined Cardiac Arrhythmias Leading to Electrical Chaos Developed in the Convalescent Phase of SARS-CoV-2 Infection: A Case Report and Literature Review" Journal of Clinical Medicine 14, no. 17: 6053. https://doi.org/10.3390/jcm14176053
APA StyleHan, E., Hasimbegovic, E., Schönbauer, R., Beitzke, D., & Gyöngyösi, M. (2025). Combined Cardiac Arrhythmias Leading to Electrical Chaos Developed in the Convalescent Phase of SARS-CoV-2 Infection: A Case Report and Literature Review. Journal of Clinical Medicine, 14(17), 6053. https://doi.org/10.3390/jcm14176053








