Late Recurrence of High-Grade Vulvar Leiomyosarcoma After 5 Years: A Rare Case Report and Expanded Review of Reported Cases
Abstract
1. Introduction
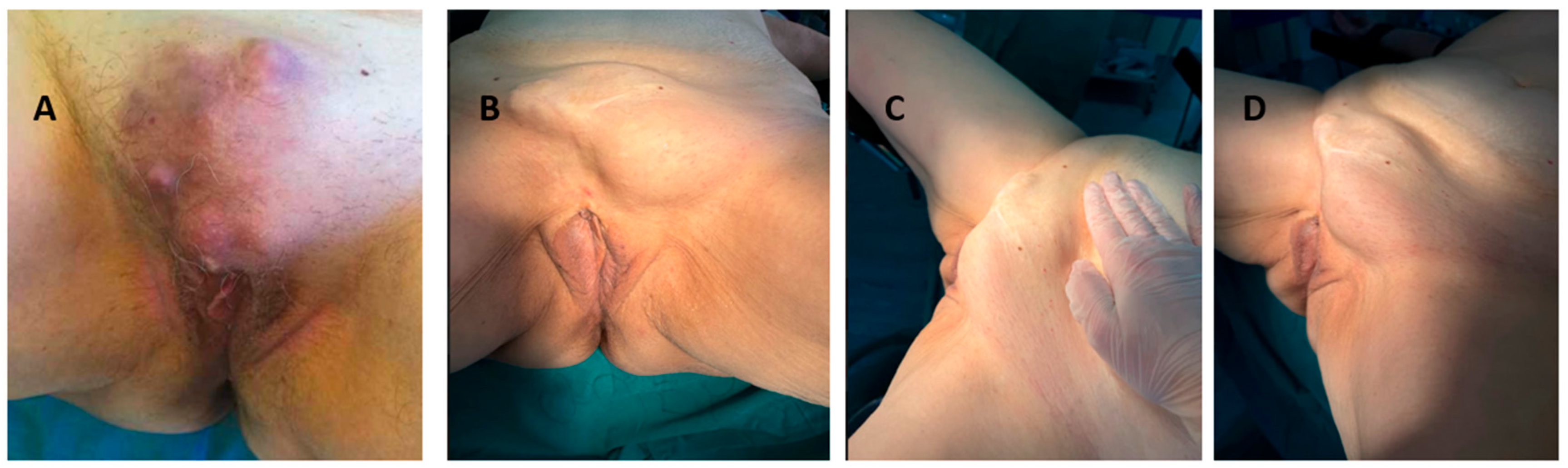
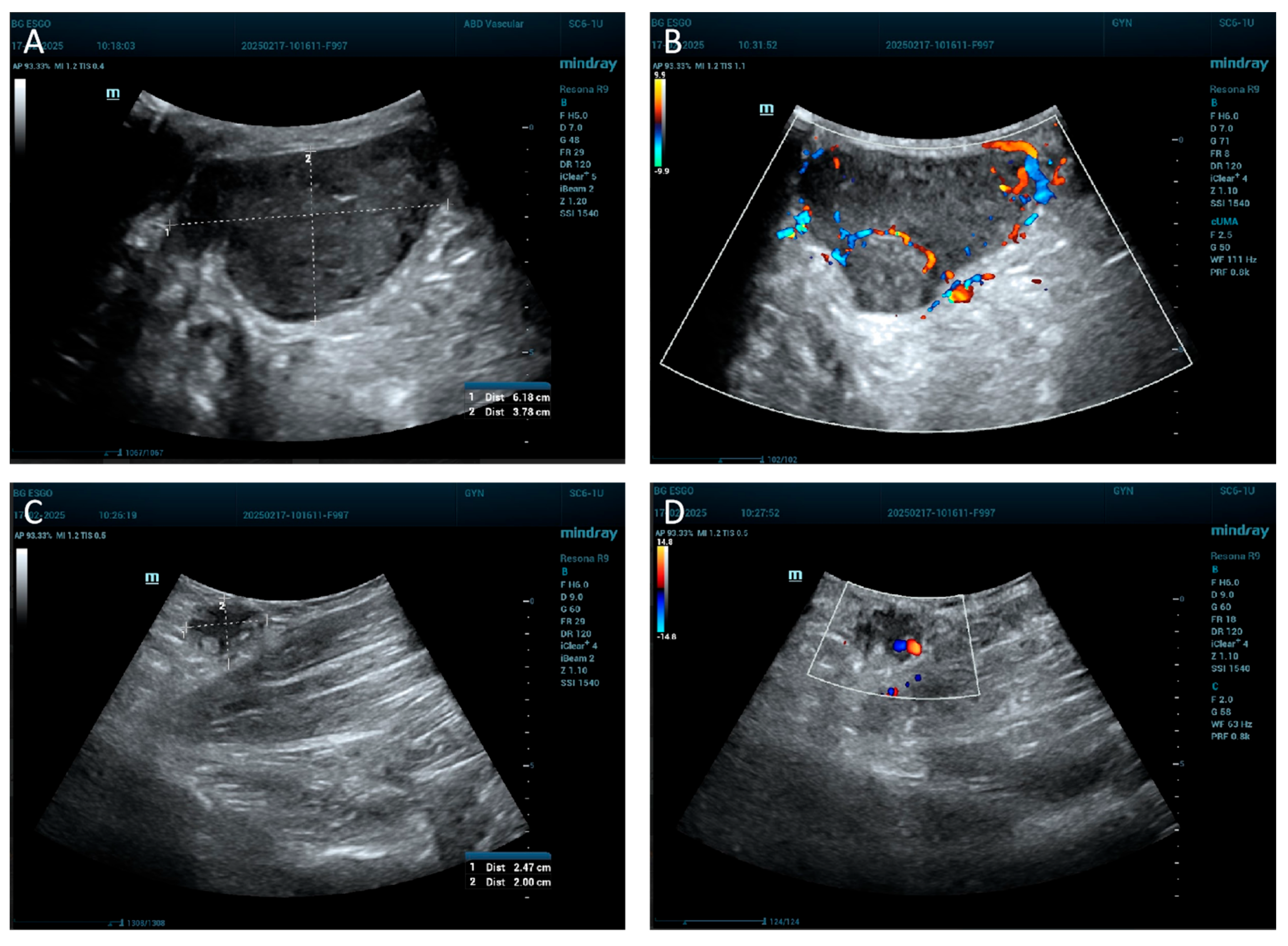
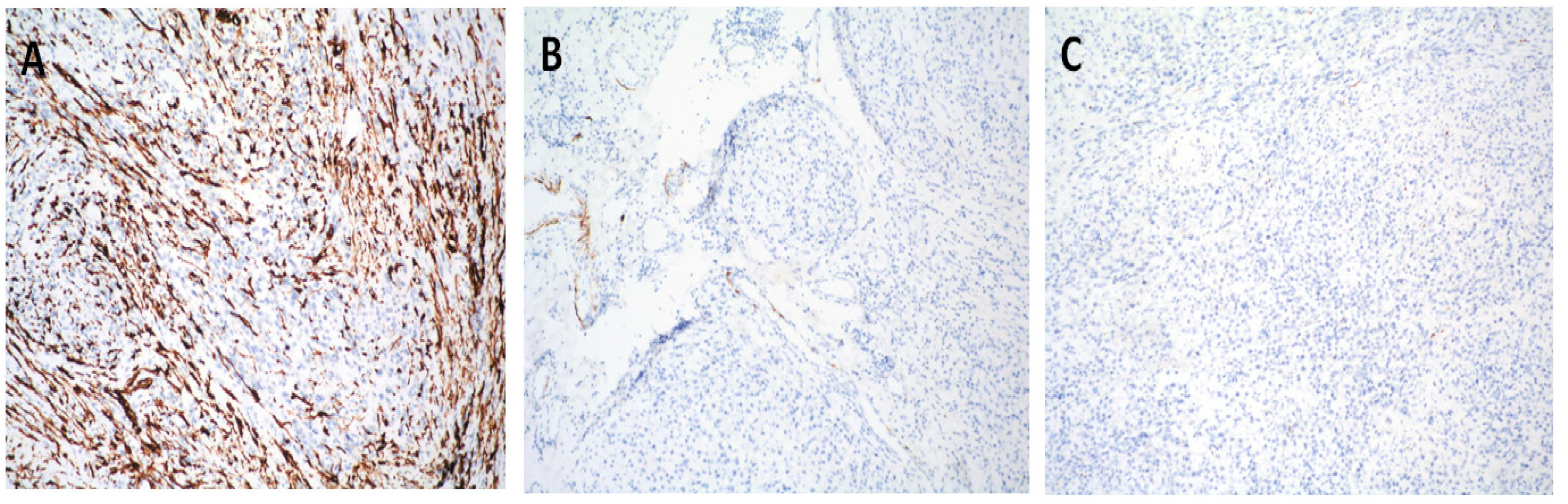
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chokoeva, A.A.; Tchernev, G.; Cardoso, J.C.; Patterson, J.W.; Dechev, I.; Valkanov, S.; Zanardelli, M.; Lotti, T.; Wollina, U. Vulvar sarcomas: Short guideline for histopathological recognition and clinical management. Part 1. Int. J. Immunopathol. Pharmacol. 2015, 28, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, V.; Kurdoğlu, Z.; Kardag, B.; Arslanca, T.; Caydere, M.; Ergun, Y. A rare case of leiomyosarcoma localized in the Bartholin’s gland area and review of the literature. J. Obstet. Gynaecol. Res. 2016, 42, 589–592. [Google Scholar] [CrossRef]
- Yordanov, A.; Slavchev, S.; Kostov, S.; Strashilov, S.; Ivanov, I.; Nikolova, M. Leiomyosarcoma of the vulva: A case report. Prz Menopauzalny 2020, 19, 184–187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Audet-Lapointe, P.; Paquin, F.; Guerard, M.J.; Charbonneau, A.; Methot, F.; Morand, G. Leiomyosarcoma of the vulva. Gynecol. Oncol. 1980, 10, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Smit, W.L.; Knobel, J.; van der Merwe, J.V. Leiomioom en leiomiosarkoom van die vulva [Leiomyoma and leiomyosarcoma of the vulva]. S Afr. Med. J. 1984, 66, 961–962. (In Afrikaans) [Google Scholar] [PubMed]
- Krag Møller, L.B.; Nygaard Nielsen, M.; Trolle, C. Leiomyosarcoma vulvae. Acta Obstet. Gynecol. Scand. 1990, 69, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Kuller, J.A.; Zucker, P.K.; Peng, T.C. Vulvar leiomyosarcoma in pregnancy. Am. J. Obstet. Gynecol. 1990, 162, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Bakri, Y.N.; Akhtar, M.; el-Senoussi, M.; Wierzbicki, R. Vulvar sarcoma: A report of four cases. Gynecol. Oncol. 1992, 46, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kapadia, A.; Desai, A.; Dave, K.S. Leiomyosarcoma of the vulva. Eur. J. Gynaecol. Oncol. 1993, 14, 406–407. [Google Scholar] [PubMed]
- Dhar, K.K.; Sau, A.K.; Dey, P.; Vasishta, K. Leiomyosarcoma of the labium minus. Int. J. Gynaecol. Obstet. 1994, 44, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, O.; Huntrakoon, M.; Collins, J.; Owiety, T.; Seoud, M.A.; Weed, J., Jr. Leiomyosarcoma of the vulva: Report of a case. Gynecol. Oncol. 1994, 54, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Lösch, A.; Joura, E.A.; Stani, J.; Breitenecker, G.; Lahodny, J. Leiomyosarcoma of the vulva, A case report. J. Reprod. Med. 2001, 46, 609–612. [Google Scholar] [PubMed]
- Rawal, N.; Saridogan, E.; Khan, N.; Weekes, A. Leiomyosarcoma of the vulva in association with lichen sclerosus. J. Obstet. Gynaecol. 2005, 25, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, G.; Adonakis, G.; Ravazoula, P.; Tsapanos, V.; Kourounis, G. Leiomyosarcoma of the vulva: A case report. Eur. J. Gynaecol. Oncol. 2005, 26, 577–578. [Google Scholar] [PubMed]
- Dewdney, S.; Kennedy, C.M.; Galask, R.P. Leiomyosarcoma of the vulva: A case report. J. Reprod. Med. 2005, 50, 630–632. [Google Scholar] [PubMed]
- Shankar, S.; Todd, P.M.; Rytina, E.; Crawford, R.A. Leiomyosarcoma of the vulva. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 116–117. [Google Scholar] [CrossRef] [PubMed]
- González-Bugatto, F.; Añón-Requena, M.J.; López-Guerrero, M.A.; Báez-Perea, J.M.; Bartha, J.L.; Hervías-Vivancos, B. Vulvar leiomyosarcoma in Bartholin’s gland area: A case report and literature review. Arch. Gynecol. Obstet. 2009, 279, 171–174. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, M.; Pintilie, H.; Wilkinson, N.; Lane, G.; Orton, J.; El-Ghobashy, A. A rare case of vulval leiomyosarcoma: Management and an updated review of the literature. J. Obstet. Gynaecol. 2011, 31, 675–676. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.A.; Winham, W.M.; Bryant, C.S.; Quick, C.M. Smooth muscle neoplasms of the vulva masquerading as Bartholin gland duct cysts. Proc. (Bayl. Univ. Med. Cent.) 2014, 27, 25–27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alnafisah, F.; Alfieri, J. Lung Metastasis in a Case of Recurrent Poorly Differentiated Leiomyosarcoma of the Bartholin Gland: A Case Report and Review of the Literature. Cureus 2016, 8, e550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saquib, S.; Keloth, T.R. Letter in Reply: Leiomyosarcoma of the Vulva Mimicking as Chronic Bartholin Cyst: A Case Report. Oman Med. J. 2021, 36, e258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aljehani, A.M.; Quatei, A.; Qattea, L.; Aljohani, R.M.; Alkushi, A. Vulvar Leiomyosarcoma in Pregnancy. Cureus 2021, 13, e18772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reinicke, T.; Anderson, D.J.; Kumar, D.; Griggs, C. Vulvar Leiomyosarcoma Masquerading as a Bartholin’s Gland Cyst in an Adolescent. Cureus 2022, 14, e21674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Capalbo, G.; Logoteta, A.; Gallo, R.; Cuccu, I.; Gentile, G.; Arienzo, F.; Musella, A.; Pernazza, A.; Perniola, G.; Di Donato, V.; et al. Chemo-surgical approach in vulvar leiomyosarcoma: A case report. Tumori 2022, 108, NP26–NP29. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Singh, A.; Bhatla, N.; Mathur, S. Primary leiomyosarcoma of the vulva—A rare occurrence. Pol. J. Pathol. 2023, 74, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bae, H.; Kim, H.-S. Dedifferentiation in uterine leiomyosarcoma: Diagnostic pitfalls and emerging molecular insights. Diagnostics 2024, 14, 160. [Google Scholar] [CrossRef]



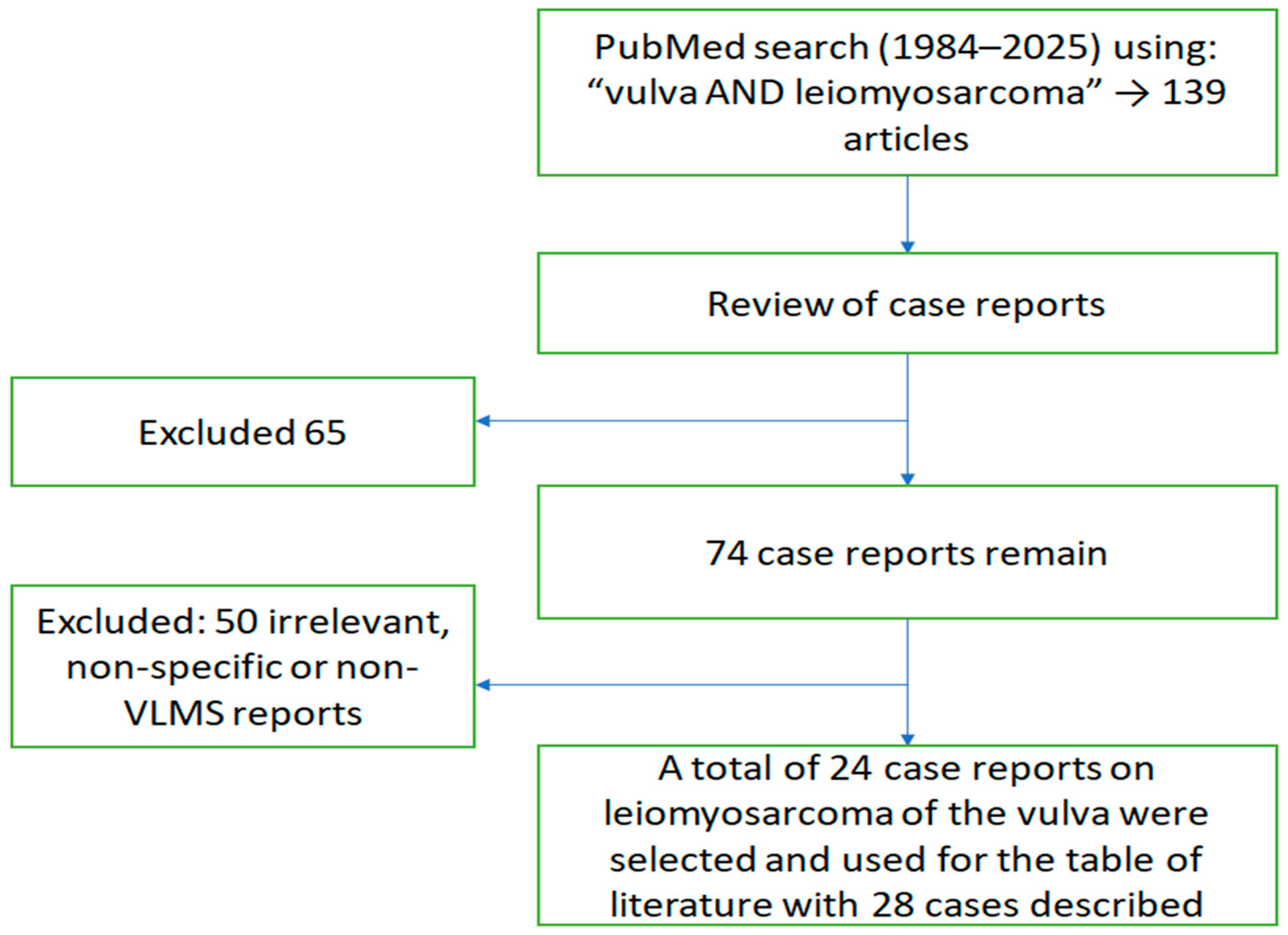
| № | Author/Year | Age | Location | Symptoms | Initial Tumor Size (cm) | Recurrence Size (cm) | Primary Treatment | Recurrence Treatment | Recurrence (Yes/No) | Follow-Up (months) | Metastasis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Audet-Lapointe et al., 1980 [4] | 48 | Left labium majus | Painless growing nodule | 2 × 4 | 7 × 2 → 3 | TE | TE, Radical Vulvectomy + BILND | Yes (2×) | 24 | No |
| 2 | Smith et al., 1984 [5] | 49 | n.a. | n.a. | 7 | n.a. | WLE + RT | Not specified | Yes | 18 | No |
| 3 | KragMoller et al., 1990 [6] | 54 | Left labium majus | Growing tender swelling | 8 × 7 × 4 | - | TE, Radical Vulvectomy + BILND | - | No | 30 | No |
| 4 | Kuller et al., 1990 [7] | 17 | Hymeneal ring, left | No | 4 × 4 × 4 | - | TE, WLE, BLINK | - | No | 20 | No |
| 5 | Bakri et al., 1992 Case 1 [8] | 29 | Right labium majus | No | 9 | Multiple | WLE | 4 × WLE | Yes (4×) | 120 | Lung |
| 6 | Bakri et al., 1992 Case 2 [8] | 41 | Left labium majus | Painless growing nodule | 10 × 8 | Multiple | WLE | 3 × WLE | Yes (3×) | 90 | Lung |
| 7 | Bakri et al., 1992 Case 3 [8] | 17 | Left labium majus | Lump | 10 × 15 | Multiple | WLE | 2 × WLE | Yes (2×) | 108- death | Lung |
| 8 | Bakri et al., 1992 Case 4 [8] | 20 | Left vulva/ groin | Pain/swelling | 8 × 10; 5 × 3 (ILN) | n.a. | WLE, Laparotomy | n.a. | n.a. | n.a. | Internal iliac nodes |
| 9 | Patel et al., 1993 [9] | n.a. | n.a. | n.a. | n.a. | n.a. | TE | Radical Vulvectomy, BLINK, WLE | Yes (1×) | n.a. | No |
| 10 | Dhar et al., 1994 [10] | 45 | Left labium minus | Itching, pain | 4 × 6 × 3 | - | FNA ILN, Simple Vulvectomy | - | No | 12 | No |
| 11 | Tawfik et al., 1994 [11] | 52 | Right labia majora/minora | Pain, swelling | 12 × 15 | - | TE | - | No | 14 | No |
| 12 | Lösch et al., 2001 [12] | 38 | Vulva | Slowly growing mass | n.a. | - | TE | - | No | 24 | No |
| 13 | Rawal et al., 2005 [13] | 81 | Right vulva | Lichen sclerosus over 30 yr, 1 yr postmenopausal, bleeding | 5×2 | - | WLE | - | No | 9 | No |
| 14 | Androutsopoulos et al., 2005 [14] | 55 | Right labia majora | 5 yr enlargement | 8×9 | n.a. | n.a. | n.a. | n.a. | 6 (Death) | Multiple |
| 15 | Dewdney et al., 2005 [15] | 36 | Vulva | Slowly growing painless mass | n.a. | - | Modified Radical Vulvectomy | - | No | 13 | No |
| 16 | Shankar et al., 2006 [16] | 58 | Right vulva | Enlarging asymptomatic lump | 3 | - | TE | - | No | 42 | No |
| 17 | Gonzalez-Bugatto et al., 2009 [17] | 52 | Left Bartholin gland area | Painless nodule rapid growth | 6 | 3–4 | Excision → Hemivulvectomy+ ILND → Adjuvant RT + Chemo | WLE | Yes | 48 | No |
| 18 | McKenzie et al., 2011 [18] | 45 | Right labium majus | n.a. | n.a. | - | TE | - | No | 24 | No |
| 19 | Levy et al., 2014 Case 1 [19] | 50 | Left labia inBartholin area | No | 4×6 | n.a. | TE | - | n.a. | n.a. | n.a. |
| 20 | Levy et al., 2014 Case 2 [19] | 57 | Bartholin area | No | 4×2 | n.a. | TE | - | n.a. | n.a. | n.a. |
| 21 | Alnafisah et al., 2016 [20] | 37 | Left vulva | Rapid enlarging mass | 5.0 × 3.9 × 2.9 | Lung | WLE → Radical Vulvectomy + ILND → Chemo → Brachytherapy + EBRT | Lung lobectomy + Chemo | Yes | 24+ | Lung |
| 22 | Korkmaz et al., 2016 [2] | 65 | Left vulvar Bartholin area | Enlarging vulvar mass | 5 × 6 | - | TE | - | No | 6 | No |
| 23 | Saquib et al., 2020 [21] | 63 | Left labia majora | Painless swelling | 2.8 × 2.4× 1.4 | - | TE | - | No | 6 | No |
| 24 | Yordanov et al., 2020 [3]/Present Case 2025 | 73/78 | Symphysis | Pain, rapid growth | 7 × 5 | 15×10 | WLE | Re-excision + LND | Yes | 60 | No |
| 25 | Aljehani et al., 2021 [22] | 38 (pregnant) | Left labia majora/minora | Vulvar mass | 15 × 10 | n.a. | TE | - | No | 12 | No |
| 26 | Reinicke et al., 2022 [23] | 14 | Left labia majora | Growing mass | 6 × 6 | n.a. | TE | n.a. | n.a. | n.a. | n.a. |
| 27 | Capalbo et al., 2022 [24] | 74 | Vulva | n.a. | 12.5 | n.a. | Neoadjuvant Chemo → Radical Vulvectomy → Chemo | - | No | 12 | No |
| 28 | Rathore et al., 2023 [25] | 47 | Left lower of the vulva | Vulvar swelling | 8 × 8 | n.a. | Radical Vulvectomy | - | No | 8 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yordanov, A.; Ivanov, I.; Kostov, S.; Hasan, I.; Dimitrova, V. Late Recurrence of High-Grade Vulvar Leiomyosarcoma After 5 Years: A Rare Case Report and Expanded Review of Reported Cases. J. Clin. Med. 2025, 14, 6032. https://doi.org/10.3390/jcm14176032
Yordanov A, Ivanov I, Kostov S, Hasan I, Dimitrova V. Late Recurrence of High-Grade Vulvar Leiomyosarcoma After 5 Years: A Rare Case Report and Expanded Review of Reported Cases. Journal of Clinical Medicine. 2025; 14(17):6032. https://doi.org/10.3390/jcm14176032
Chicago/Turabian StyleYordanov, Angel, Ivan Ivanov, Stoyan Kostov, Ihsan Hasan, and Vasilena Dimitrova. 2025. "Late Recurrence of High-Grade Vulvar Leiomyosarcoma After 5 Years: A Rare Case Report and Expanded Review of Reported Cases" Journal of Clinical Medicine 14, no. 17: 6032. https://doi.org/10.3390/jcm14176032
APA StyleYordanov, A., Ivanov, I., Kostov, S., Hasan, I., & Dimitrova, V. (2025). Late Recurrence of High-Grade Vulvar Leiomyosarcoma After 5 Years: A Rare Case Report and Expanded Review of Reported Cases. Journal of Clinical Medicine, 14(17), 6032. https://doi.org/10.3390/jcm14176032








