Retinal Vessel Coronal Displacement in Intermediate Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Optical Coherence Tomography Image Acquisition
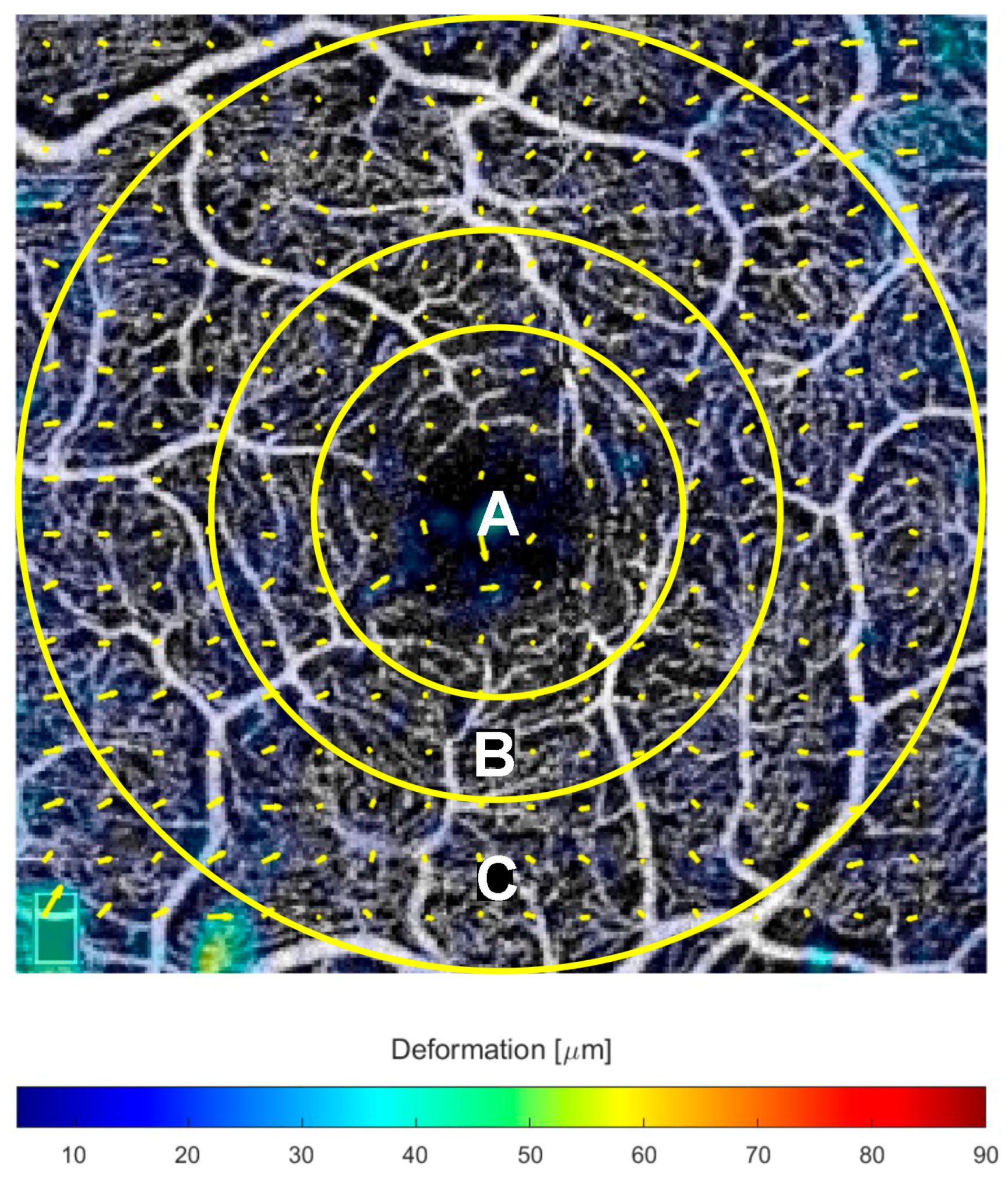
2.3. Displacement Measures
2.4. Statistical Analysis
3. Results
3.1. En-Face Retinal Displacement
3.2. Qualitative Features Associated with a Tangential Vascular Displacement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| CC | Choriocapillaris |
| DCP | Deep capillary plexus |
| dPED | Drusenoid pigment epithelial detachment |
| OCTA | Optical coherence tomography angiography |
| RPD | Reticular pseudodrusen |
| SDD | Subretinal drusenoid deposits |
| SCP | Superficial capillary plexus |
References
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Lutty, G.A.; McLeod, D.S.; Bhutto, I.A.; Edwards, M.M.; Seddon, J.M. Choriocapillaris dropout in early age-related macular degeneration. Exp. Eye Res. 2020, 192, 107939. [Google Scholar] [CrossRef] [PubMed]
- Biesemeier, A.; Taubitz, T.; Julien, S.; Yoeruek, E.; Schraermeyer, U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol. Aging 2014, 35, 2562–2573. [Google Scholar] [CrossRef]
- Sacconi, R.; Corbelli, E.; Borrelli, E.; Capone, L.; Carnevali, A.; Gelormini, F.; Querques, L.; Bandello, F.; Querques, G. Choriocapillaris flow impairment could predict the enlargement of geographic atrophy lesion. Br. J. Ophthalmol. 2021, 105, 97–102. [Google Scholar] [CrossRef]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Toto, L.; Borrelli, E.; Mastropasqua, R.; Di Antonio, L.; Doronzo, E.; Carpineto, P.; Mastropasqua, L. Association between outer retinal alterations and microvascular changes in intermediate stage age-related macular degeneration: An optical coherence tomography angiography study. Br. J. Ophthalmol. 2017, 101, 774–779. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Rabiolo, A.; Sacconi, R.; Lamanna, F.; Querques, L.; Bandello, F.; Querques, G. Retinal vascular alterations in reticular pseudodrusen with and without outer retinal atrophy assessed by optical coherence tomography angiography. Br. J. Ophthalmol. 2018, 102, 1192–1198. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Fragiotta, S.; Bassis, L.; Abdolrahimzadeh, B.; Marino, A.; Sepe, M.; Abdolrahimzadeh, S. Exploring Current Molecular Targets in the Treatment of Neovascular Age-Related Macular Degeneration toward the Perspective of Long-Term Agents. Int. J. Mol. Sci. 2024, 25, 4433. [Google Scholar] [CrossRef]
- Schierling, W.; Troidl, K.; Troidl, C.; Schmitz-Rixen, T.; Schaper, W.; Eitenmüller, I.K. The role of angiogenic growth factors in arteriogenesis. J. Vasc. Res. 2009, 46, 365–374. [Google Scholar] [CrossRef]
- Sacconi, R.; Fragiotta, S.; Sarraf, D.; Sadda, S.R.; Freund, K.B.; Parravano, M.; Corradetti, G.; Cabral, D.; Capuano, V.; Miere, A.; et al. Towards a better understanding of non-exudative choroidal and macular neovascularization. Prog. Retin. Eye Res. 2022, 92, 101113. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, X.; Zhu, W.; Cai, W.J.; Schaper, J.; Schaper, W. Immunohistochemical study of the growth factors, aFGF, bFGF, PDGF-AB, VEGF-A and its receptor (Flk-1) during arteriogenesis. Mol. Cell. Biochem. 2010, 343, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Birol, G.; Wang, S.; Budzynski, E.; Wangsa-Wirawan, N.D.; Linsenmeier, R.A. Oxygen distribution and consumption in the macaque retina. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1696–H1704. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J. Neovascular Remodeling and Subretinal Fibrosis as Biomarkers for Predicting Incomplete Response to Anti-VEGF Therapy in Neovascular Age-Related Macular Degeneration. Front. Biosci. 2022, 27, 135. [Google Scholar] [CrossRef]
- Savastano, M.C.; Rizzo, C.; Gambini, G.; Savastano, A.; Falsini, B.; Bacherini, D.; Caputo, C.G.; Kilian, R.; Faraldi, F.; De Vico, U.; et al. Choriocapillaris Vascular Density Changes: Healthy vs. Advanced Exudative Age-Related Macular Degeneration Previously Treated with Multiple Anti-VEGF Intravitreal Injections. Diagnostics 2021, 11, 1958. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Yanagi, Y.; Agrawal, R.; Teo, H.Y.; Seen, S.; Yeo, I.Y.S.; Mathur, R.; Chan, C.M.; Lee, S.Y.; Wong, E.Y.M.; et al. Choroidal Remodeling in Age-related Macular Degeneration and Polypoidal Choroidal Vasculopathy: A 12-month Prospective Study. Sci. Rep. 2017, 7, 7868. [Google Scholar] [CrossRef] [PubMed]
- Fragiotta, S.; Costanzo, E.; Viggiano, P.; De Geronimo, D.; Scuderi, G.; Varano, M.; Parravano, M. Functional Correlates of Outer Retina Remodeling in Intermediate Age-Related Macular Degeneration Using Microperimetry. Investig. Ophthalmol. Vis. Sci. 2022, 63, 16. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Mukwaya, A.; Biesemeier, A.; Ntzouni, M.; Ramskold, D.; Giatrellis, S.; Mammadzada, P.; Cao, R.; Lennikov, A.; Marass, M.; et al. Intussusceptive Vascular Remodeling Precedes Pathological Neovascularization. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1402–1418. [Google Scholar] [CrossRef]
- Rossi, T.; Querzoli, G.; Cosimi, P.; Ripandelli, G.; Steel, D.H.; Romano, M.R. TANGENTIAL RETINAL DISPLACEMENT INCREASES AFTER MACULAR PUCKER SURGERY: An Apparent Nonsense. Retina 2024, 44, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Scarinci, F.; Querzoli, G.; Cosimi, P.; Ripandelli, G.; Romano, M.R.; Cacciamani, A.; Munk, M.R.; Rossi, T. RETINAL TECTONICS AFTER MACULAR PUCKER SURGERY: Thickness Changes and En Face Displacement Recovery. Retina 2024, 44, 102–110. [Google Scholar] [CrossRef]
- Rossi, T.; Querzoli, G.; Ripandelli, G.; Placentino, L.; Parravano, M.; Steel, D.H.; Romano, M.R. RETINAL DISPLACEMENT AFTER IDIOPATHIC MACULAR HOLE SURGERY: Layer by Layer Analysis. Retina 2025, 45, 410–419. [Google Scholar] [CrossRef]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express 2012, 20, 4710–4725. [Google Scholar] [CrossRef]
- Campbell, J.P.; Zhang, M.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Jia, Y.; Huang, D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, 42201. [Google Scholar] [CrossRef]
- Faes, L.; Bijon, J.; Bacci, T.; Freund, K.B. Review of type 3 macular neovascularization in age-related macular degeneration: No DRAMA (Deep Retinal Age-related Microvascular Anomalies). Eye 2025, 39, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Zanzottera, E.C.; Ach, T.; Balaratnasingam, C.; Freund, K.B. Activated retinal pigment epithelium, an optical coherence tomography biomarker for progression in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO211–BIO226. [Google Scholar]
- Cabral, D.; Pereira, T.; Ledesma-Gil, G.; Rodrigues, C.; Coscas, F.; Sarraf, D.; Freund, K.B. Volume Rendering of Dense B-Scan Optical Coherence Tomography Angiography to Evaluate the Connectivity of Macular Blood Flow. Investig. Ophthalmol. Vis. Sci. 2020, 61, 44. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Cukras, C.; Agron, E.; Klein, M.L.; Ferris, F.L., 3rd; Chew, E.Y.; Gensler, G.; Wong, W.T.; Age-Related Eye Disease Study Research Group. Natural history of drusenoid pigment epithelial detachment in age-related macular degeneration: Age-Related Eye Disease Study Report No. 28. Ophthalmology 2010, 117, 489–499. [Google Scholar] [CrossRef]
- Farnebäck, G. Two-Frame Motion Estimation Based on Polynomial Expansion. In Proceedings of the Image Analysis, Halmstad, Sweden, 29 June–2 July 2003; pp. 363–370. [Google Scholar]
- Hartnett, M.E.; Weiter, J.J.; Staurenghi, G.; Elsner, A.E. Deep retinal vascular anomalous complexes in advanced age-related macular degeneration. Ophthalmology 1996, 103, 2042–2053. [Google Scholar] [CrossRef]
- Chen, L.; Messinger, J.D.; Kar, D.; Duncan, J.L.; Curcio, C.A. Biometrics, Impact, and Significance of Basal Linear Deposit and Subretinal Drusenoid Deposit in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2021, 62, 33. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Martens, C.; Kosanetzky, S.; Brinkmann, C.K.; Hageman, G.S.; Holz, F.G. Fundus autofluorescence and spectral-domain optical coherence tomography characteristics in a rapidly progressing form of geographic atrophy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3761–3766. [Google Scholar] [CrossRef]
- Fragiotta, S.; Dysli, C.; Parravano, M.; Sacconi, R.; Fantaguzzi, F.; Servillo, A.; Severo, A.A.; Tombolini, B.; Costanzo, E.; De Geronimo, D.; et al. Phenotypic characterization of predictors for development and progression of geographic atrophy using optical coherence tomography. Retina 2024, 44, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. New proposal for the pathophysiology of type 3 neovascularization as based on multimodal imaging findings. Retina 2019, 39, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.; Cabral, D.; Chen, L.; Messinger, J.D.; Balaratnasingam, C.; Mendis, R.; Ferrara, D.; Freund, K.B.; Curcio, C.A. Histology of Type 3 Macular Neovascularization and Microvascular Anomalies in Treated Age-Related Macular Degeneration: A Case Study. Ophthalmol. Sci. 2023, 3, 100280. [Google Scholar] [CrossRef]
- You, Q.S.; Wang, J.; Guo, Y.; Flaxel, C.J.; Hwang, T.S.; Huang, D.; Jia, Y.; Bailey, S.T. Detection of Reduced Retinal Vessel Density in Eyes with Geographic Atrophy Secondary to Age-Related Macular Degeneration Using Projection-Resolved Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2020, 209, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Guo, Y.; Hormel, T.T.; Wang, J.; White, E.; Park, D.W.; Hwang, T.S.; Bailey, S.T.; Jia, Y. Nonperfused Retinal Capillaries-A New Method Developed on OCT and OCTA. Investig. Ophthalmol. Vis. Sci. 2025, 66, 22. [Google Scholar] [CrossRef]
- Kalaw, F.G.P.; Alex, V.; Walker, E.; Bartsch, D.U.; Freeman, W.R.; Borooah, S. Inner Retinal Thickness and Vasculature in Patients with Reticular Pseudodrusen. Ophthalmic Res. 2023, 66, 885–891. [Google Scholar] [CrossRef]
- Abdolrahimzadeh, S.; Zweifel, S.A.; Di Pippo, M.; Bajka, A.; Scuderi, G.; Lotery, A.J. Central macular choriocapillaris impairment as a manifestation of microvascular disease in eyes with subretinal drusenoid deposits. Eye 2024, 38, 173–178. [Google Scholar] [CrossRef]
- Alten, F.; Heiduschka, P.; Clemens, C.R.; Eter, N. Exploring choriocapillaris under reticular pseudodrusen using OCT-Angiography. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 2165–2173. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Lu, J.; Shen, M.; Cheng, Y.; Siddiqui, N.; Zhou, H.; Zhang, Q.; Liu, J.; Herrera, G.; et al. Decreased Macular Choriocapillaris Perfusion in Eyes With Macular Reticular Pseudodrusen Imaged With Swept-Source OCT Angiography. Investig. Ophthalmol. Vis. Sci. 2023, 64, 15. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Nivison-Smith, L.; Trinh, M. Spatial Analysis Reveals Vascular Changes in Retinal and Choroidal Vessel Perfusion in Intermediate AMD With Reticular Pseudodrusen. Investig. Ophthalmol. Vis. Sci. 2024, 65, 33. [Google Scholar] [CrossRef]
- Trinh, M.; Eshow, N.; Alonso-Caneiro, D.; Kalloniatis, M.; Nivison-Smith, L. Reticular Pseudodrusen Are Associated With More Advanced Para-Central Photoreceptor Degeneration in Intermediate Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2022, 63, 12. [Google Scholar] [CrossRef]
- Digsby, K.; Zhang, Q.; Miller, J.M.L. Basic science observations link subretinal drusenoid deposit formation to retinal pigment epithelial hypoxia. Eye 2025, 39, 790–792. [Google Scholar] [CrossRef]
- Curcio, C.A.; Goerdt, L. A cell culture system for RPE hypoxia, a physiologic stressor relevant to AMD deposit formation. Eye 2025, 39, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Trinh, M.; Kalloniatis, M.; Nivison-Smith, L. Vascular Changes in Intermediate Age-Related Macular Degeneration Quantified Using Optical Coherence Tomography Angiography. Transl. Vis. Sci. Technol. 2019, 8, 20. [Google Scholar] [CrossRef]
- Corvi, F.; Tiosano, L.; Corradetti, G.; Nittala, M.G.; Lindenberg, S.; Alagorie, A.R.; McLaughlin, J.A.; Lee, T.K.; Sadda, S.R. Choriocapillaris Flow Deficit as a risk factor for progression of Age-Related Macular Degeneration. Retina 2020, 41, 686–693. [Google Scholar] [CrossRef]
- Nassisi, M.; Baghdasaryan, E.; Borrelli, E.; Ip, M.; Sadda, S.R. Choriocapillaris flow impairment surrounding geographic atrophy correlates with disease progression. PLoS ONE 2019, 14, e0212563. [Google Scholar] [CrossRef]
- Kar, D.; Amjad, M.; Corradetti, G.; Swain, T.A.; Clark, M.E.; McGwin, G., Jr.; Sloan, K.R.; Owsley, C.; Sadda, S.R.; Curcio, C.A. Choriocapillaris Impairment, Visual Function, and Distance to Fovea in Aging and Age-Related Macular Degeneration: ALSTAR2 Baseline. Investig. Ophthalmol. Vis. Sci. 2024, 65, 40. [Google Scholar] [CrossRef]
- Berni, A.; Cheng, Y.; Shen, M.; El-Mulki, O.S.; Herrera, G.; Beqiri, S.; Kastner, J.D.; Zhang, Q.; Gregori, G.; Wang, R.K.; et al. Updated Guidelines for Imaging the Choriocapillaris in Eyes with Age-Related Macular Degeneration Using Swept-Source Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2025, 278, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Berni, A.; Foti, C.; Ulla, L.; Vyas, C.; Chhablani, J.; Chaudhary, V.; Subhi, Y.; Gregori, G.; Wang, R.K.; Rosenfeld, P.J.; et al. Choriocapillaris in Age-Related Macular Degeneration: A Systematic Review of Optical Coherence Tomography Angiography-Based Assessments and Challenges in Standardization. Am. J. Ophthalmol. 2025, 277, 482–496. [Google Scholar] [CrossRef]
- Balaratnasingam, C.; Yannuzzi, L.A.; Curcio, C.A.; Morgan, W.H.; Querques, G.; Capuano, V.; Souied, E.; Jung, J.; Freund, K.B. Associations between retinal pigment epithelium and drusen volume changes during the lifecycle of large drusenoid pigment epithelial detachments. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5479–5489. [Google Scholar] [CrossRef]
- Hilely, A.; Au, A.; Freund, K.B.; Loewenstein, A.; Souied, E.H.; Zur, D.; Sacconi, R.; Borrelli, E.; Peiretti, E.; Iovino, C.; et al. Non-neovascular age-related macular degeneration with subretinal fluid. Br. J. Ophthalmol. 2020, 105, 1415–1420. [Google Scholar] [CrossRef]
- Dolz-Marco, R.; Balaratnasingam, C.; Gattoussi, S.; Ahn, S.; Yannuzzi, L.A.; Freund, K.B. Long-term Choroidal Thickness Changes in Eyes With Drusenoid Pigment Epithelium Detachment. Am. J. Ophthalmol. 2018, 191, 23–33. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Grebe, R.; Bhutto, I.; Merges, C.; Baba, T.; Lutty, G.A. Relationship between RPE and choriocapillaris in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4982–4991. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; Ling, J.X.; Wallace, T.M.; Dithmar, S.; Lawson, D.H.; Cohen, C.; Elner, V.M.; Elner, S.G.; Sternberg, P., Jr. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol. Vis. 2002, 8, 119–126. [Google Scholar]
- Elner, S.G.; Strieter, R.M.; Elner, V.M.; Rollins, B.J.; Del Monte, M.A.; Kunkel, S.L. Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab. Investig. 1991, 64, 819–825. [Google Scholar]


| Time Points | ||||
|---|---|---|---|---|
| Variables | T0 | T6 | CI 95% | p (T0–T6) |
| BCVA (ETDRS letters) | 82.17 ± 4.54 | 81.78 ± 5 | −3.59, 2.81 | 0.80 |
| Snellen equivalent | 20/25 | 20/25 | - | - |
| Macular Thickness (µm) | 287.8 ± 32.3 | 287.9 ± 33.4 | −16.49, 16.69 | 0.99 |
| Vascular Displacement (µm) | ||||||
|---|---|---|---|---|---|---|
| Radius | SCP | 95% CI | DCP | 95% CI | CC | 95% CI |
| <0.5 mm | 11.9 ± 7.6 | 8.1–15.7 | 10.4 ± 6.3 | 7.3–13.5 | 14.2 ± 9.2 | 9.6–18.8 |
| <0.75 mm | 11 ± 6.5 | 7.8–14.2 | 10.7 ± 5.9 | 7.8–13.6 | 14.9 ± 9.5 | 10.2–19.6 |
| <1.5 mm | 12.7 ± 6.7 | 9.4–16 | 13.5 ± 7.9 | 9.6–17.4 | 17.6 ± 10.6 | 12.3–22.9 |
| 95% CI | ||||
|---|---|---|---|---|
| Microvascular Slabs | Estimate | SE | Lower | Upper |
| Superficial | 13.393 | 1.273 | 10.897 | 15.888 |
| Deep | 14.590 | 1.614 | 11.426 | 17.754 |
| Choriocapillaris | 18.517 | 1.182 | 16.200 | 20.834 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parravano, M.; Fragiotta, S.; Polito, M.S.; Varano, M.; Querzoli, G.; Rossi, T. Retinal Vessel Coronal Displacement in Intermediate Age-Related Macular Degeneration. J. Clin. Med. 2025, 14, 6030. https://doi.org/10.3390/jcm14176030
Parravano M, Fragiotta S, Polito MS, Varano M, Querzoli G, Rossi T. Retinal Vessel Coronal Displacement in Intermediate Age-Related Macular Degeneration. Journal of Clinical Medicine. 2025; 14(17):6030. https://doi.org/10.3390/jcm14176030
Chicago/Turabian StyleParravano, Mariacristina, Serena Fragiotta, Maria Sole Polito, Monica Varano, Giorgio Querzoli, and Tommaso Rossi. 2025. "Retinal Vessel Coronal Displacement in Intermediate Age-Related Macular Degeneration" Journal of Clinical Medicine 14, no. 17: 6030. https://doi.org/10.3390/jcm14176030
APA StyleParravano, M., Fragiotta, S., Polito, M. S., Varano, M., Querzoli, G., & Rossi, T. (2025). Retinal Vessel Coronal Displacement in Intermediate Age-Related Macular Degeneration. Journal of Clinical Medicine, 14(17), 6030. https://doi.org/10.3390/jcm14176030





