Revealing New Trends in the Global Burden of Hepatocellular Cancer Related to Hepatitis C Virus by Region, Sociodemographic Index, and Sex
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Data Analysis
3. Results
3.1. Global Trends
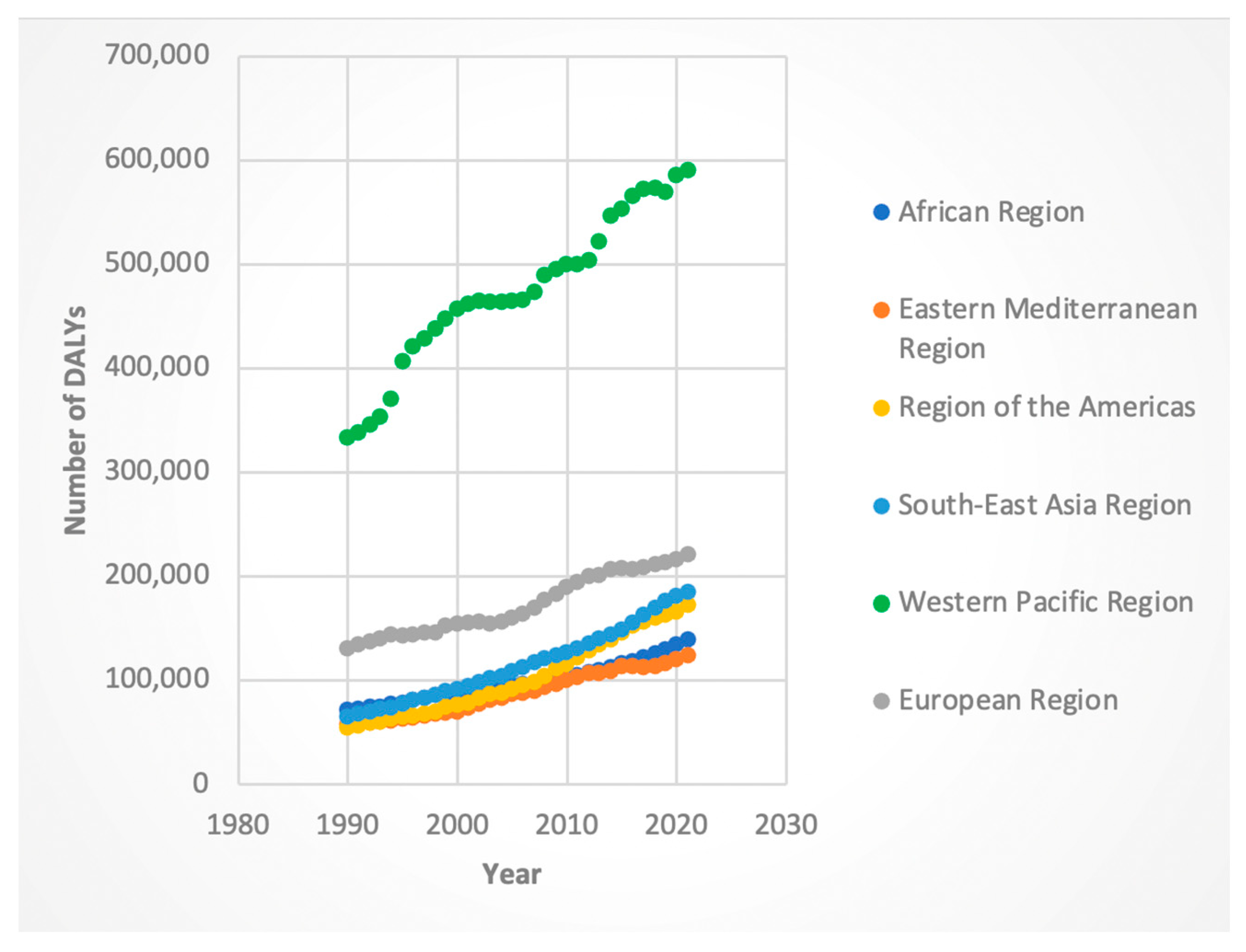
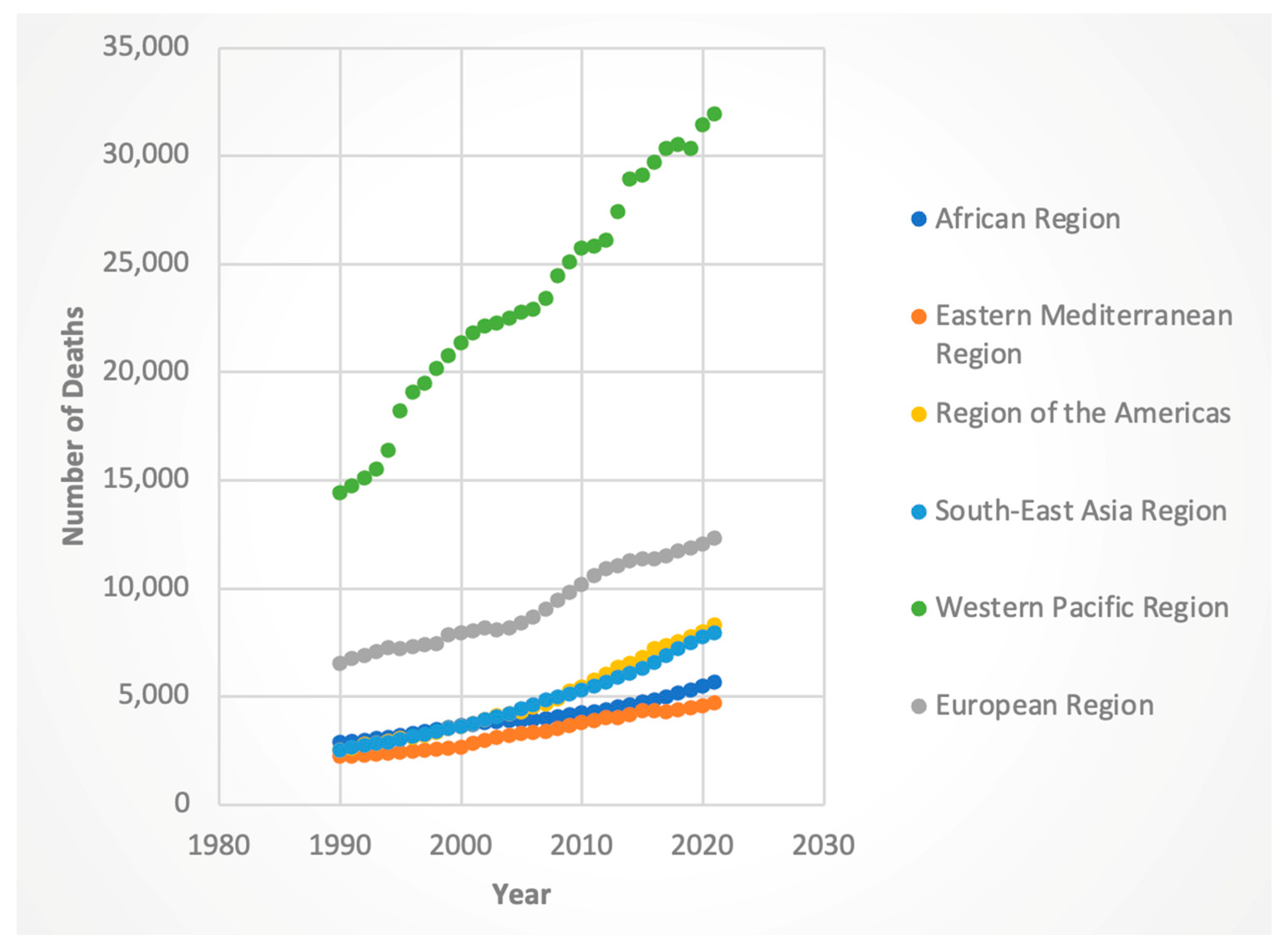
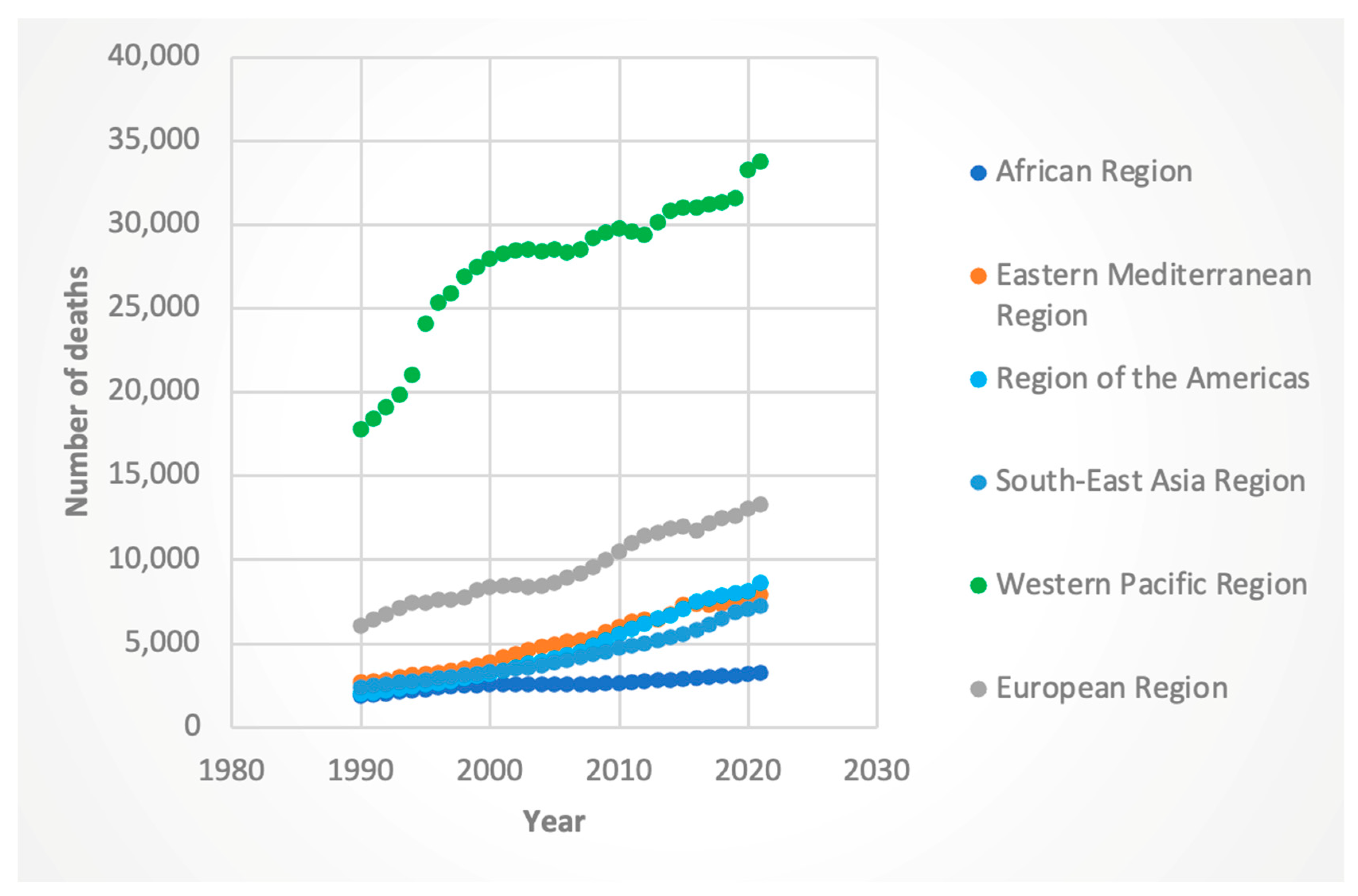
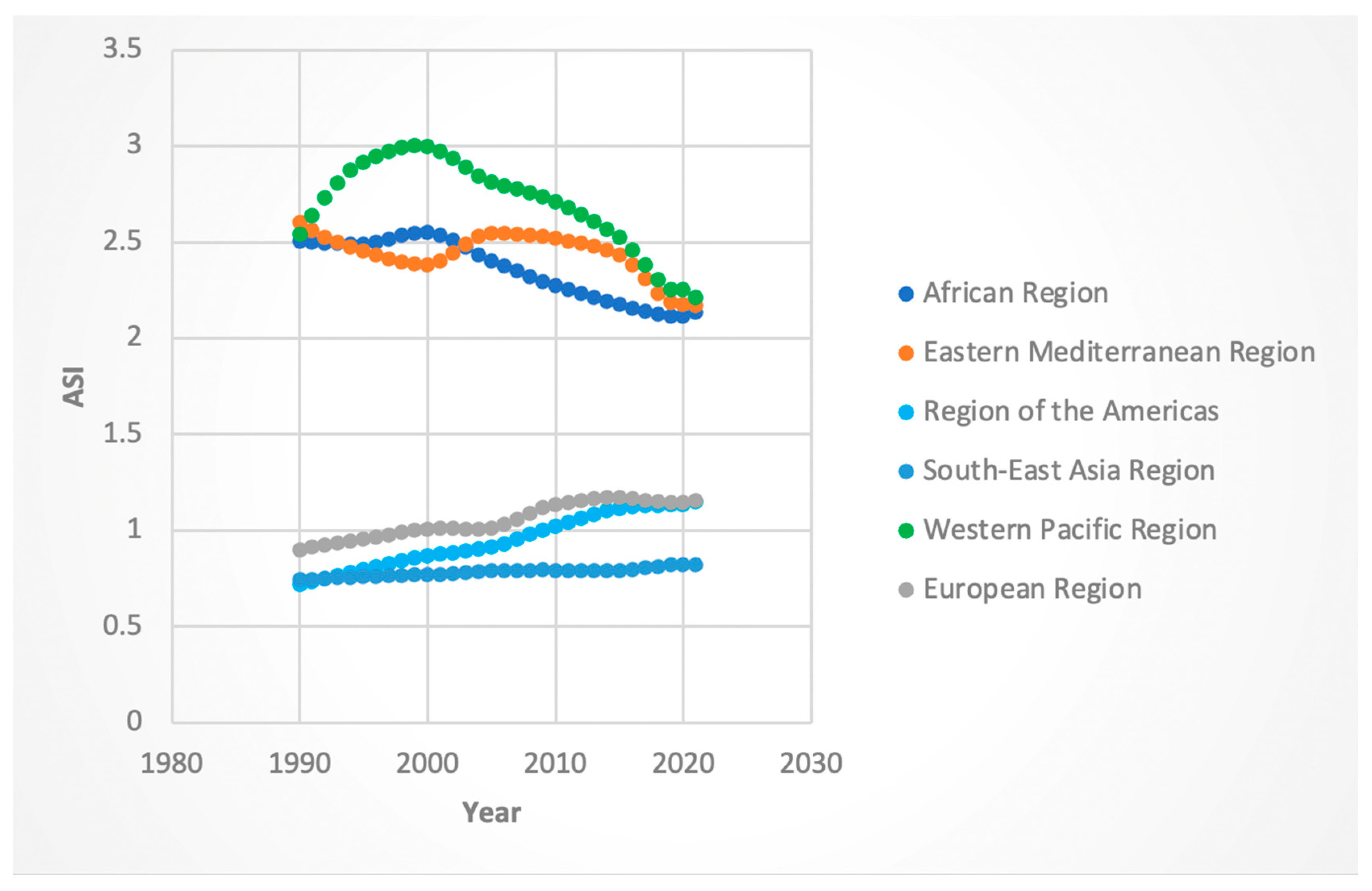
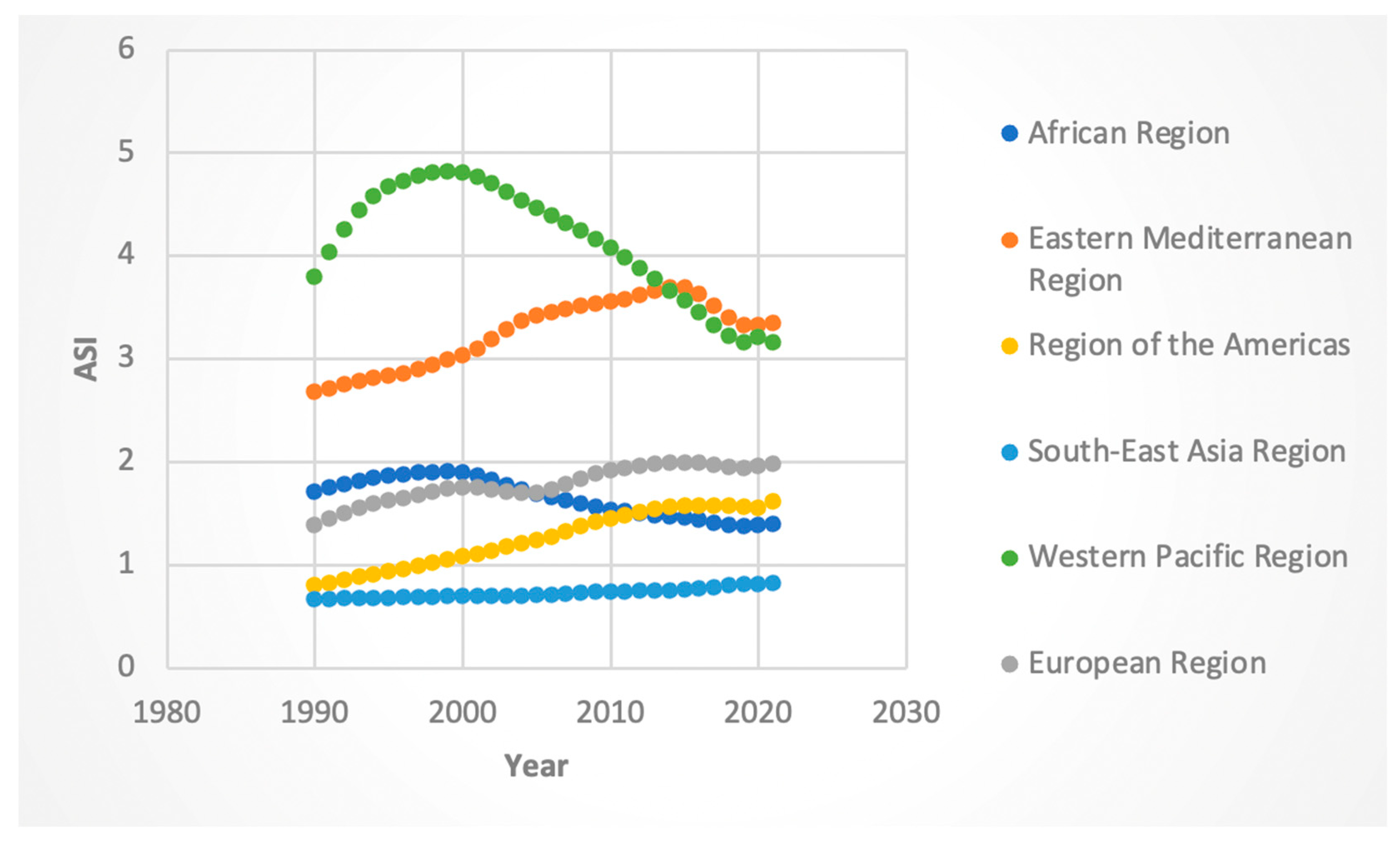
3.2. Trends by WHO Region
3.2.1. DALYs
3.2.2. Deaths
3.2.3. ASI Rates
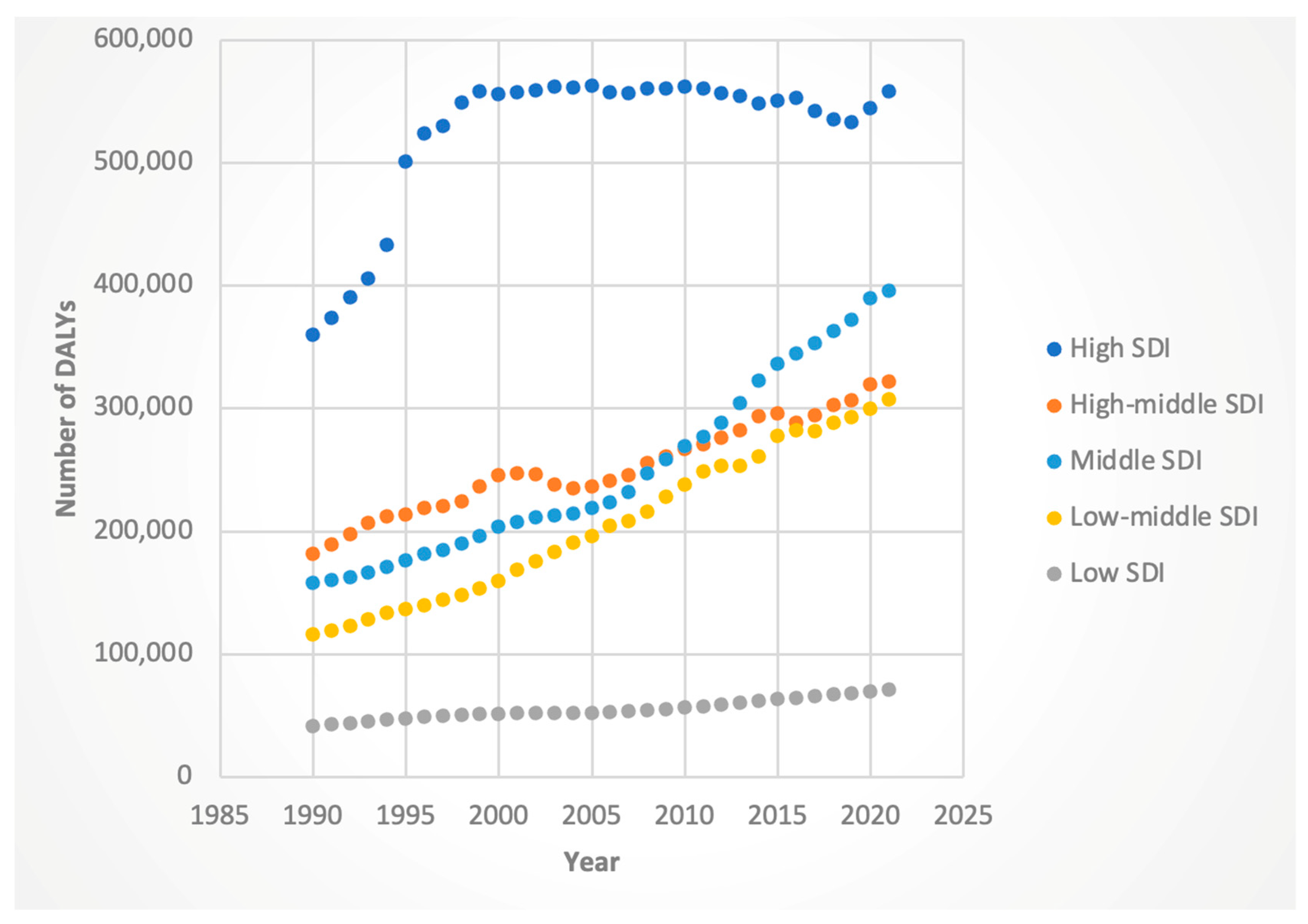
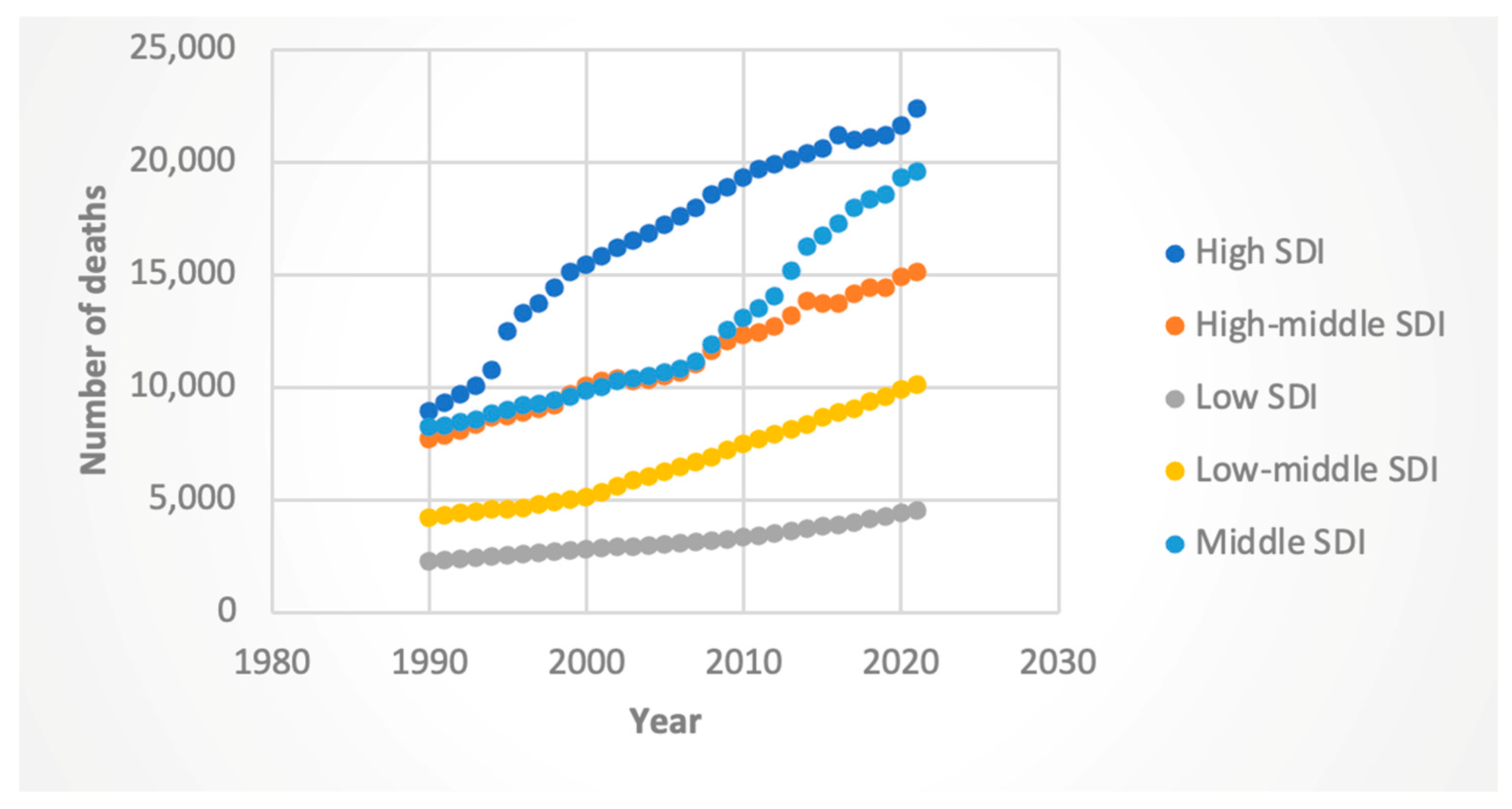
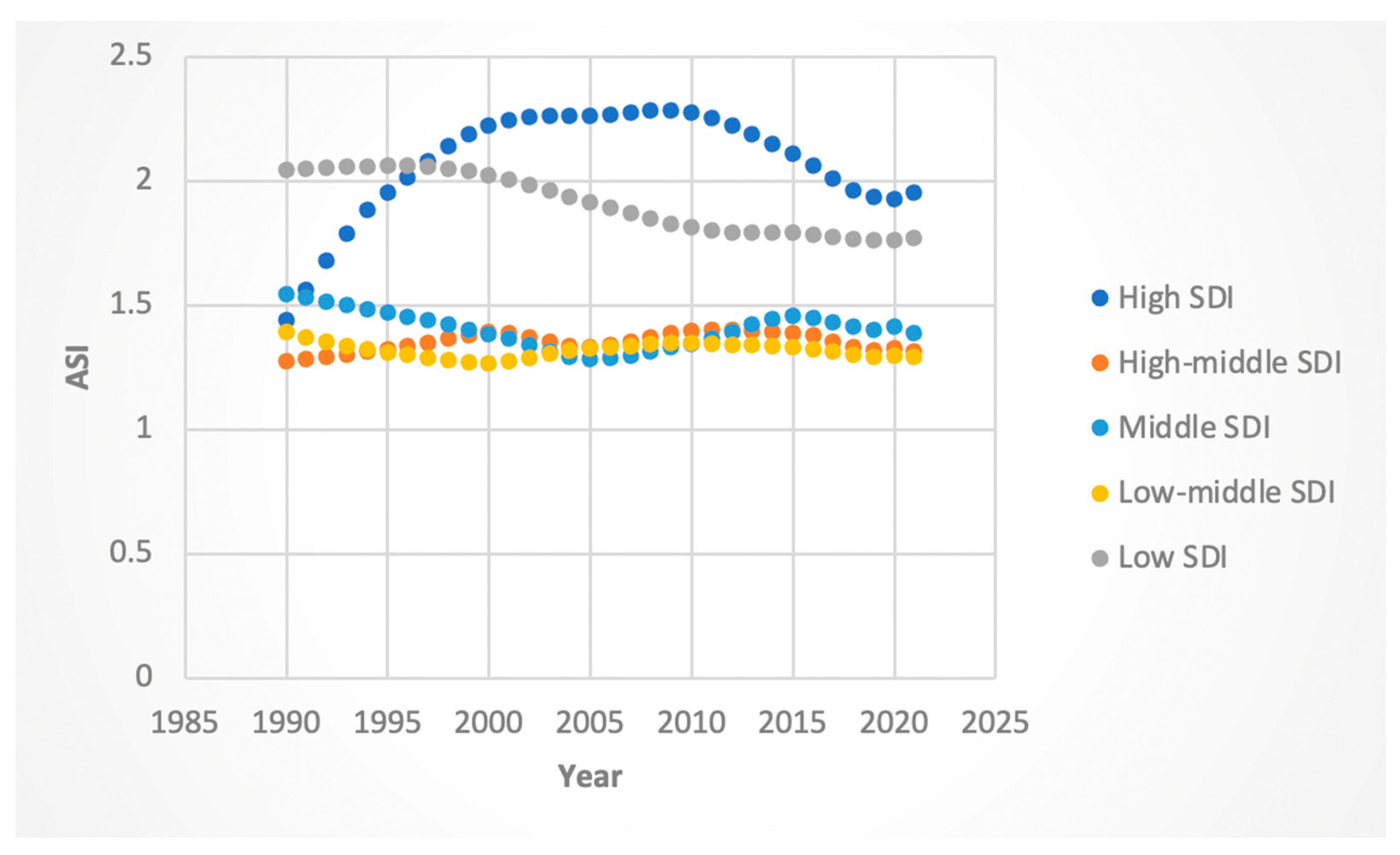
3.3. Trends by Sociodemographic Index (SDI)
3.3.1. DALYs
3.3.2. Deaths
3.3.3. ASI Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| DAA | Direct-acting antiviral |
| SVR | Sustained viral response |
| HCV-HCC | Hepatocellular cancer related to HCV |
| WHO | World Health Organization |
| SDI | Sociodemographic index |
| DALY | Disability-adjusted life year |
| GBD | Global Burden of Disease 2021 Study |
| ASI | Age-standardized incidence |
| APC | Annual percent change |
| AAPC | Average annual percent change |
| CI | Confidence interval |
References
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef]
- de Oliveria Andrade, L.J.; D’Oliveira, A.; Melo, R.C.; De Souza, E.C.; Costa Silva, C.A.; Paraná, R. Association Between Hepatitis C and Hepatocellular Carcinoma. J. Glob. Infect. Dis. 2009, 1, 33–37. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Chen, R.; Jia, C.; Liu, H.; Tang, M.; Zhou, S.; Fu, D.; Tang, X.; Chen, L.; et al. Global, Regional and National Burden of Chronic Hepatitis C, 1990–2021: A Systematic Analysis for the GBD Study 2021. J. Viral Hepat. 2025, 32, e70053. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Kamath, P.S. Model for End-stage Liver Disease. J. Clin. Exp. Hepatol. 2013, 3, 50–60. [Google Scholar] [CrossRef]
- Applegate, T.L.; Fajardo, E.; Sacks, J.A. Hepatitis C Virus Diagnosis and the Holy Grail. Infect. Dis. Clin. N. Am. 2018, 32, 425–445. [Google Scholar] [CrossRef] [PubMed]
- Nahon, P.; Bourcier, V.; Layese, R.; Audureau, E.; Cagnot, C.; Marcellin, P.; Guyader, D.; Fontaine, H.; Larrey, D.; De Lédinghen, V.; et al. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology 2017, 152, 142–156.e2. [Google Scholar] [CrossRef] [PubMed]
- Farhang Zangneh, H.; Wong, W.W.L.; Sander, B.; Bell, C.M.; Mumtaz, K.; Kowgier, M.; van der Meer, A.J.; Cleary, S.P.; Janssen, H.L.A.; Chan, K.K.W.; et al. Cost Effectiveness of Hepatocellular Carcinoma Surveillance After a Sustained Virologic Response to Therapy in Patients With Hepatitis C Virus Infection and Advanced Fibrosis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 1840–1849.e16. [Google Scholar] [CrossRef]
- Yew, K.C.; Tan, Q.R.; Lim, P.C.; Low, W.Y.; Lee, C.Y. Assessing the impact of direct-acting antivirals on hepatitis C complications: A systematic review and meta-analysis. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 1421–1431. [Google Scholar] [CrossRef]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922. [Google Scholar] [CrossRef]
- Wei, J.; Ouyang, G.; Huang, G.; Wang, Y.; Li, S.; Liu, J.; Zhang, Y.; Yuan, G.; He, S. Burden of liver cancer due to hepatitis C from 1990 to 2019 at the global, regional, and national levels. Front. Oncol. 2023, 13, 1218901. [Google Scholar] [CrossRef]
- Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis. Available online: https://www.who.int/publications/i/item/WHO-HIV-2016.06 (accessed on 4 February 2025).
- Joinpoint Regression Program. Available online: https://surveillance.cancer.gov/joinpoint/index.html (accessed on 1 June 2025).
- Calvaruso, V.; Craxì, A. Hepatic benefits of HCV cure. J. Hepatol. 2020, 73, 1548–1556. [Google Scholar] [CrossRef]
- Kåberg, M.; Weiland, O. Hepatitis C elimination—Macro-elimination. Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40 (Suppl. S1), 61–66. [Google Scholar] [CrossRef]
- Hiebert-Suwondo, L.; Manning, J.; Tohme, R.A.; Buti, M.; Kondili, L.A.; Spearman, C.W.; Hajarizadeh, B.; Turnier, V.; Lazarus, J.V.; Grebely, J.; et al. A 2024 global report on national policies, programmes, and progress towards hepatitis C elimination: Findings from 33 hepatitis elimination profiles. Lancet Gastroenterol. Hepatol. 2025, 10, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.L. Global Elimination of Chronic Hepatitis. N. Engl. J. Med. 2019, 380, 2041–2050. [Google Scholar] [CrossRef]
- Heffernan, A.; Cooke, G.S.; Nayagam, S.; Thursz, M.; Hallett, T.B. Scaling up prevention and treatment towards the elimination of hepatitis C: A global mathematical model. Lancet Lond. Engl. 2019, 393, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Handanagic, S.; Shadaker, S.; Drobeniuc, J.; Tsereteli, M.; Alkhazashvili, M.; Adesigbin, C.; Adamu, I.; Adabe, R.; Agwuocha, C.; Adisa, O.; et al. Lessons Learned From Global Hepatitis C Elimination Programs. J. Infect. Dis. 2024, 229, S334–S341. [Google Scholar] [CrossRef] [PubMed]
- Umutesi, J.; Yu, M.-L.; Lesi, O.; Ward, J.W.; Serumondo, J. Strategies for Removal of Barriers to Hepatitis C Elimination in Sub-Saharan Africa. J. Infect. Dis. 2023, 228, S221–S225. [Google Scholar] [CrossRef]
- Ward, J.W.; Hinman, A.R. What Is Needed to Eliminate Hepatitis B Virus and Hepatitis C Virus as Global Health Threats. Gastroenterology 2019, 156, 297–310. [Google Scholar] [CrossRef]
- Boerekamps, A.; van den Berk, G.E.; Lauw, F.N.; Leyten, E.M.; van Kasteren, M.E.; van Eeden, A.; Posthouwer, D.; Claassen, M.A.; Dofferhoff, A.S.; Verhagen, D.W.M.; et al. Declining Hepatitis C Virus (HCV) Incidence in Dutch Human Immunodeficiency Virus-Positive Men Who Have Sex with Men After Unrestricted Access to HCV Therapy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 66, 1360–1365. [Google Scholar] [CrossRef]
- Shiha, G.; Soliman, R.; Mikhail, N.N.H.; Easterbrook, P. An educate, test and treat model towards elimination of hepatitis C infection in Egypt: Feasibility and effectiveness in 73 villages. J. Hepatol. 2020, 72, 658–669. [Google Scholar] [CrossRef]
- Danpanichkul, P.; Suparan, K.; Sukphutanan, B.; Kaeosri, C.; Tothanarungroj, P.; Sirimangklanurak, S.; Kalligeros, M.; Polpichai, N.; Pang, Y.; Wijarnpreecha, K.; et al. Changes in the epidemiological trends of primary liver cancer in the Asia-Pacific region. Sci. Rep. 2024, 14, 19544. [Google Scholar] [CrossRef]
- Le, L.-V.; Blach, S.; Rewari, B.; Chan, P.; Fuqiang, C.; Ishikawa, N.; Sharma, M.; Mangadan-Konath, N.; Razavi, H.; Low-Beer, D.; et al. Progress towards achieving viral hepatitis B and C elimination in the Asia and Pacific region: Results from modelling and global reporting. Liver Int. Off. J. Int. Assoc. Study Liver 2022, 42, 1930–1934. [Google Scholar] [CrossRef]
- Pham, Y.T.-H.; Huang, D.Q.; Zhang, Z.; Ng, C.H.; Tan, D.J.H.; Nguyen, H.C.; Nguyen, T.C.; Behari, J.; Yuan, J.-M.; Luu, H.N. Changing global epidemiology of chronic hepatitis C virus-related outcomes from 2010 to 2019: Cirrhosis is the growing burden of hepatitis C virus-related disease. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2024, 33, 512–524. [Google Scholar] [CrossRef]
- Yang, J.; Qi, J.-L.; Wang, X.-X.; Li, X.-H.; Jin, R.; Liu, B.-Y.; Liu, H.-X.; Rao, H.-Y. The burden of hepatitis C virus in the world, China, India, and the United States from 1990 to 2019. Front. Public Health 2023, 11, 1041201. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Khaderi, S.; Singal, A.G.; Marrero, J.A.; Asrani, S.K.; Amos, C.I.; Thrift, A.P.; Kramer, J.R.; Yu, X.; Cao, Y.; et al. Risk Stratification Model for Hepatocellular Cancer in Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2023, 21, 3296–3304.e3. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.-L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.; Price, J.C.; Tien, P.C. Hepatitis C Virus Infection in the Older Patient. Infect. Dis. Clin. N. Am. 2017, 31, 827–838. [Google Scholar] [CrossRef]
- Jiang, J.; Shiels, M.S.; Rivera, D.; Ghany, M.G.; Engels, E.A.; O’Brien, T.R. Trends in hepatocellular carcinoma and viral hepatitis treatment in older Americans. PLoS ONE 2024, 19, e0307746. [Google Scholar] [CrossRef] [PubMed]
- Altekruse, S.F.; Henley, S.J.; Cucinelli, J.E.; McGlynn, K.A. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am. J. Gastroenterol. 2014, 109, 542–553. [Google Scholar] [CrossRef]
- Njei, B.; Rotman, Y.; Ditah, I.; Lim, J.K. Emerging Trends in Hepatocellular Carcinoma Incidence and Mortality. Hepatology 2015, 61, 191–199. [Google Scholar] [CrossRef]
- Kassa, G.M.; Walker, J.G.; Alamneh, T.S.; Tamiru, M.T.; Bivegete, S.; Adane, A.; Amogne, W.; Dillon, J.F.; Vickerman, P.; Dagne, E.; et al. Prevalence, trends, and distribution of hepatitis C virus among the general population in sub-Saharan Africa: A systematic review and meta-analysis. Liver Int. 2024, 44, 3238–3249. [Google Scholar] [CrossRef]
- Salomon, I.; Olivier, S.; Egide, N. Advancing Hepatitis C Elimination in Africa: Insights from Egypt. Hepatic Med. Evid. Res. 2024, 16, 37–44. [Google Scholar] [CrossRef]
- Ridruejo, E.; Soza, A. Which Strategies Should Be Implemented in Latin America to Eradicate Hepatitis C Virus by 2030? Clin. Liver Dis. 2019, 13, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, R.; Huang, A.S.; Gurmessa, K.; Hsu, E.B. Understanding Barriers to Hepatitis C Antiviral Treatment in Low-Middle-Income Countries. Healthcare 2024, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Saab, S.; Kullar, R.; Gounder, P. The Urgent Need for Hepatitis C Screening in Pregnant Women: A Call to Action. Obstet. Gynecol. 2020, 135, 773–777. [Google Scholar] [CrossRef]
- Ruggieri, A.; Gagliardi, M.C.; Anticoli, S. Sex-Dependent Outcome of Hepatitis B and C Viruses Infections: Synergy of Sex Hormones and Immune Responses? Front. Immunol. 2018, 9, 2302. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, A.; Barbati, C.; Malorni, W. Cellular and molecular mechanisms involved in hepatocellular carcinoma gender disparity. Int. J. Cancer 2010, 127, 499–504. [Google Scholar] [CrossRef]
- Valente, J.Y.; Sohi, I.; Garcia-Cerde, R.; Monteiro, M.G.; Sanchez, Z.M. What is associated with the increased frequency of heavy episodic drinking during the COVID-19 pandemic? Data from the PAHO regional web-based survey. Drug Alcohol Depend. 2021, 221, 108621. [Google Scholar] [CrossRef]
- Sohi, I.; Chrystoja, B.R.; Rehm, J.; Wells, S.; Monteiro, M.; Ali, S.; Shield, K.D. Changes in alcohol use during the COVID-19 pandemic and previous pandemics: A systematic review. Alcohol. Clin. Exp. Res. 2022, 46, 498–513. [Google Scholar] [CrossRef]
- Roberts, A.; Rogers, J.; Mason, R.; Siriwardena, A.N.; Hogue, T.; Whitley, G.A.; Law, G.R. Alcohol and other substance use during the COVID-19 pandemic: A systematic review. Drug Alcohol Depend. 2021, 229, 109150. [Google Scholar] [CrossRef]
- Global Burden of Disease 2019 Cancer Collaboration Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [CrossRef]




| WHO Region | Female DALYs AAPC (95% CI) | Male DALYs AAPC (95% CI) | Female Deaths AAPC (95% CI) | Male Deaths AAPC (95% CI) | Female ASI AAPC (95% CI) | Male ASI AAPC (95% CI) |
|---|---|---|---|---|---|---|
| Americas | 3.73 (3.63–3.81) | 4.63 (4.55–4.70) | 3.93 (3.87–3.99) | 4.81 (4.74–4.87) | 1.5 (1.49–1.51) | 2.20 (2.15–2.26) |
| Southeast Asia | 3.43 (3.39–3.46) | 3.38 (3.35–3.41) | 3.76 (3.71–3.79) | 3.65 (3.61–3.69) | 0.32 (0.30–0.33) | 0.68 (0.66–0.69) |
| Europe | 1.65 (1.61–1.70) | 2.05 (1.97–2.15) | 1.97 (1.90–2.02) | 2.53 (2.44–2.63) | 0.80 (0.78–0.81) | 1.14 (1.14–1.17) |
| Western Pacific | 1.86 (1.79–1.92) | 1.17 (1.08–1.27) | 2.57 (2.48–2.66) | 2.12 (2.0–2.21) | −0.51 (−0.53 to −0.51) | −0.57 (−0.61 to −0.53) |
| Eastern Mediterranean | 2.51 (2.45–2.56) | 3.51 (3.37–3.62) | 2.49 (2.43–2.54) | 3.56 (3.43–3.64) | −0.58 (−0.60 to −0.56) | 0.71 (0.68–0.73) |
| Africa | 2.17 (2.15–2.18) | 1.80 (1.79–1.82) | 2.23 (2.22–2.25) | 1.83 (1.80–1.84) | −0.52 (−0.53 to −0.51) | −0.64 (−0.67 to −0.62) |
| SDI Level | Female DALYs AAPC (95% CI) | Male DALYs AAPC (95% CI) | Female Deaths AAPC (95% CI) | Male Deaths AAPC (95% CI) | Female ASI AAPC (95% CI) | Male ASI AAPC (95% CI) |
|---|---|---|---|---|---|---|
| High | 1.77 (1.71–1.83) | 1.34 (1.25–1.43) | 2.94 (2.86–3.02) | 2.33 (2.24–2.40) | 0.97 (0.94 to 1.0) | 0.22 (0.18–0.25) |
| High–Middle | 2.49 (2.42–2.55) | 1.80 (1.73–1.91) | 2.23 (2.16–2.30) | 2.26 (2.13–2.37) | 0.11 (0.09 to 0.13) | 0.17 (0.12–0.20) |
| Middle | 2.49 (2.43–2.55) | 3.02 (2.97–3.09) | 2.88 (2.81–2.94) | 3.39 (3.33–3.46) | −0.35 (−0.38 to −0.33) | 0.44 (0.39–0.48) |
| Middle-Low | 2.76 (2.72–2.79) | 3.19 (3.11–3.27) | 2.89 (2.85–2.92) | 3.28 (3.19–3.36) | −0.23 (−0.24 to −0.22) | 0.61 (0.60–0.62) |
| Low | 2.22 (2.21–2.23) | 1.76 (1.74–1.78) | 2.31 (2.29–2.33) | 1.78 (1.76–1.80) | −0.47 (−0.48 to −0.46) | −0.73 (−0.75 to −0.71) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sequeira, L.; Ying, X.; Ozturk, N.B.; Reidy, D.; Jesudian, A.B.; Gurakar, A. Revealing New Trends in the Global Burden of Hepatocellular Cancer Related to Hepatitis C Virus by Region, Sociodemographic Index, and Sex. J. Clin. Med. 2025, 14, 6006. https://doi.org/10.3390/jcm14176006
Sequeira L, Ying X, Ozturk NB, Reidy D, Jesudian AB, Gurakar A. Revealing New Trends in the Global Burden of Hepatocellular Cancer Related to Hepatitis C Virus by Region, Sociodemographic Index, and Sex. Journal of Clinical Medicine. 2025; 14(17):6006. https://doi.org/10.3390/jcm14176006
Chicago/Turabian StyleSequeira, Lynette, Xiaohan Ying, Nazli Begum Ozturk, Deirdre Reidy, Arun B. Jesudian, and Ahmet Gurakar. 2025. "Revealing New Trends in the Global Burden of Hepatocellular Cancer Related to Hepatitis C Virus by Region, Sociodemographic Index, and Sex" Journal of Clinical Medicine 14, no. 17: 6006. https://doi.org/10.3390/jcm14176006
APA StyleSequeira, L., Ying, X., Ozturk, N. B., Reidy, D., Jesudian, A. B., & Gurakar, A. (2025). Revealing New Trends in the Global Burden of Hepatocellular Cancer Related to Hepatitis C Virus by Region, Sociodemographic Index, and Sex. Journal of Clinical Medicine, 14(17), 6006. https://doi.org/10.3390/jcm14176006






