Infectious Keratitis Management: 10-Year Update
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Corneal Culture Results
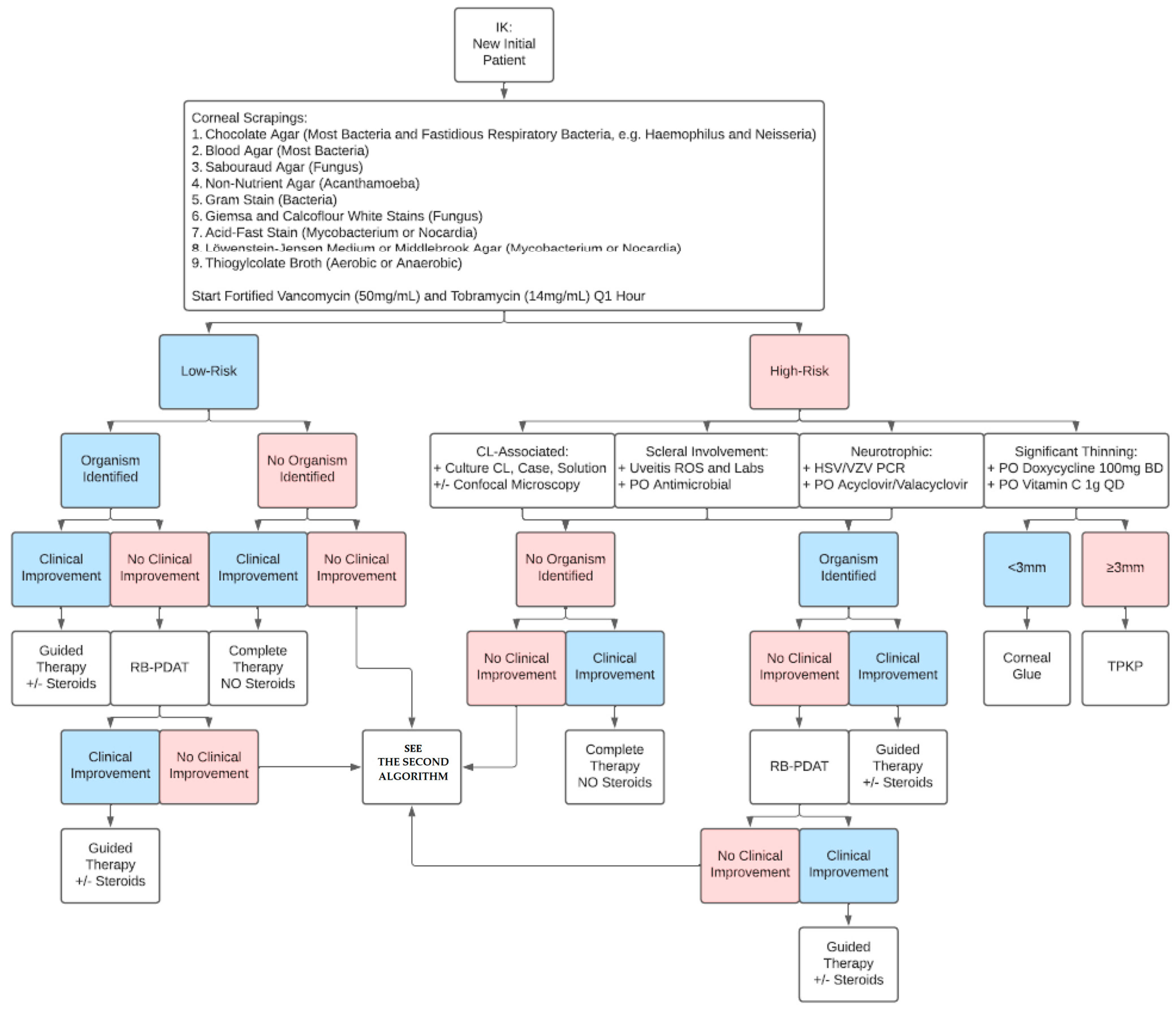
3.2. The First Algorithm: New IK Patient
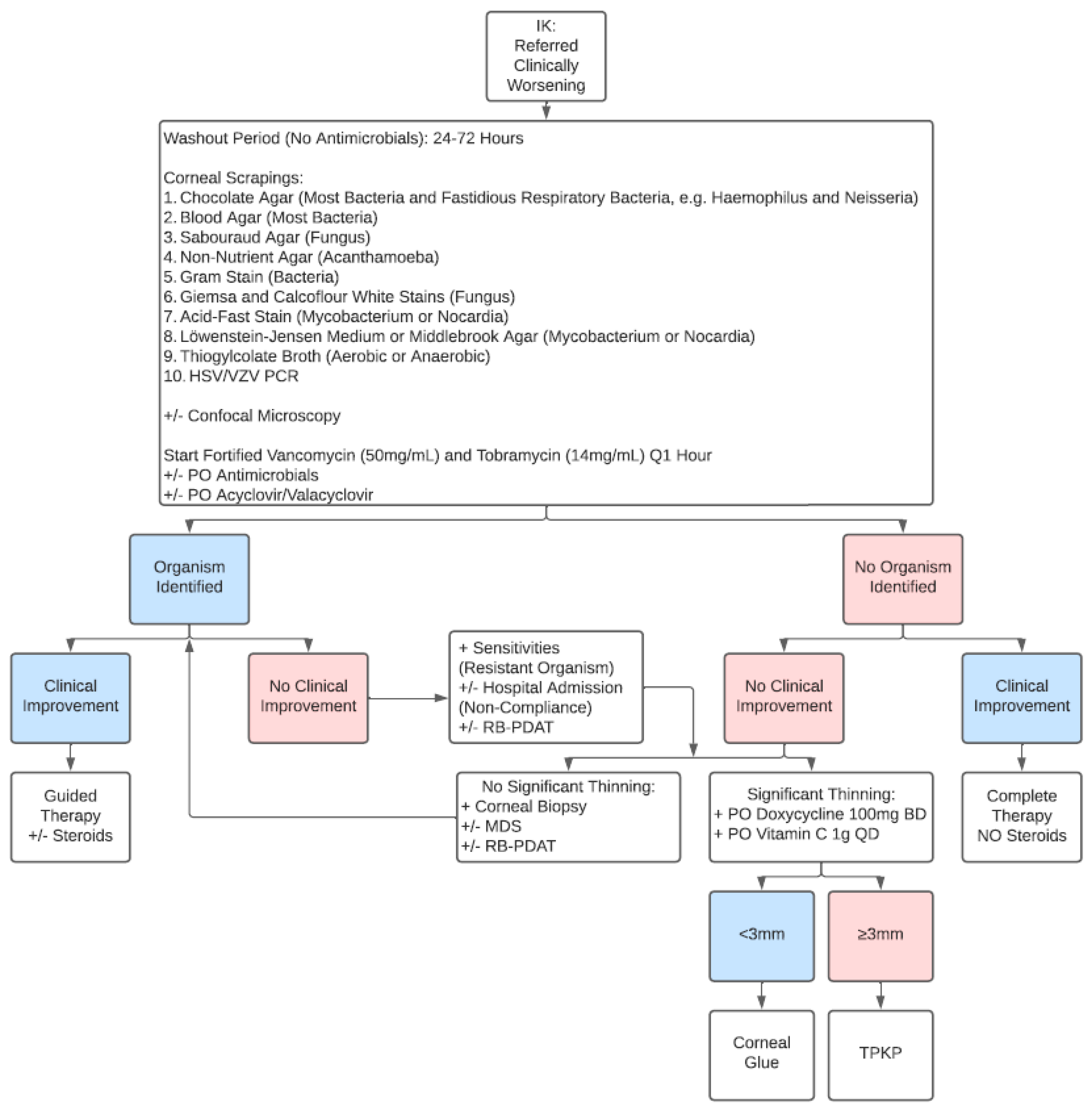
3.3. The Second Algorithm: Referred Clinically Worsening IK Patient
3.4. Corticosteroid Therapy
3.5. PACK-CXL
3.6. RB-PDAT
4. Discussion
Funding
Conflicts of Interest
References
- Flaxman, S.R.; Bourne, R.R.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Ung, L.; Acharya, N.R.; Agarwal, T.; Alfonso, E.C.; Bagga, B.; Bispo, P.J.; Burton, M.J.; Dart, J.K.; Doan, T.; Fleiszig, S.M.; et al. Infectious corneal ulceration: A proposal for neglected tropical disease status. Bull. World Health Organ. 2019, 97, 854–856. [Google Scholar] [CrossRef]
- Durand, M.L.; Barshak, M.B.; Chodosh, J. Infectious Keratitis in 2021. JAMA 2021, 326, 1319–1320. [Google Scholar] [CrossRef] [PubMed]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar]
- Stapleton, F. The epidemiology of infectious keratitis. Ocul. Surf. 2021, 28, 351–363. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Ho, C.S.; Deshmukh, R.; Said, D.G.; Dua, H.S. Infectious keratitis: An update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 2021, 35, 1084–1101. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.A.; Gronostaj, M.P.; MacGurn, A.K.; Cope, J.R.; Awsumb, K.L.; Yoder, J.S.; Beach, M.J. Estimated burden of keratitis—United States, 2010. MMWR Morb. Mortal Wkly. Rep. 2014, 63, 1027–1030. [Google Scholar] [PubMed]
- Singh, R.B.; Dohlman, T.H.; Ivanov, A.; Hall, N.; Ross, C.; Elze, T.; Miller, J.W.; Lorch, A.; Yuksel, E.; Yin, J.; et al. Corneal Opacity in the United States: An American Academy of Ophthalmology IRIS® Registry (Intelligent Research in Sight) Study. Ophthalmology 2024, 132, 52–61. [Google Scholar] [CrossRef]
- Prajna, N.V.; Srinivasan, M.; Mascarenhas, J.; Lalitha, P.; Rajaraman, R.; McClintic, S.M.; O’BRien, K.S.; Ray, K.J.; Acharya, N.R.; Lietman, T.M.; et al. Visual Impairment in Fungal Versus Bacterial Corneal Ulcers 4 Years After Successful Antimicrobial Treatment. Am. J. Ophthalmol. 2019, 204, 124–129. [Google Scholar] [CrossRef]
- Lalitha, P.; Prajna, N.V.; Sikha, M.; Gunasekaran, R.; Hinterwirth, A.; Worden, L.; Chen, C.; Zhong, L.; Liu, Z.; Lietman, T.M.; et al. Evaluation of Metagenomic Deep Sequencing as a Diagnostic Test for Infectious Keratitis. Ophthalmology 2021, 128, 473–475. [Google Scholar] [CrossRef]
- Ting, D.S.; Gopal, B.P.; Deshmukh, R.; Seitzman, G.D.; Said, D.G.; Dua, H.S. Diagnostic armamentarium of infectious keratitis: A comprehensive review. Ocul. Surf. 2022, 23, 27–39. [Google Scholar] [CrossRef]
- Uddaraju, M.; Mascarenhas, J.; Das, M.R.; Radhakrishnan, N.; Keenan, J.D.; Prajna, L.; Prajna, V.N. Corneal Cross-linking as an Adjuvant Therapy in the Management of Recalcitrant Deep Stromal Fungal Keratitis: A Randomized Trial. Am. J. Ophthalmol. 2015, 160, 131–134.e5. [Google Scholar] [CrossRef]
- Prajna, N.V.; Radhakrishnan, N.; Lalitha, P.; Rajaraman, R.; Narayana, S.; Austin, A.F.; Liu, Z.; Keenan, J.D.; Porco, T.C.; Lietman, T.M.; et al. Cross-Linking-Assisted Infection Reduction: A Randomized Clinical Trial Evaluating the Effect of Adjuvant Cross-Linking on Outcomes in Fungal Keratitis. Ophthalmology 2020, 127, 159–166. [Google Scholar] [CrossRef]
- Gulias-Cañizo, R.; Benatti, A.; De Wit-Carter, G.; Hernández-Quintela, E.; Sánchez-Huerta, V. Photoactivated Chromophore for Keratitis-Corneal Collagen Cross-Linking (PACK-CXL) Improves Outcomes of Treatment-Resistant Infectious Keratitis. Clin. Ophthalmol. 2020, 14, 4451–4457. [Google Scholar] [CrossRef]
- Amescua, G.; Arboleda, A.; Nikpoor, N.; Durkee, H.; Relhan, N.; Aguilar, M.C.; Flynn, H.W., Jr.; Miller, D.; Parel, J.-M. Rose Bengal Photodynamic Antimicrobial Therapy: A Novel Treatment for Resistant Fusarium Keratitis. Cornea 2017, 36, 1141–1144. [Google Scholar] [CrossRef]
- Naranjo, A.; Arboleda, A.; Martinez, J.D.; Durkee, H.; Aguilar, M.C.; Relhan, N.; Nikpoor, N.; Galor, A.; Dubovy, S.R.; Leblanc, R.; et al. Rose Bengal Photodynamic Antimicrobial Therapy for Patients With Progressive Infectious Keratitis: A Pilot Clinical Study. Am. J. Ophthalmol. 2019, 208, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Bagga, B.; Sharma, S.; Ahirwar, L.K.; Sheba, E.; Vaddavalli, P.K.; Mishra, D.K. Clinical Outcomes of Rose Bengal Mediated Photodynamic Antimicrobial Therapy on Fungal Keratitis with Their Microbiological and Pathological Correlation. Curr. Eye Res. 2022, 47, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Beltran, P.A.; Levine, H.; Altamirano, D.S.; Martinez, J.D.; Durkee, H.; Mintz, K.; Leblanc, R.; Tóthová, J.D.; Miller, D.; Parel, J.-M.; et al. Rose Bengal Photodynamic Antimicrobial Therapy: A Review of the Intermediate-Term Clinical and Surgical Outcomes. Am. J. Ophthalmol. 2022, 243, 125–134. [Google Scholar] [CrossRef]
- Amescua, G.; Miller, D.; Alfonso, E.C. What is causing the corneal ulcer? Management strategies for unresponsive corneal ulceration. Eye 2012, 26, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Enzor, R.; Bowers, E.M.; Perzia, B.; Perera, C.; Palazzolo, L.; Mammen, A.; Dhaliwal, D.K.; Kowalski, R.P.; Jhanji, V. Comparison of Clinical Features and Treatment Outcomes of Pseudomonas aeruginosa Keratitis in Contact Lens and Non-Contact Lens Wearers. Am. J. Ophthalmol. 2021, 227, 1–11. [Google Scholar] [CrossRef]
- Singh, R.B.; Zhu, S.; Yung, A.; Dohlman, T.H.; Dana, R.; Yin, J. Efficacy of cyanoacrylate tissue adhesive in the management of corneal thinning and perforation due to microbial keratitis. Ocul. Surf. 2020, 18, 795–800. [Google Scholar] [CrossRef]
- Mathews, P.M.; Fogla, R.; Samayoa, E.; VanCourt, S.; Akpek, E.K. Long-term clinical outcomes of keratoplasty using gamma-irradiated corneal lenticules. BMJ Open Ophthalmol. 2019, 4, e000396. [Google Scholar] [CrossRef]
- Rohowetz, L.J.; Hudson, J.; Sayed-Ahmed, I.O.; Amescua, G.; Flynn, H.W.J. Cataract wound infection and endophthalmitis treated with partial conjunctival pedicle flap and pars plana vitrectomy. JCRS Online Case Rep. 2024, 12, e00111. [Google Scholar] [CrossRef]
- Palioura, S.; Henry, C.R.; Amescua, G.; Alfonso, E.C. Role of steroids in the treatment of bacterial keratitis. Clin. Ophthalmol. 2016, 10, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef]
- Vaddavalli, P.K.; Garg, P.; Sharma, S.; Sangwan, V.S.; Rao, G.N.; Thomas, R. Role of confocal microscopy in the diagnosis of fungal and acanthamoeba keratitis. Ophthalmology 2011, 118, 29–35. [Google Scholar] [CrossRef]
- Hudson, J.; Al-Khersan, H.; Carletti, P.; Miller, D.; Dubovy, S.R.; Amescua, G. Role of corneal biopsy in the management of infectious keratitis. Curr. Opin. Ophthalmol. 2022, 33, 290–295. [Google Scholar] [CrossRef]
- Carnt, N.; Robaei, D.; Watson, S.L.; Minassian, D.C.; Dart, J.K. The Impact of Topical Corticosteroids Used in Conjunction with Antiamoebic Therapy on the Outcome of Acanthamoeba Keratitis. Ophthalmology 2016, 123, 984–990. [Google Scholar] [CrossRef]
- Naranjo, A.; Martinez, J.D.; Miller, D.; Tonk, R.; Amescua, G. Systemic Miltefosine as an Adjunct Treatment of Progressive Acanthamoeba Keratitis. Ocul. Immunol. Inflamm. 2021, 29, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Thulasi, P.; Saeed, H.N.; Rapuano, C.J.; Hou, J.H.; Appenheimer, A.B.; Chodosh, J.; Kang, J.J.; Morrill, A.M.; Vyas, N.; Zegans, M.E.; et al. Oral Miltefosine as Salvage Therapy for Refractory Acanthamoeba Keratitis. Am. J. Ophthalmol. 2021, 223, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Peng, L.; Wang, B.; Zhang, H.; Li, S.; Yang, R.; Deng, Y.; Huang, H.; Yuan, J. Tacrolimus interacts with voriconazole to reduce the severity of fungal keratitis by suppressing IFN-related inflammatory responses and concomitant FK506 and voriconazole treatment suppresses fungal keratitis. Mol. Vis. 2018, 24, 187–200. [Google Scholar]
- Chatterjee, S.; Agrawal, D. Use of Topical Cyclosporine 0.1% in Therapeutic Penetrating Keratoplasty for Fungal Keratitis. Cornea 2022, 41, 1116–1121. [Google Scholar] [CrossRef]
- Srinivasan, M.; Mascarenhas, J.; Rajaraman, R.; Ravindran, M.; Lalitha, P.; Glidden, D.V.; Ray, K.J.; Hong, K.C.; Oldenburg, C.E.; Lee, S.M.; et al. Corticosteroids for bacterial keratitis: The Steroids for Corneal Ulcers Trial (SCUT). Arch. Ophthalmol. 2012, 130, 143–150. [Google Scholar] [CrossRef]
- Girgis, D.O.; Karp, C.L.; Miller, D. Ocular infections caused by non-tuberculous mycobacteria: Update on epidemiology and management. Clin. Exp. Ophthalmol. 2012, 40, 467–475. [Google Scholar] [CrossRef]
- Knutsson, K.A.; Iovieno, A.; Matuska, S.; Fontana, L.; Rama, P. Topical Corticosteroids and Fungal Keratitis: A Review of the Literature and Case Series. J. Clin. Med. 2021, 10, 1178. [Google Scholar] [CrossRef] [PubMed]
- White, M.L.; Chodosh, J. Herpes Simplex Virus Keratitis: A Treatment Guideline—2014; American Academy of Ophthalmology: San Francisco, CA, USA, 2014. [Google Scholar]
- Wilhelmus, K.R.; Gee, L.; Hauck, W.W.; Kurinij, N.; Dawson, C.R.; Jones, D.B.; Barron, B.A.; Kaufman, H.E.; Sugar, J.; Hyndiuk, R.A.; et al. Herpetic Eye Disease Study. A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology 1994, 101, 1883–1895. [Google Scholar] [CrossRef]
- Hirano, K.; Tanaka, H.; Kato, K.; Araki-Sasaki, K. Topical Corticosteroids for Infectious Keratitis Before Culture-Proven Diagnosis. Clin. Ophthalmol. 2021, 15, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Said, D.G.; Elalfy, M.S.; Gatzioufas, Z.; El-Zakzouk, E.S.; Hassan, M.A.; Saif, M.Y.; Zaki, A.A.; Dua, H.S.; Hafezi, F. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology 2014, 121, 1377–1382. [Google Scholar] [CrossRef]
- Hafezi, F.; Hosny, M.; Shetty, R.; Knyazer, B.; Chen, S.; Wang, Q.; Hashemi, H.; Torres-Netto, E.A.; the PACK-CXL Working Group; Zhang, H.; et al. PACK-CXL vs. antimicrobial therapy for bacterial, fungal, and mixed infectious keratitis: A prospective randomized phase 3 trial. Eye Vis. 2022, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Prajna, N.V.; Radhakrishnan, N.; Lalitha, P.; Rajaraman, R.; Narayana, S.; Austin, A.F.; Liu, Z.; Keenan, J.D.; Porco, T.C.; Lietman, T.M.; et al. Cross-Linking Assisted Infection Reduction (CLAIR): A Randomized Clinical Trial Evaluating the Effect of Adjuvant Cross-Linking on Bacterial Keratitis. Cornea 2021, 40, 837–841. [Google Scholar] [CrossRef]
- Radhakrishnan, N.; Prajna, V.N.; Prajna, L.S.; Venugopal, A.; Narayana, S.; Rajaraman, R.; Amescua, G.; Porco, T.C.; Lietman, T.M.; Rose-Nussbaumer, J. Double-masked, sham and placebo-controlled trial of corneal cross-linking and topical difluprednate in the treatment of bacterial keratitis: Steroids and Cross-linking for Ulcer Treatment Trial (SCUT II) study protocol. BMJ Open Ophthalmol. 2021, 6, e000811. [Google Scholar] [CrossRef]
- Kling, S.; Hufschmid, F.S.; Torres-Netto, E.A.; Randleman, J.B.; Willcox, M.; Zbinden, R.; Hafezi, F.M. High Fluence Increases the Antibacterial Efficacy of PACK Cross-Linking. Cornea 2020, 39, 1020–1026. [Google Scholar] [CrossRef]
- Achiron, A.; Elhaddad, O.M.B.; Regev, T.; Krakauer, Y.; Tsumi, E.; Hafezi, F.M.; Knyazer, B. PACK Cross-Linking as Adjuvant Therapy Improves Clinical Outcomes in Culture-Confirmed Bacterial Keratitis. Cornea 2022, 41, 1069–1073. [Google Scholar] [CrossRef]
- Ozbek-Uzman, S.; Yalniz-Akkaya, Z.; Burcu, A. Corneal Collagen Cross-Linking With Photoactivated Chromophore for Infectious Keratitis After Penetrating Keratoplasty. Cornea 2020, 39, 283–289. [Google Scholar] [CrossRef]
- Levine, H.; Sepulveda-Beltran, P.A.; Amescua, G. Rose Bengal Photodynamic Antimicrobial Therapy as potential adjuvant treatment for Serratia marcescens corneal ulcer. Am. J. Ophthalmol. 2021, 231, e1–e2. [Google Scholar] [CrossRef]
- Ung, L.; Wang, Y.; Vangel, M.; Davies, E.C.; Gardiner, M.; Bispo, P.J.; Gilmore, M.S.; Chodosh, J. Validation of a Comprehensive Clinical Algorithm for the Assessment and Treatment of Microbial Keratitis. Am. J. Ophthalmol. 2020, 214, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Redd, T.K.; Lalitha, P.; Prajna, N.V.; Sikha, M.; Gunasekaran, R.M.; Hinterwirth, A.; Chen, C.; Zhong, L.B.; Liu, Z.; Lietman, T.M.; et al. Impact of Sample Collection Order on the Diagnostic Performance of Metagenomic Deep Sequencing for Infectious Keratitis. Cornea 2022, 41, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Seitzman, G.D.; Hinterwirth, A.; Zhong, L.; Cummings, S.; Chen, C.; Driver, T.H.; Lee, M.D.; Doan, T. Metagenomic Deep Sequencing for the Diagnosis of Corneal and External Disease Infections. Ophthalmology 2019, 126, 1724–1726. [Google Scholar] [CrossRef] [PubMed]
- Redd, T.K.; Prajna, N.V.; Srinivasan, M.; Lalitha, P.; Krishnan, T.; Rajaraman, R.; Venugopal, A.; Lujan, B.; Acharya, N.; Seitzman, G.D.; et al. Expert Performance in Visual Differentiation of Bacterial and Fungal Keratitis. Ophthalmology 2022, 129, 227–230. [Google Scholar] [CrossRef]
- Koyama, A.; Miyazaki, D.; Nakagawa, Y.; Ayatsuka, Y.; Miyake, H.; Ehara, F.; Sasaki, S.-I.; Shimizu, Y.; Inoue, Y. Determination of probability of causative pathogen in infectious keratitis using deep learning algorithm of slit-lamp images. Sci. Rep. 2021, 11, 22642. [Google Scholar] [CrossRef]
- Kuo, M.-T.; Hsu, B.W.-Y.; Lin, Y.-S.; Fang, P.-C.; Yu, H.-J.; Chen, A.; Yu, M.-S.; Tseng, V.S. Comparisons of deep learning algorithms for diagnosing bacterial keratitis via external eye photographs. Sci. Rep. 2021, 11, 24227. [Google Scholar] [CrossRef] [PubMed]
- Redd, T.K.; Prajna, N.V.; Srinivasan, M.; Lalitha, P.; Krishnan, T.; Rajaraman, R.; Venugopal, A.; Acharya, N.; Seitzman, G.D.; Lietman, T.M.; et al. Image-Based Differentiation of Bacterial and Fungal Keratitis Using Deep Convolutional Neural Networks. Ophthalmol. Sci. 2022, 2, 100119. [Google Scholar] [CrossRef]
- Klug, D.M.; Idiris, F.I.M.; Blaskovich, M.A.T.; von Delft, F.; Dowson, C.G.; Kirchhelle, C.; Roberts, A.P.; Singer, A.C.; Todd, M.H. There is no market for new antibiotics: This allows an open approach to research and development. Wellcome Open Res. 2021, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Shrestha, G.S.; Vijay, A.K.; Carnt, N. Epidemiology, Microbiology, and Genetics of Contact Lens-Related and Non-Contact Lens-Related Infectious Keratitis. Eye Contact Lens. 2022, 48, 127–133. [Google Scholar] [CrossRef] [PubMed]
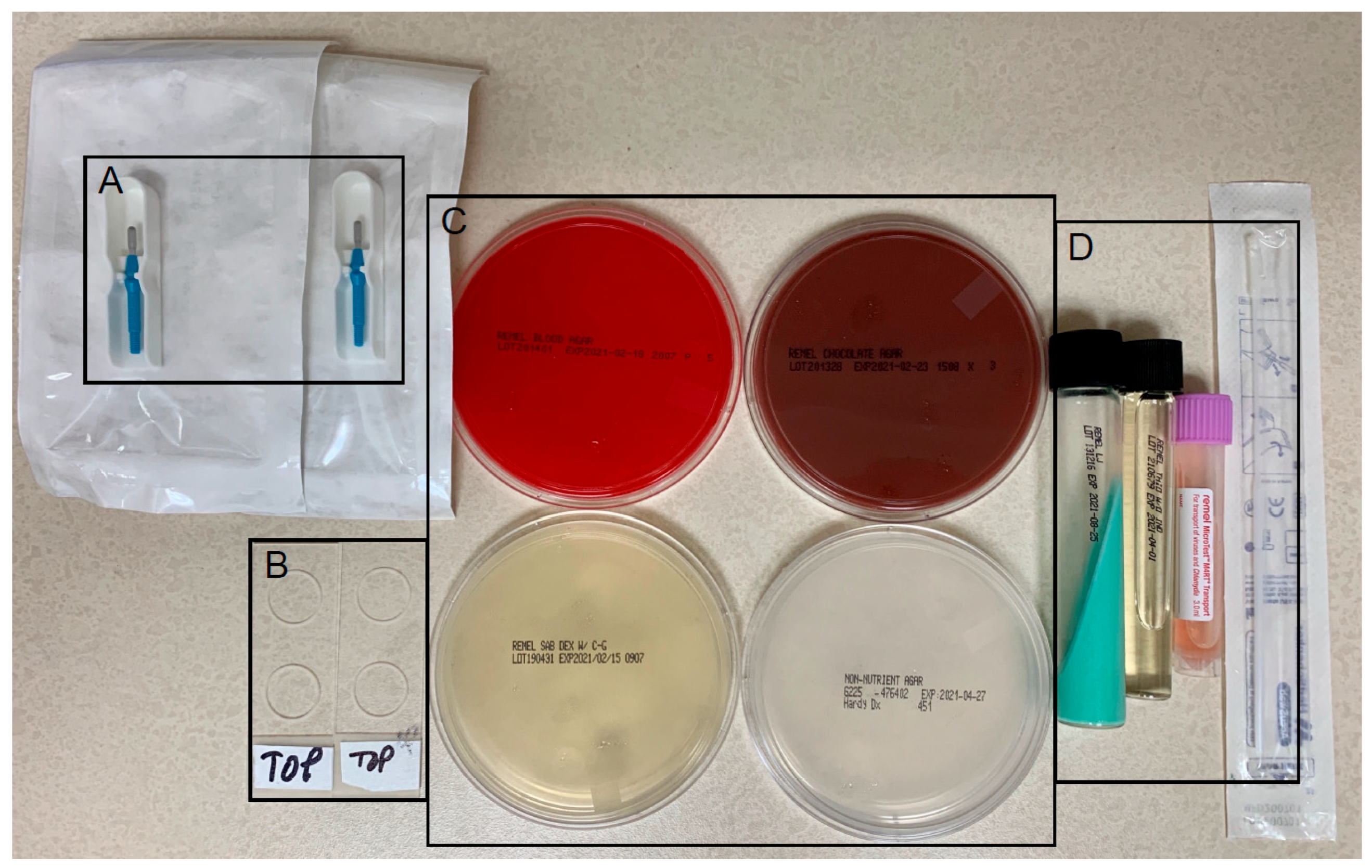
| Isolates | Top Pathogen | |
|---|---|---|
| Bacteria | 3010 (83.4%) | |
| Gram-negative | 1693 (46.9%) | Pseudomonas aeruginosa |
| Gram-positive | 1231 (34.1%) | Staphylococcus aureus |
| Mycobacterium species | 50 (1.4%) | Mycobacterium chelonae |
| Nocardia species | 36 (1%) | Nocardia asteroides |
| Fungus | 486 (13.4%) | |
| Mold | 387 (10.7%) | Fusarium species |
| Yeast | 99 (2.7%) | Candida albicans |
| Parasite | 113 (3.13%) | |
| Acanthamoeba | 113 (3.13%) | N/A |
| Pathogen | Corticosteroid Summary |
|---|---|
| BACTERIAL | |
| Nocardia |
|
| Mycobacteria |
|
| Other | |
| FUNGAL |
|
| PARASITIC | |
| Acanthamoeba |
|
| VIRAL | |
| Epithelial |
|
| Stromal |
|
| Endothelial |
|
| UNKNOWN |
|
| Author (Year) | Pathogen (n) | Study Type | Photosensitizer | Light Source (λ) Irradiance (Time) Fluence | Conclusions |
|---|---|---|---|---|---|
| Hafezi et al. (2022) [40] |
| Randomized clinical trial | Hypo-osmolar riboflavin 0.1% | UV-A (365 nm) 9 mW/cm2 (10 min or 13 min and 20 s) 5.4 J/cm2 or 7.2 J/cm2 |
|
| Achiron et al. (2022) [44] |
| Retrospective interventional cohort | Hypo-osmolar riboflavin 0.1% | UV-A (365 nm) 30 mW/cm2 (3 min) 5.4 J/cm2 |
|
| Prajna et al. (2021) [41] |
| Randomized clinical trial | Iso-osmolar riboflavin 0.1%, 20% dextran | UV-A (365 nm) 3 mW/cm2 (30 min) 5.4 J/cm2 |
|
| Gulias-Cañizo et al. (2020) [14] |
| Prospective observational cohort | Hypo-osmolar riboflavin 0.1% | UV-A (370 nm) 3 mW/cm2 (30 min) 5.4 J/cm2 |
|
| Ozbek-Uzman et al. (2020) [45] |
| Retrospective interventional cohort | Iso-osmolar riboflavin 0.1%, 1.1% HPMC (CCT > 400 μm) Hypo-osmolar riboflavin 0.1% (CCT 350–400 μm) | UV-A (365 nm) 3 mW/cm2 (30 min) 5.4 J/cm2 |
|
| Prajna et al. (2020) [13] |
| Randomized clinical trial | Iso-osmolar riboflavin 0.1%, 20% dextran | UV-A (365 nm) 3 mW/cm2 (30 min) 5.4 J/cm2 |
|
| Author (Year) | Pathogen (n) | Study Type | Photosensitizer | Fluence | Conclusions |
|---|---|---|---|---|---|
| Sepulveda-Beltran et al. (2022) [18] and Naranjo et al. (2019) [16] |
| Retrospective interventional cohort | 0.1% OR 0.2% Rose Bengal | 5.4 J/cm2 (15 min) |
|
| Bagga et al. (2022) [17] |
| Prospective case series | 0.1% Rose Bengal | 5.4 J/cm2 (15 min) |
|
| Levine et al. (2021) [46] |
| Case report | 0.1% Rose Bengal | 5.4 J/cm2 (15 min) |
|
| Amescua et al. (2017) [15] |
| Case report | 0.1% Rose Bengal | 0.9 J/cm2 (#1) and 1.8 J/cm2 (#2) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasricha, N.D.; Larco, P.; Miller, D.; Altamirano, D.S.; Rose-Nussbaumer, J.R.; Alfonso, E.C.; Amescua, G. Infectious Keratitis Management: 10-Year Update. J. Clin. Med. 2025, 14, 5987. https://doi.org/10.3390/jcm14175987
Pasricha ND, Larco P, Miller D, Altamirano DS, Rose-Nussbaumer JR, Alfonso EC, Amescua G. Infectious Keratitis Management: 10-Year Update. Journal of Clinical Medicine. 2025; 14(17):5987. https://doi.org/10.3390/jcm14175987
Chicago/Turabian StylePasricha, Neel D., Pablo Larco, Darlene Miller, Diego S. Altamirano, Jennifer R. Rose-Nussbaumer, Eduardo C. Alfonso, and Guillermo Amescua. 2025. "Infectious Keratitis Management: 10-Year Update" Journal of Clinical Medicine 14, no. 17: 5987. https://doi.org/10.3390/jcm14175987
APA StylePasricha, N. D., Larco, P., Miller, D., Altamirano, D. S., Rose-Nussbaumer, J. R., Alfonso, E. C., & Amescua, G. (2025). Infectious Keratitis Management: 10-Year Update. Journal of Clinical Medicine, 14(17), 5987. https://doi.org/10.3390/jcm14175987








