Is There a Link Between TSH Levels and Schizophrenia? A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Reporting and Quality Assessment
2.5. Data Extraction
2.6. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Qualitative Analysis
3.3. Quantitative Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FEDN | First-episode drug-naïve |
| SHZ | Schizophrenia |
| T3 | Triiodothyronine |
| T4 | Tetraiodothyronine |
| TH | Thyroid hormone |
| TRH | Thyrotropin-releasing hormone |
| TSH | Thyroid stimulating hormone |
References
- Bernal, J. Thyroid hormone receptors in brain development and function. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 249–259. [Google Scholar] [CrossRef]
- Talhada, D.; Santos, C.R.A.; Gonçalves, I.; Ruscher, K. Thyroid Hormones in the Brain and Their Impact in Recovery Mechanisms After Stroke. Front. Neurol. 2019, 10, 1103. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Toro, F.; Jialal, I. Physiology, Thyroid Stimulating Hormone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sheehan, M.T. Biochemical Testing of the Thyroid: TSH is the Best and, Oftentimes, Only Test Needed—A Review for Primary Care. Clin. Med. Res. 2016, 14, 83–92. [Google Scholar] [CrossRef]
- World Health Organization (WHO). ICD-11 for Mortality and Morbidity Statistics. Available online: http://id.who.int/icd/entity/334423054 (accessed on 1 July 2025).
- Murray, C.J.L. The Global Burden of Disease Study at 30 years. Nat. Med. 2022, 28, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Freuer, D.; Meisinger, C. Causal link between thyroid function and schizophrenia: A two-sample Mendelian randomization study. Eur. J. Epidemiol. 2023, 38, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Nandeesha, H.; Kattimani, S.; Meiyappan, K.; Sarkar, S.; Sivasankar, D. Association between prolactin and thyroid hormones with severity of psychopathology and suicide risk in drug free male schizophrenia. Clin. Chim. Acta 2015, 444, 78–80. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Calvin, S.; Singh, J.K.; Thomas, B.; Srinivasan, K. Thyroid dysfunction in major psychiatric disorders in a hospital based sample. Indian J. Med. Res. 2013, 138, 888–893. [Google Scholar] [PubMed]
- Bunevicius, R.; Steibliene, V.; Prange, A.J. Thyroid axis function after in-patient treatment of acute psychosis with antipsychotics: A naturalistic study. BMC Psychiatry 2014, 14, 279. [Google Scholar] [CrossRef]
- Rasmussen, M.J.; Robbins, H.; Fipps, D.C.; Rustad, J.K.; Pallais, J.C.; Stern, T.A. Neuropsychiatric Sequelae of Thyroid Dysfunction: Evaluation and Management. Prim. Care Companion CNS Disord. 2023, 25, 23f03570. [Google Scholar] [CrossRef]
- Cavaleri, D.; Capogrosso, C.A.; Guzzi, P.; Bernasconi, G.; Re, M.; Misiak, B.; Crocamo, C.; Bartoli, F.; Carrà, G. Blood concentrations of anterior pituitary hormones in drug-naïve people with first-episode psychosis: A systematic review and meta-analysis. Psychoneuroendocrinology 2023, 158, 106392. [Google Scholar] [CrossRef]
- Melamed, S.B.; Farfel, A.; Gur, S.; Krivoy, A.; Weizman, S.; Matalon, A.; Feldhamer, I.; Hermesh, H.; Weizman, A.; Meyerovitch, J. Thyroid function assessment before and after diagnosis of schizophrenia: A community-based study. Psychiatry Res. 2020, 293, 113356. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Banki, C.M.; Arató, M.; Papp, Z. Thyroid stimulation test in healthy subjects and psychiatric patients. Acta Psychiatr. Scand. 1984, 70, 295–303. [Google Scholar] [CrossRef]
- Wahby, V.S.; Ibrahim, G.A.; Giller, E.L.; Mason, J.W.; Saddik, F.W.; Adams, J.R.; Martin, R.P.; Milad, E.R. Thyrotropin response to thyrotropin-releasing hormone in RDC schizodepressed men. J. Affect. Disord. 1988, 15, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Wolkowitz, O.; Doran, A.; Pickar, D. TRH test in schizophrenic patients and controls. Biol. Psychiatry 1989, 25, 523–526. [Google Scholar] [CrossRef]
- Rao, M.L.; Gross, G.; Strebel, B.; Bräunig, P.; Huber, G.; Klosterkötter, J. Serum amino acids, central monoamines, and hormones in drug-naive, drug-free, and neuroleptic-treated schizophrenic patients and healthy subjects. Psychiatry Res. 1990, 34, 243–257. [Google Scholar] [CrossRef]
- Baumgartner, A.; Pietzcker, A.; Gaebel, W. The hypothalamic-pituitary-thyroid axis in patients with schizophrenia. Schizophr. Res. 2000, 44, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Yazici, K.; Yazici, A.E.; Taneli, B. Different neuroendocrine profiles of remitted and nonremitted schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Bicikova, M.; Hampl, R.; Hill, M.; Ripova, D.; Mohr, P.; Putz, Z. Neuro- and immunomodulatory steroids and other biochemical markers in drug-naive schizophrenia patients and the effect of treatment with atypical antipsychotics. Neuroendocrinol. Lett. 2011, 32, 141–147. [Google Scholar]
- Garcia-Rizo, C.; Fernandez-Egea, E.; Oliveira, C.; Justicia, A.; Parellada, E.; Bernardo, M.; Kirkpatrick, B. Prolactin concentrations in newly diagnosed, antipsychotic-naïve patients with nonaffective psychosis. Schizophr. Res. 2012, 134, 16–19. [Google Scholar] [CrossRef]
- Wysokiński, A.; Kłoszewska, I. Level of thyroid-stimulating hormone (TSH) in patients with acute schizophrenia, unipolar depression or bipolar disorder. Neurochem. Res. 2014, 39, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Bratek, A.; Koźmin-Burzyńska, A.; Krysta, K.; Cierpka-Wiszniewska, K.; Krupka-Matuszczyk, I. Effects of hormones on cognition in schizophrenic male patients--preliminary results. Psychiatr. Danub. 2015, 27 (Suppl. 1), 261–265. [Google Scholar]
- Degner, D.; Haust, M.; Meller, J.; Rüther, E.; Reulbach, U. Association between autoimmune thyroiditis and depressive disorder in psychiatric outpatients. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 265, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Huang, J.; Tian, J.B.; Cao, Y.Y.; Zhang, G.L.; Wang, C.G.; Cao, Y.; Li, J.R. Factors associated with decreased bone mineral density in postmenopausal women with schizophrenia. Clin. Interv. Aging 2016, 11, 153–157. [Google Scholar] [CrossRef]
- Petrikis, P.; Tigas, S.; Tzallas, A.T.; Archimandriti, D.T.; Skapinakis, P.; Mavreas, V. Prolactin levels in drug-naïve patients with schizophrenia and other psychotic disorders. Int. J. Psychiatry Clin. Pract. 2016, 20, 165–169. [Google Scholar] [CrossRef]
- Telo, S.; Bilgic, S.; Karabulut, N. Thyroid Hormone Levels in Chronic Schizophrenic Patients: Association with Psychopathology. West. Indian Med. J. 2016, 65, 312–315. [Google Scholar] [CrossRef]
- Del Cacho, N.; Butjosa, A.; Vila-Badia, R.; Cuadras, D.; Kaplan, M.; Rubio-Abadal, E.; Pardo, M.; Muñoz-Samons, D.; Cuevas-Esteban, J.; Saenz-Navarrete, G.; et al. Prolactin levels in drug-naïve first episode nonaffective psychosis patients compared with healthy controls. Sex differences. Psychiatry Res. 2019, 276, 218–222. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, C.Y.; Wang, H.S.; Lane, H.Y. Long-term Use of Clozapine is Protective for Bone Density in Patients with Schizophrenia. Sci. Rep. 2019, 9, 3895. [Google Scholar] [CrossRef]
- Kalinowska, S.; Trześniowska-Drukała, B.; Safranow, K.; Pełka-Wysiecka, J.; Kłoda, K.; Misiak, B.; Samochowiec, J. Association between thyroid function and metabolic syndrome in male and female schizophrenia patients. Psychiatry Res. 2019, 274, 167–175. [Google Scholar] [CrossRef]
- Kornetova, E.G.; Kornetov, A.N.; Mednova, I.A.; Lobacheva, O.A.; Gerasimova, V.I.; Dubrovskaya, V.V.; Tolmachev, I.V.; Semke, A.V.; Loonen, A.J.M.; Bokhan, N.A.; et al. Body Fat Parameters, Glucose and Lipid Profiles, and Thyroid Hormone Levels in Schizophrenia Patients with or without Metabolic Syndrome. Diagnostics 2020, 10, 683. [Google Scholar] [CrossRef]
- Petruzzelli, M.G.; Marzulli, L.; Giannico, O.V.; Furente, F.; Margari, M.; Matera, E.; Margari, F. Glucose Metabolism, Thyroid Function, and Prolactin Level in Adolescent Patients With First Episode of Schizophrenia and Affective Disorders. Front. Psychiatry 2020, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ji, H.; Tao, L.; Cai, Q.; Wang, F.; Ji, W.; Li, G.; Fang, Y. Functional Status of Hypothalamic-Pituitary-Thyroid and Hypothalamic-Pituitary-Adrenal Axes in Hospitalized Schizophrenics in Shanghai. Front. Psychiatry 2020, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Duval, F.; Mokrani, M.C.; Erb, A.; Danila, V.; Gonzalez Lopera, F.; Jeanjean, L. Dopaminergic, Noradrenergic, Adrenal, and Thyroid Abnormalities in Psychotic and Affective Disorders. Front. Psychiatry 2020, 11, 533872. [Google Scholar] [CrossRef]
- Li, C.; Shi, Z.; Ji, J.; Niu, G.; Liu, Z. Associations of C-Reactive Protein, Free Triiodothyronine, Thyroid Stimulating Hormone and Creatinine Levels with Agitation in Patients with Schizophrenia: A Comparative Cross-Sectional Study. Neuropsychiatr. Dis. Treat. 2021, 17, 2575–2585. [Google Scholar] [CrossRef]
- Makarow-Gronert, A.; Margulska, A.; Strzelecki, D.; Krajewska, K.; Gmitrowicz, A.; Gawlik-Kotelnicka, O. Comparison of thyroid-stimulating hormone levels in adolescents with schizophrenia, bipolar disorder, unipolar depression, conduct disorders, and hyperkinetic disorders. Medicine 2021, 100, e28160. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ullah, S.; Subhan, F.; Nazar, Z.; Hussain, S.M.; Khuda, F.; Khan, A.; Khusro, A.; Sahibzada, M.U.K.; Albogami, S.; et al. Clinical Investigation on the Impact of Cannabis Abuse on Thyroid Hormones and Associated Psychiatric Manifestations in the Male Population. Front. Psychiatry 2021, 12, 730388. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, S.W.; Li, M.; Sun, Z.; Yuan, X.; Retnakaran, R.; Zhang, R.; Zhai, D. Dose-response association of acute-phase quetiapine treatment with risk of new-onset hypothyroidism in schizophrenia patients. Br. J. Clin. Pharmacol. 2021, 87, 4823–4830. [Google Scholar] [CrossRef]
- Ouyang, H.; Huang, M.; Chen, S.; Wu, X.; Zhou, D. Peripheral lower triiodothyronine levels related to interleukin-6 in patients with first-episode schizophrenia. Psychiatry Res. 2022, 312, 114546. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Bing, J.; Shan, X.; Luo, H.; Wen, W.; Gao, S.; Niu, K.; Cui, T.; Li, H.; Retnakaran, R.; et al. Higher central set point of thyroid homeostasis in drug-naïve patients affected by first episode schizophrenia. Schizophr. Res. 2022, 250, 62–66. [Google Scholar] [CrossRef]
- Zhao, Y.; Guan, Q.; Shi, J.; Sun, J.; Wang, Q.; Yang, J.; Retnakaran, R.; Han, J.; Zhang, X.; Hao, W.; et al. Impaired central set point of thyroid homeostasis during quetiapine treatment in the acute phase of schizophrenia. Schizophr. Res. 2022, 241, 244–250. [Google Scholar] [CrossRef]
- Esposito, C.M.; De Cagna, F.; Caldiroli, A.; Capuzzi, E.; Ceresa, A.; Di Paolo, M.; Auxilia, A.M.; Capellazzi, M.; Tagliabue, I.; Cirella, L.; et al. Gender differences in clinical and biochemical parameters among patients hospitalized for schizophrenia: Towards precision medicine. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 274, 1093–1103. [Google Scholar] [CrossRef]
- Li, K.; Long, J.; Cao, M.; Xue, R.; Chen, J.; Cheng, B.; Wang, J.; Guo, W.; Deng, W.; Li, T. Thyroid hormone optimize management in electroconvulsive therapy treatment of schizophrenia. Schizophr. Res. 2023, 252, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Głodek, M.; Skibinska, M.; Suwalska, A. Diet and physical activity and metabolic disorders in patients with schizophrenia and bipolar affective disorder in the Polish population. PeerJ 2023, 11, e15617. [Google Scholar] [CrossRef]
- Cui, T.; Qi, Z.; Wang, M.; Zhang, X.; Wen, W.; Gao, S.; Zhai, J.; Guo, C.; Zhang, N.; Zhang, X.; et al. Thyroid allostasis in drug-free affective disorder patients. Psychoneuroendocrinology 2024, 162, 106962. [Google Scholar] [CrossRef]
- Chen, J.; Ge, H.; Liu, N.; Li, Y.; Dong, Y.; Wang, X.; Xun, Z.; Li, S. Sex-specific differences in the relationship between thyroid hormones and neurocognition in schizophrenia: A large-scale cross-sectional study. Psychoneuroendocrinology 2025, 172, 107249. [Google Scholar] [CrossRef]
- Jiang, Q.H.; Gong, W.D. Correlation analyse between thyroid hormone levels and severity of schizophrenia symptoms. World J. Psychiatry 2025, 15, 100880. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Y.; Wu, Q.; Wang, Q.; Yang, W.F.Z.; Wang, Y.; Yang, D.; Luo, Y.; Tang, K.; Liu, T.; et al. Comparison of Thyroid Hormone Levels Between Patients With Major Depressive Disorder and Healthy Individuals in China. Front. Psychiatry 2021, 12, 750749. [Google Scholar] [CrossRef] [PubMed]
- Medici, M.; Direk, N.; Visser, W.E.; Korevaar, T.I.; Hofman, A.; Visser, T.J.; Tiemeier, H.; Peeters, R.P. Thyroid function within the normal range and the risk of depression: A population-based cohort study. J. Clin. Endocrinol. Metab. 2014, 99, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Galán, J.R.; Pedraza, P.; Santacana, M.; del Ray, F.E.; de Escobar, G.M.; Ruiz-Marcos, A. Early effects of iodine deficiency on radial glial cells of the hippocampus of the rat fetus. A model of neurological cretinism. J. Clin. Investig. 1997, 99, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, T.I.; Muetzel, R.; Medici, M.; Chaker, L.; Jaddoe, V.W.; de Rijke, Y.B.; Steegers, E.A.; Visser, T.J.; White, T.; Tiemeier, H.; et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: A population-based prospective cohort study. Lancet Diabetes Endocrinol. 2016, 4, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R. Neurodevelopmental and neurophysiological actions of thyroid hormone. J. Neuroendocrinol. 2008, 20, 784–794. [Google Scholar] [CrossRef]
- George, K.M.; Lutsey, P.L.; Selvin, E.; Palta, P.; Windham, B.G.; Folsom, A.R. Association Between Thyroid Dysfunction and Incident Dementia in the Atherosclerosis Risk in Communities Neurocognitive Study. J. Endocrinol. Metab. 2019, 9, 82–89. [Google Scholar] [CrossRef]
- Rieben, C.; Segna, D.; da Costa, B.R.; Collet, T.H.; Chaker, L.; Aubert, C.E.; Baumgartner, C.; Almeida, O.P.; Hogervorst, E.; Trompet, S.; et al. Subclinical Thyroid Dysfunction and the Risk of Cognitive Decline: A Meta-Analysis of Prospective Cohort Studies. J. Clin. Endocrinol. Metab. 2016, 101, 4945–4954. [Google Scholar] [CrossRef]
- Dayu, L.; Yubo, X.; Bin, J. Acute schizophrenia-like psychosis as major clinical presentation of Graves’ disease successfully treated by radioiodine in combination with antipsychotics: A case report. Actas Esp. Psiquiatr. 2018, 46, 242–248. [Google Scholar]
- da Silva, J.A.; Almeida, J.T.; Corrêa, B.B.; Narigão, M.; Xavier, M. Acute psychotic episode in a patient with thyrotoxicosis factitia. BMJ Case Rep. 2009, 2009, bcr0820080676. [Google Scholar] [CrossRef] [PubMed]
- Sumi, Y.; Kawahara, S.; Fujii, K.; Yamaji, M.; Nakajima, K.; Nakamura, T.; Horikawa, O.; Fujita, Y.; Ozeki, Y. Case report: Impact of hyperthyroidism on psychotic symptoms in schizophrenia comorbid with Graves’ disease. Front. Psychiatry 2023, 14, 1219049. [Google Scholar] [CrossRef]
- Bennett, B.; Mansingh, A.; Fenton, C.; Katz, J. Graves’ disease presenting with hypomania and paranoia to the acute psychiatry service. BMJ Case Rep. 2021, 14, e236089. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, H.; Yoshimi, T.; Yanai, H. A patient with Graves’ disease showing only psychiatric symptoms and negativity for both TSH receptor autoantibody and thyroid stimulating antibody. Thyroid. Res. 2012, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Lee, Y.C.; Tsai, S.J.; Hu, P.G.; Sim, C.B. Psychiatric disturbances associated with hyperthyroidism: An analysis report of 30 cases. Zhonghua Yi Xue Za Zhi 1995, 56, 393–398. [Google Scholar]
- Misiak, B.; Stańczykiewicz, B.; Wiśniewski, M.; Bartoli, F.; Carra, G.; Cavaleri, D.; Samochowiec, J.; Jarosz, K.; Rosińczuk, J.; Frydecka, D. Thyroid hormones in persons with schizophrenia: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 111, 110402. [Google Scholar] [CrossRef]
- Santos, N.C.; Costa, P.; Ruano, D.; Macedo, A.; Soares, M.J.; Valente, J.; Pereira, A.T.; Azevedo, M.H.; Palha, J.A. Revisiting thyroid hormones in schizophrenia. J. Thyroid. Res. 2012, 2012, 569147. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; London, E.D.; Silverman, D.H.; Rasgon, N.; Kirchheiner, J.; Whybrow, P.C. Thyroid, brain and mood modulation in affective disorder: Insights from molecular research and functional brain imaging. Pharmacopsychiatry 2003, 36 (Suppl. 3), 215–221. [Google Scholar] [CrossRef]
- Martin, J.V.; Sarkar, P.K. Nongenomic roles of thyroid hormones and their derivatives in adult brain: Are these compounds putative neurotransmitters? Front. Endocrinol. 2023, 14, 1210540. [Google Scholar] [CrossRef]
- Kringlen, E. Twin studies in schizophrenia with special emphasis on concordance figures. Am. J. Med. Genet. 2000, 97, 4–11. [Google Scholar] [CrossRef]
- Hanif, F.; Amir, Q.; Washdev, W. Effect of DIO2 Gene Polymorphism on Thyroid Hormone Levels and Its Correlation with the Severity of Schizophrenia in a Pakistani Population. Int. J. Mol. Sci. 2024, 25, 1915. [Google Scholar] [CrossRef]
- Palha, J.A.; Goodman, A.B. Thyroid hormones and retinoids: A possible link between genes and environment in schizophrenia. Brain Res. Rev. 2006, 51, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Estrada, F.; Crosas, J.M.; Ahuir, M.; Pérez-Muñoz, S.; Zabala, W.; Aguayo, R.; Barbero, J.D.; Montalvo, I.; Tost, M.; Llauradó, L.; et al. Free Thyroxine Concentrations Moderate the Response to a Cognitive Remediation Therapy in People with Early Psychosis: A Pilot Randomized Clinical Trial. Front. Psychiatry 2020, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Sharif, K.; Tiosano, S.; Watad, A.; Comaneshter, D.; Cohen, A.D.; Shoenfeld, Y.; Amital, H. The link between schizophrenia and hypothyroidism: A population-based study. Immunol. Res. 2018, 66, 663–667. [Google Scholar] [CrossRef]
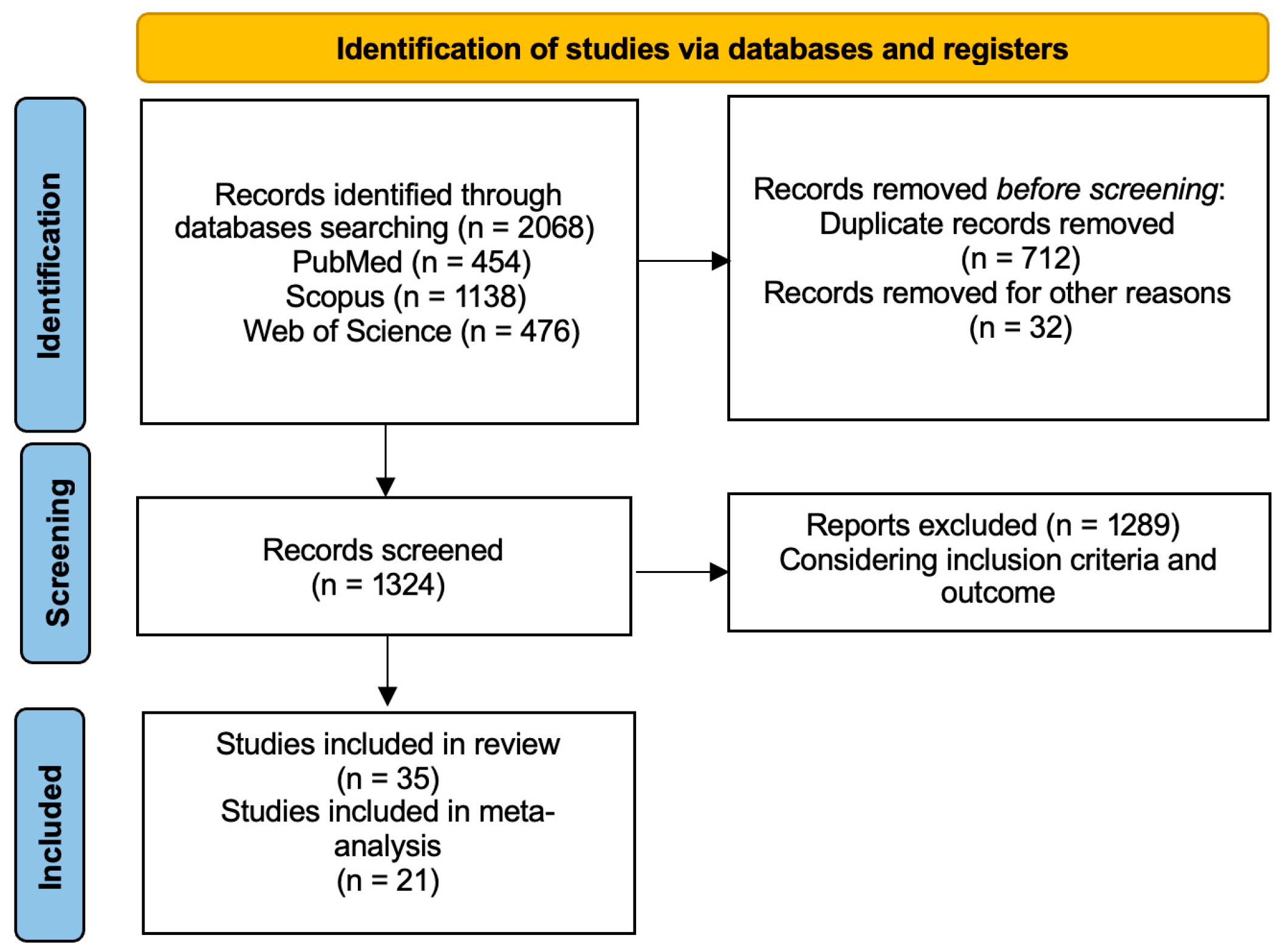
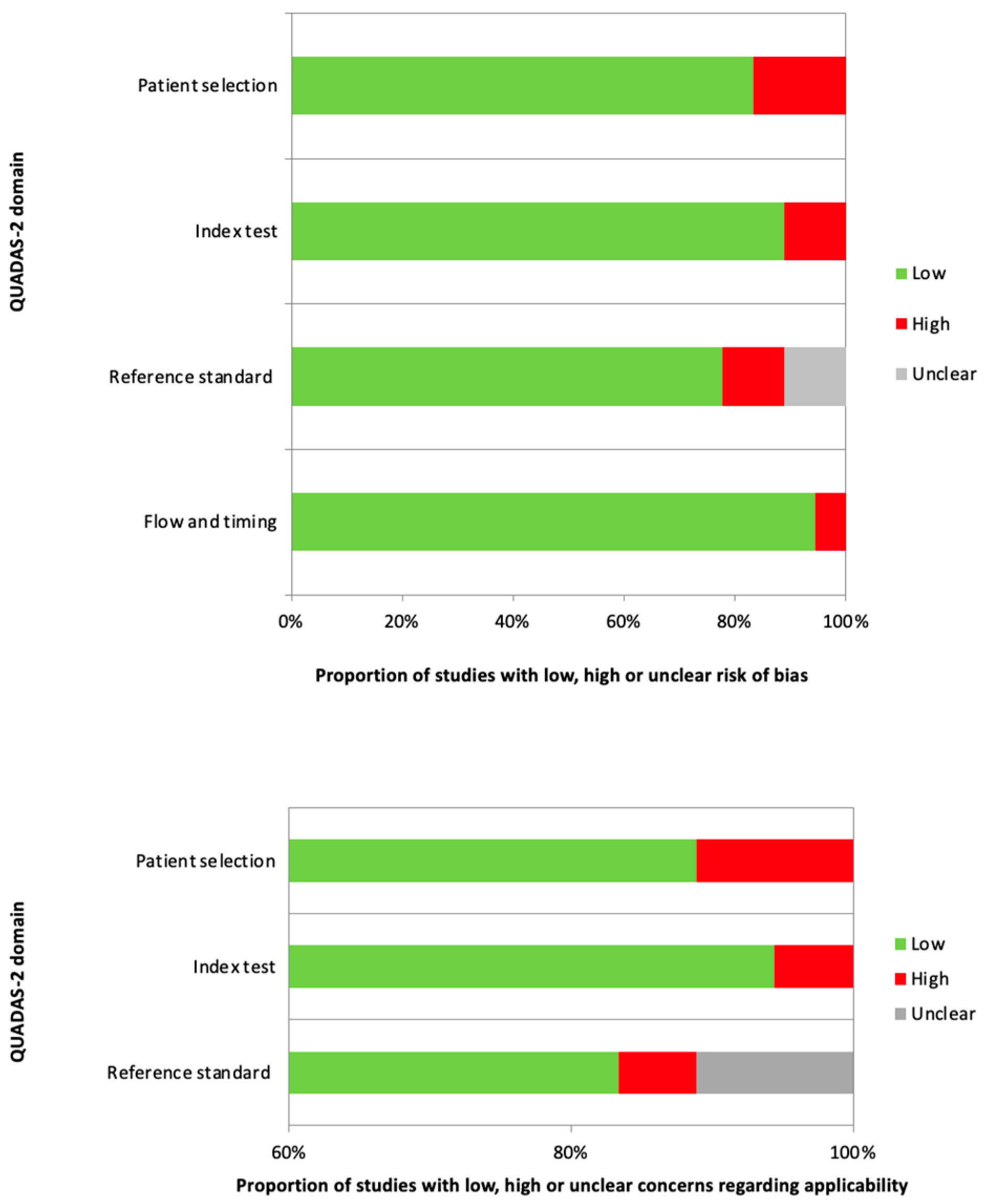
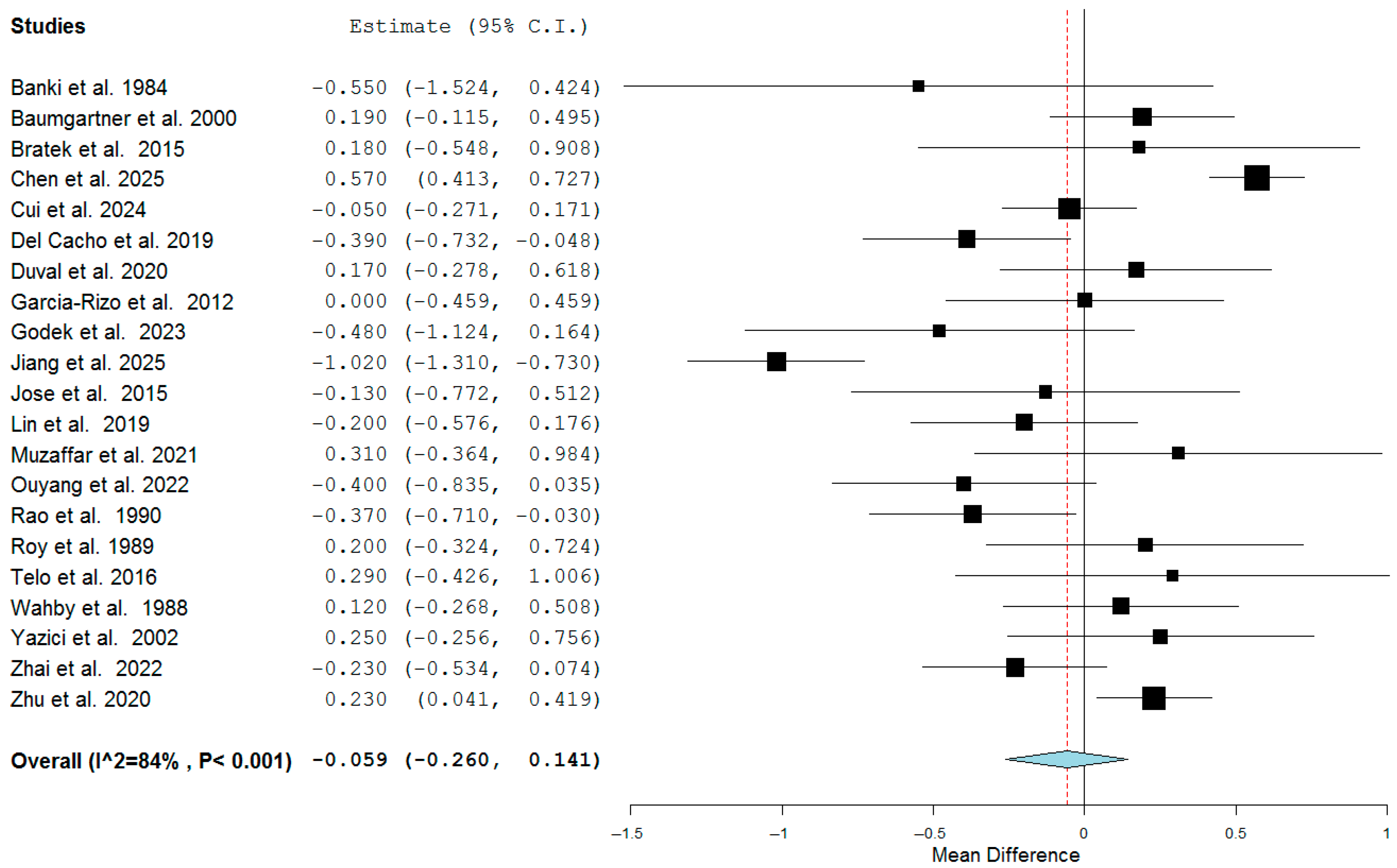
| First Author | Ref. N. | Year | Country | Study Design | N. Pts. | Age | Sex M:F |
|---|---|---|---|---|---|---|---|
| Banki | [15] | 1984 | Hungary | Interventional | 24 | 41.0 ± 13.0 | 0/24 |
| Wahby | [16] | 1988 | United States | Interventional | 37 | 35.5 ± 3.9 | 37/0 |
| Roy | [17] | 1989 | United States | Interventional | 14 | 25.4 ± 4.4 | 7/7 |
| Rao | [18] | 1990 | German | Retrospective cross-sectional | 110 | 34 ± 13 | 58/52 |
| Baumgartner | [19] | 2000 | Germany | Prospective cross-sectional | 31 | 29.2 ± 9.1 | 22/9 |
| Yazici | [20] | 2002 | Turkey | Interventional | 58 | 32.5 ± 11.4 | 35/23 |
| Bicikova | [21] | 2011 | Czech Republic | Interventional | 22 | 32.6 ± 7.4 | 13/9 |
| Garcia-Rizo | [22] | 2012 | Spain | Prospective cross-sectional | 33 | 28.6 ± 7.1 | 20/13 |
| Wysokiński | [23] | 2014 | Poland | Retrospective cross-sectional | 769 | 40.0 ± 16.2 | 381/388 |
| Bratek | [24] | 2015 | Poland | Prospective cross-sectional | 15 | 36.6 ± 7.5 | 15/0 |
| Degner | [25] | 2015 | Germany | Prospective cross-sectional | 19 | 43.5 | 7/12 |
| Jose | [8] | 2015 | India | Retrospective cross-sectional | 38 | 26.8 ± 4.4 | 38/0 |
| Liang | [26] | 2016 | China | Retrospective observational | 219 | 61.1 ± 6.6 | 0/219 |
| Petrikis | [27] | 2016 | Greece | Retrospective observational | 40 | 32.5 ± 9.8 | 27/13 |
| Telo | [28] | 2016 | Turkey | Retrospective cross-sectional | 63 | 44.7 ± 10.4 | 31/32 |
| Del Cacho | [29] | 2019 | Spain | Prospective cross-sectional | 61 | 24.6 ± 9.3 | 38/23 |
| Lin | [30] | 2019 | Taiwan | Prospective cross-sectional | 69 | 41.8 ± 10.4 | 40/29 |
| Kalinowska | [31] | 2019 | Poland | Retrospective observational | 106 | 41.89 ± 9.7 | 42/64 |
| Kornetova | [32] | 2020 | Russia | Retrospective cross-sectional | 156 | NA | 68/88 |
| Petruzzelli | [33] | 2020 | Italy | Retrospective observational | 30 | 15.4 ± 1.7 | 6/16 |
| Zhu | [34] | 2020 | China | Retrospective cross-sectional | 486 | 39.3 ± 12.6 | 292/194 |
| Duval | [35] | 2021 | France | Interventional | 13 | 31.1 ± 10.3 | 13/0 |
| Li | [36] | 2021 | China | Retrospective cross-sectional | 83 | 34 (IQR 29–47) | 37/46 |
| Makarow-Gronert | [37] | 2021 | Poland | Retrospective cross-sectional | 59 | NA | 23/36 |
| Muzaffar | [38] | 2021 | United States | Prospective cross-sectional | 19 | NA | 19/0 |
| Zhao | [39] | 2021 | China | Retrospective observational | 2022 | 31.3 ± 10.8 | 801/1221 |
| Ouyang | [40] | 2022 | China | Prospective observational | 46 | 25.33 ± 5.75 | 22/24 |
| Zhai | [41] | 2022 | China | Retrospective observational | 235 | 26.5 ± 9.5 | 87/148 |
| Zhao | [42] | 2022 | China | Retrospective observational | 1302 | 32.5 ± 11.1 | 455/847 |
| Esposito | [43] | 2023 | Italy | Retrospective observational | 555 | 43.4 ± 13.9 | 322/233 |
| Li | [44] | 2023 | China | Retrospective observational | 89 | 21.83 ± 7.94 | 50/39 |
| Głodek | [45] | 2023 | Poland | Prospective cross-sectional | 47 | 39.1 ± 11.4 | 25/22 |
| Cui | [46] | 2024 | China | Prospective cross-sectional | 1186 | 29.27 ± 9.35 | 536/650 |
| Chen | [47] | 2025 | China | Prospective cross-sectional | 1007 | 47.01 ± 13.02 | 602/405 |
| Jiang | [48] | 2025 | China | Retrospective observational | 100 | 34.6 ± 10.4 | 46/54 |
| First Author | Patients’ Characteristics | TSH Levels | Main Findings |
|---|---|---|---|
| Banki [15] | Hospitalized adult women | 1.89 ± 1.37 | No difference in TRH–TSH response was found between SHZ and controls. |
| Baumgartner [19] | Hospitalized adult patients for acute SHZ | 1.2 ± 0.7 | No difference in TSH was found among SHZ patients and healthy controls. |
| Bicikova [21] | Patients diagnosed with SHZ | 1.21 (IQR 0.8–1.8) | SHZ patients showed lower TSH levels and higher AbTPO titers. |
| Bratek [24] | Hospitalized adult patients | 1.76 ± 1.08 | No difference in TSH was found among SHZ patients and healthy controls. |
| Chen [47] | Hospitalized adult patients | 2.05 ± 2.25 | Sex differences exist in thyroid hormone T3 levels in people with schizophrenia. |
| Cui [46] | Aged below 50 adult patients | 1.89 ± 1.74 | No differences in TSH levels were found among SHZ and healthy controls. |
| Degner [25] | Adult outpatients without previously diagnosed thyroidal diseases. | 1.6 ± 1.5 | No differences in TSH levels were found among BD, MDD, and SHZ patients; AbTPO levels were higher in MDD and BD compared with SHZ. |
| Del Cacho [29] | Inpatients hospitalized for acute illness | 1.47 ± 0.95 | Significantly lower TSH levels were found in acute SHZ patients. |
| Duval [35] | Adult male hospitalized patients who underwent TRH–TSH stimulation | 1.30 ± 0.69 | TSH response is unaltered in schizophrenia patients. |
| Esposito [43] | Adult inpatients diagnosed with SHZ | 2.33 ± 2.56 | TSH levels were significantly higher, and hypothyroidism was more frequent, in women inpatients than men. |
| Garcia-Rizo [22] | SHZ outpatients | 1.8 ± 1.0 | No differences in TSH levels were found among SHZ patients and healthy controls. |
| Głodek [45] | Adults hospitalised in the Department of Adult Psychiatry with age between 18 and 65 | 1.50 ± 0.78 | No significant differences in thyroid function were found between BD and schizophrenia patients. |
| Jiang [48] | Adult inpatients diagnosed with SHZ | 3.10 ± 0.90 | The serum levels of T3, FT3, FT4, TSH, and cortisol in the schizophrenia group were lower than those in the control group (p < 0.05). |
| Jose [8] | SHZ patients aged 18 to 45 years | 1.82 ± 1.61 | fT4 increased in patients with schizophrenia as compared with controls and in those with suicidal ideation. |
| Kalinowska [31] | Outpatients aged 18 to 70 years during the remitted state of the disease | 2.28 ± 1.46 | An association between TSH values and metabolic syndrome criteria was found in patients with SHZ. |
| Kornetova [32] | Inpatients aged 18 to 55 years living in Western Siberia. | 1.38 (IQR 0.81–2.03) | SHZ patients showed lower TSH levels than controls. |
| Li [36] | Inpatients aged 18 to 60 years. | 2.54 (IQR 1.67–4.20) | Increased fT3 and decreased serum TSH levels were independent risk factors for agitation in hospitalized patients with SHZ. |
| Li [44] | Adult inpatients diagnosed with SHZ | 1.85 ± 0.93 | Electroconvulsive therapy impaired hypothalamus–pituitary–thyroid axis: y THRT may help prevent amnesia. |
| Liang [26] | Chinese post-menopausal SHZ women patients | 3.2 ± 2.9 | TSH levels were superimposable on the prevalence of abnormal bone mineral density in SHZ women. |
| Lin [30] | SHZ outpatients and inpatients | 1.5 ± 0.8 | No differences in TSH levels were found among SHZ and healthy controls. |
| Makarow-Gronert [37] | Caucasian patients aged 12 to 18 years who were hospitalized in the Department of Adolescent Psychiatry. | 2.12 ± 1.01 | There may be a higher prevalence of thyroid dysfunctions in BD and MDD subgroups among adolescents than in SHZ. |
| Muzaffar [38] | Cannabis related SHZ in male patients aged 18 to 60. | 1.61 ± 1.38 | No differences in TSH, fT4, and fT3 serum levels were found among patients and healthy controls. |
| Ouyang [40] | Outpatients with first episode SHZ | 1.69 ± 0.87 | There was no significant difference in TSH, fT4, and fT3 levels between SHZ patients and healthy controls. |
| Petrikis [27] | Adult inpatients with SHZ | 1.45 (IQR 0.26–3.49) | No differences in TSH levels among SHZ and healthy subjects were found; patients had lower levels of fT3 than controls. |
| Petruzzelli [33] | Adolescent inpatients admitted to Child and Adolescent Psychiatric Unit for first episode of SHZ. | 2.0 ± 1.1 | fT4 levels were significantly higher in SHZ patients than in those diagnosed with affective spectrum disorder. |
| Rao [18] | Adult SHZ inpatients | 1.53 ± 1.11 | Dopaminergic hyperactivity in SHZ may be related to a decrease in TSH hormone levels. |
| Roy [17] | SHZ outpatients who underwent TRH-TSH stimulation | 2.6 ± 0.8 | No differences were found between SHZ patients and healthy controls in TSH basal levels and TRH–TSH response. |
| Telo [28] | Adult patients with SHZ | 2.15 ± 1.53 | No differences were found between SHZ patients and healthy controls in TSH levels |
| Wahby [16] | Adult male patients who underwent TRH–TSH stimulation | 2.92 ± 0.24 | Schizodepressed patients appeared significantly different from MDD but closer to SHZ and healthy controls on the TRH test. |
| Wysokiński [23] | Hospitalized patients in acute phase evaluated at first entry | 1.71 ± 1.49 | In patients with schizophrenia, older patients had the lowest level of TSH. |
| Yazici [20] | Patients admitted to the psychiatric clinic for SHZ and followed up for 1 year. | 1.35 ± 1.62 | No differences in basal TSH levels were found among SHZ patients and healthy controls. |
| Zhai [41] | Patients with first episode of SHZ | 1.72 ± 1.69 | A higher central set point of thyroid homeostasis may be involved in the underlying mechanism of thyroid allostatic load in drug-naïve patients affected by first-episode of SHZ |
| Zhao [39] | Inpatients with a diagnosis of SHZ admitted with normal thyroid function tests | 1.80 ± 1.52 | Acute phase quetiapine treatment for schizophrenia patients was strongly associated with increased risk of developing new-onset hypothyroidism, with a clear dose–response association. |
| Zhao [42] | Inpatients with a diagnosis of SHZ admitted with normal thyroid function tests | 1.83 ± 1.48 | Impaired central set point may be involved in the mechanism by which quetiapine affects hypothalamus–pituitary–thyroid axis in acute phase of SHZ. |
| Zhu [34] | Chinese inpatients with SHZ | 2.09 ± 1.48 | Decreased fT3 and fT4 appear to be associated with SHZ symptoms. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatta, E.; Dondi, F.; Pirola, I.; Delbarba, A.; Maltese, V.; Bellini, P.; Ugoccioni, M.; Silvestrini, I.; Rotondi, M.; Bertagna, F.; et al. Is There a Link Between TSH Levels and Schizophrenia? A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 5959. https://doi.org/10.3390/jcm14175959
Gatta E, Dondi F, Pirola I, Delbarba A, Maltese V, Bellini P, Ugoccioni M, Silvestrini I, Rotondi M, Bertagna F, et al. Is There a Link Between TSH Levels and Schizophrenia? A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(17):5959. https://doi.org/10.3390/jcm14175959
Chicago/Turabian StyleGatta, Elisa, Francesco Dondi, Ilenia Pirola, Andrea Delbarba, Virginia Maltese, Pietro Bellini, Massimiliano Ugoccioni, Irene Silvestrini, Mario Rotondi, Francesco Bertagna, and et al. 2025. "Is There a Link Between TSH Levels and Schizophrenia? A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 17: 5959. https://doi.org/10.3390/jcm14175959
APA StyleGatta, E., Dondi, F., Pirola, I., Delbarba, A., Maltese, V., Bellini, P., Ugoccioni, M., Silvestrini, I., Rotondi, M., Bertagna, F., & Cappelli, C. (2025). Is There a Link Between TSH Levels and Schizophrenia? A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(17), 5959. https://doi.org/10.3390/jcm14175959






