Visual and Predictive Assessment of Pneumothorax Recurrence in Adolescents Using Machine Learning on Chest CT
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Machine Learning Algorithms
2.3. Ethical Statement
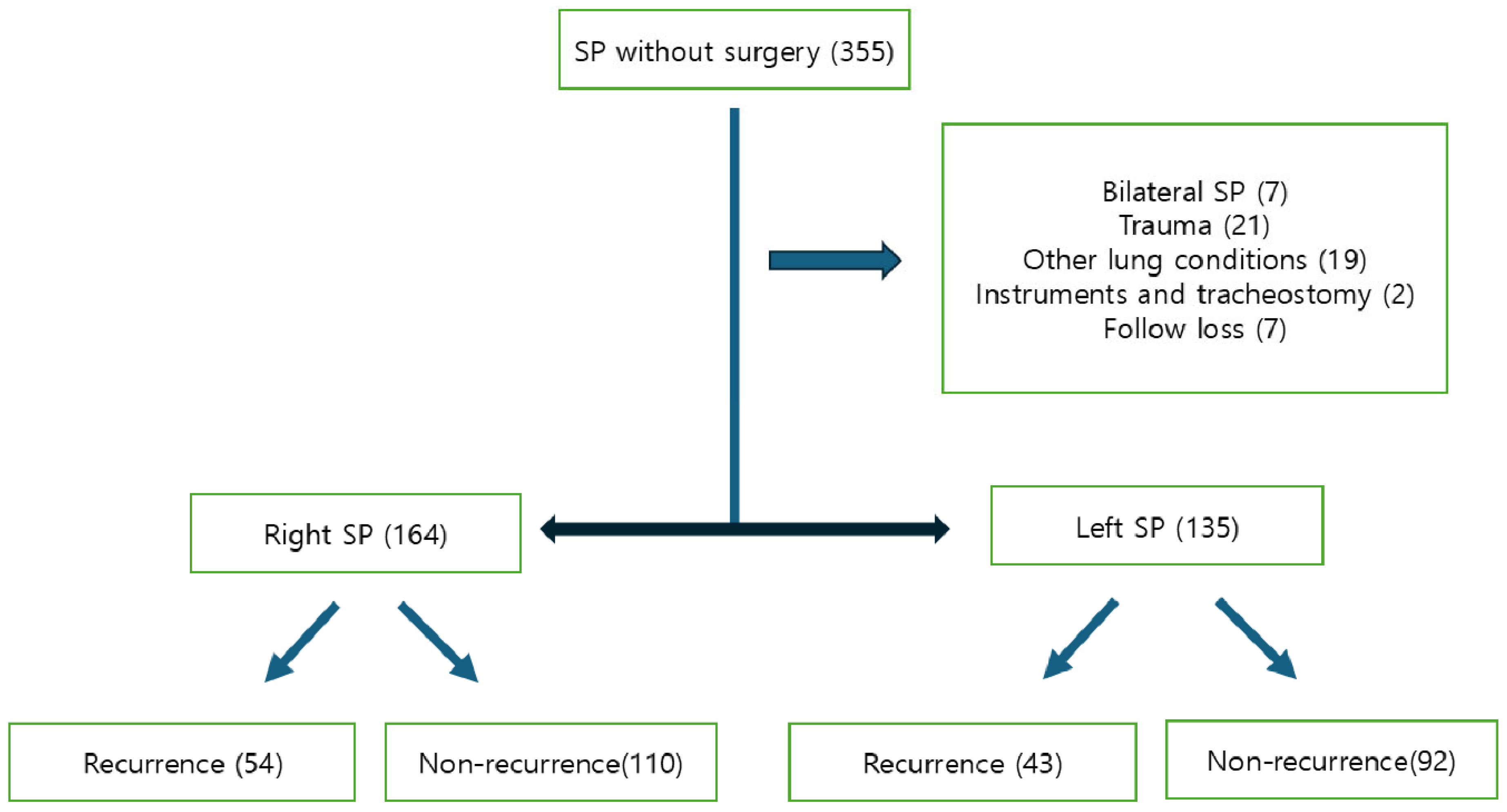
3. Results
3.1. Risk Factors for Recurrence in Young Adolescent Patients
3.2. Prediction of SP Recurrence Using Machine Learning Models with Chest CT Imaging
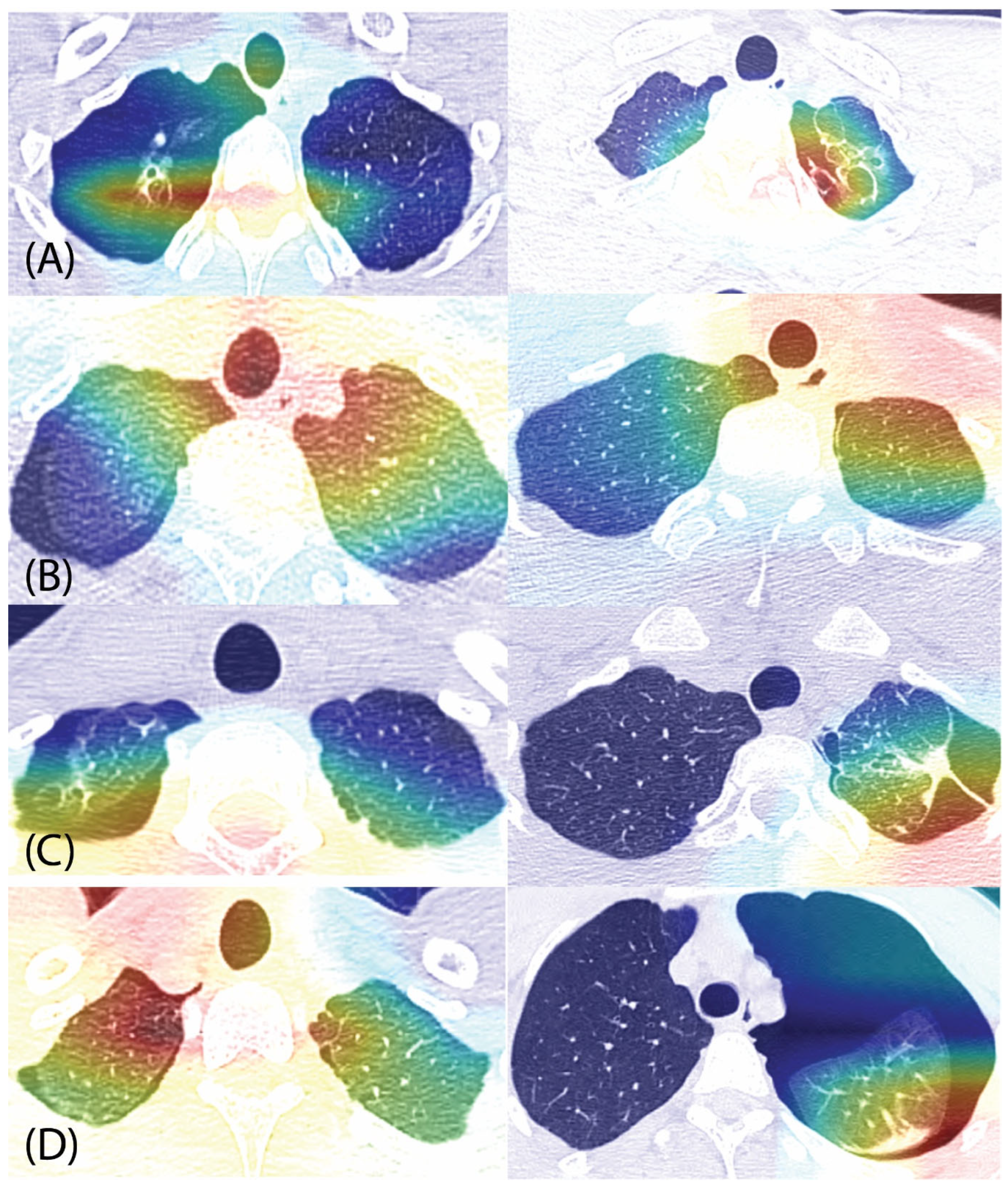
3.3. Grad-CAM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walker, S.; Hallifax, R.; Ricciardi, S.; Fitzgerald, D.; Keijzers, M.; Lauk, O.; Petersen, J.; Bertolaccini, L.; Bodtger, U.; Clive, A.; et al. Joint ERS/EACTS/ESTS clinical practice guidelines on adults with spontaneous pneumothorax. Eur. J. Cardiothorac. Surg. 2024, 65, ezae189. [Google Scholar] [CrossRef] [PubMed]
- Hallifax, R. Aetiology of Primary Spontaneous Pneumothorax. J. Clin. Med. 2022, 11, 490. [Google Scholar] [CrossRef]
- Tran, Z.; Haro, G.; Ebrahimian, S.; Verma, A.; Revels, S.; Benharash, P. Association of initial management on readmissions for spontaneous pneumothorax in adolescents. Surgery 2022, 172, 385–390. [Google Scholar] [CrossRef]
- Brown, S.G.A.; Ball, E.L.; Perrin, K.; Asha, S.E.; Braithwaite, I.; Egerton-Warburton, D.; Jones, P.G.; Keijzers, G.; Kinnear, F.B.; Kwan, B.C.H.; et al. Conservative versus Interventional Treatment for Spontaneous Pneumothorax. N. Engl. J. Med. 2020, 382, 405–415. [Google Scholar] [CrossRef]
- Hung, C.S.; Chen, Y.C.; Yang, T.F.; Huang, F.H. Systematic review and meta-analysis on juvenile primary spontaneous pneumothorax: Conservative or surgical approach first? PLoS ONE 2021, 16, e0250929. [Google Scholar] [CrossRef]
- Cattoni, M.; Rotolo, N.; Mastromarino, M.G.; Cardillo, G.; Nosotti, M.; Mendogni, P.; Rizzi, A.; Raveglia, F.; Siciliani, A.; Rendina, E.A.; et al. Analysis of pneumothorax recurrence risk factors in 843 patients who underwent videothoracoscopy for primary spontaneous pneumothorax: Results of a multicentric study. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 78–84. [Google Scholar] [CrossRef]
- Yang, H.C.; Jung, S. Bullae formation hypothesis in primary spontaneous pneumothorax. J. Thorac. Dis. 2020, 12, 2833–2837. [Google Scholar] [CrossRef]
- Soyer, T.; Dariel, A.; Dingemann, J.; Martinez, L.; Pini-Prato, A.; Morini, F.; De Coppi, P.; Gorter, R.; Doi, T.; Antunovic, S.S.; et al. European Pediatric Surgeons’ Association Survey on the Management of Primary Spontaneous Pneumothorax in Children. Eur. J. Pediatr. Surg. 2022, 32, 415–421. [Google Scholar] [CrossRef]
- Cerchia, E.; Conighi, M.L.; Bleve, C.; Chiarenza, S.F.; Sgrò, A.; Pini Prato, A.; Rotundi, F.; Parolini, F.; Bulotta, A.L.; Alberti, D.; et al. Feasibility of a Standardized Management for Primary Spontaneous Pneumothorax in Children and Adolescents: A Retrospective Multicenter Study and Review of the Literature. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 841–846. [Google Scholar] [CrossRef]
- Bennani, S.; Regnard, N.E.; Ventre, J.; Lassalle, L.; Nguyen, T.; Ducarouge, A.; Dargent, L.; Guillo, E.; Gouhier, E.; Zaimi, S.H.; et al. Using AI to Improve Radiologist Performance in Detection of Abnormalities on Chest Radiographs. Radiology 2023, 309, e230860. [Google Scholar] [CrossRef]
- Deng, L.Y.; Lim, X.Y.; Luo, T.Y.; Lee, M.H.; Lin, T.C. Application of Deep Learning Techniques for Detection of Pneumothorax in Chest Radiographs. Sensors 2023, 23, 7369. [Google Scholar] [CrossRef]
- Nagendran, M.; Chen, Y.; Lovejoy, C.A.; Gordon, A.C.; Komorowski, M.; Harvey, H.; Topol, E.J.; Ioannidis, J.P.A.; Collins, G.S.; Maruthappu, M. Artificial intelligence versus clinicians: Systematic review of design, reporting standards, and claims of deep learning studies. BMJ 2020, 368, m689. [Google Scholar] [CrossRef] [PubMed]
- Iyoda, A.; Azuma, Y.; Sakai, T.; Koezuka, S.; Otsuka, H.; Sano, A. A novel finding related to bulla and bleb formation in patients with primary spontaneous pneumothorax. BMC Pulm. Med. 2021, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.; Kim, J.J.; Choi, W.K.; Kim, Y.H.; Han, S.C. Prediction of postoperative final degree and recurrence of pectus excavatum using machine learning algorithms. J. Thorac. Dis. 2024, 16, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Reis, H.C.; Turk, V.; Khoshelham, K.; Kaya, S. MediNet: Transfer learning approach with MediNet medical visual database. Multimed. Tools Appl. 2023, 25, 39211–39254. [Google Scholar] [CrossRef]
- Yue, X.; Cui, J.; Huang, S.; Liu, W.; Qi, J.; He, K.; Li, T. An Interpretable Radiomics-Based Machine Learning Model for Predicting Reverse Left Ventricular Remodeling in STEMI Patients Using Late Gadolinium Enhancement of Myocardial Scar. Eur. Radiol. 2025. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Chen, W.; Zhang, J.; Wang, Y.; Zhou, H.; Xu, Y.; Li, H.; Ma, X. Machine Learning-Based Immune Signature for Predicting Prognosis and Immunotherapy Response in Hepatocellular Carcinoma. Front. Immunol. 2024, 15, 1446511. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Luo, G.; Liu, Z.; Huang, J.; Zhou, Y.; Zhou, Y.; Xu, W.; Cheng, J.Z. Automated segmentation and diagnosis of pneumothorax on chest X-rays with fully convolutional multi-scale ScSE-DenseNet: A retrospective study. BMC Med. Inf. Decis. Mak. 2020, 20, 317. [Google Scholar] [CrossRef]
- Shamrat, F.; Azam, S.; Karim, A.; Islam, R.; Tasnim, Z.; Ghosh, P.; De Boer, F. LungNet22: A Fine-Tuned Model for Multiclass Classification and Prediction of Lung Disease Using X-ray Images. J. Pers. Med. 2022, 12, 680. [Google Scholar] [CrossRef]
- Zhang, H.; Ogasawara, K. Grad-CAM-Based Explainable Artificial Intelligence Related to Medical Text Processing. Bioengineering 2023, 10, 1070. [Google Scholar] [CrossRef]
- Vallejo, W.; Díaz-Uribe, C.; Fajardo, C. Google Colab and Virtual Simulations: Practical e-Learning Tools to Support the Teaching of Thermodynamics and to Introduce Coding to Students. ACS Omega 2022, 7, 7421–7429. [Google Scholar] [CrossRef]
- Nonomura, R.; Sugawara, T.; Yabe, R.; Oshima, Y.; Sasaki, T.; Ishibashi, N. Age-Specific Body Shape Characteristics in the Onset of Spontaneous Pneumothorax: A Comparison Between Teens and 20s. Cureus 2024, 16, e71922. [Google Scholar] [CrossRef]
- Huang, N.; He, S.; Chen, S.; Zhang, G.; Ruan, L.; Huang, J. Incidence and risk factors for recurrent primary spontaneous pneumothorax after video-assisted thoracoscopic surgery: A systematic review and meta-analysis. J. Thorac. Dis. 2024, 16, 3696–3710. [Google Scholar] [CrossRef] [PubMed]
- DeMaio, A.; Semaan, R. Management of Pneumothorax. Clin. Chest Med. 2021, 42, 729–738. [Google Scholar] [CrossRef]
- Özalp, T.; Karapinar, K. Is there a change in the view of treatment for primary spontaneous pneumothorax?: The effect of thoracic CT and autologous blood pleurodesis: A retrospective cohort study. Medicine 2024, 103, e38639. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Kaneda, H.; Fukumoto, K.; Matsui, H.; Taniguchi, Y.; Saito, T.; Murakawa, T. A retrospective analysis of treatment selection and risk factors of treatment failure and recurrence in patients with spontaneous pneumothorax. J. Thorac. Dis. 2023, 15, 1217–1227. [Google Scholar] [CrossRef]
- Lind Plesner, L.; Müller, F.C.; Brejnebøl, M.W.; Laustrup, L.C.; Rasmussen, F.; Nielsen, O.W.; Boesen, M.; Brun Andersen, M. Commercially Available Chest Radiograph AI Tools for Detecting Airspace Disease, Pneumothorax, and Pleural Effusion. Radiology 2023, 308, e231236. [Google Scholar] [CrossRef]
- Guimaraes, C.V.; Donnelly, L.F.; Warner, B.W. CT findings for blebs and bullae in children with spontaneous pneumothorax and comparison with findings in normal age-matched controls. Pediatr. Radiol. 2007, 37, 879–884. [Google Scholar] [CrossRef]
- Kim, J.T.; Oh, T.Y.; Chang, W.H.; Kong, J.H.; Baek, K.S.; Lee, W.J.; Bang, Y.Y. Natural course of spontaneous pneumothorax without bullae or blebs under high-resolution computed tomography. Thorac. Cardiovasc. Surg. 2014, 62, 505–508. [Google Scholar] [CrossRef]
- Furuta, C.; Yano, M.; Kitagawa, Y.; Katsuya, R.; Ozeki, N.; Fukui, T. Prospective Observation Study for Primary Spontaneous Pneumothorax: Incidence of and Risk Factors for Postoperative Neogenesis of Bullae. Ann. Thorac. Cardiovasc. Surg. 2024, 30, oa-23. [Google Scholar] [CrossRef]

| Variables | Right Side | Left Side | ||||
|---|---|---|---|---|---|---|
| NRG (n = 110) | RG (n = 54) | p-Value | NRG (n = 92) | RG (n = 43) | p-Value | |
| Age | 17.3 ± 1.6 | 16.9 ± 1.3 | 0.080 | 17.5 ± 1.5 | 17.4 ± 1.5 | 0.561 |
| Sex (male) | 104 | 52 | 1.000 | 85 | 39 | 0.743 |
| Smoking | 10 | 5 | 1.000 | 6 | 2 | 1.000 |
| Blebs or bullae | 10 | 14 | 0.006 | 4 | 14 | <0.001 |
| Variables | Odds Ratio | p-Value | 95% CI |
|---|---|---|---|
| Age | 0.823 | 0.027 | 0.692–0.978 |
| Blebs or bullae | 6.035 | <0.001 | 2.951–12.339 |
| Lung Laterality | Model Type | AUC (95% CI) | Accuracy (95% CI) | F1 Score (95% CI) | Precision (95% CI) | Recall (95% CI) |
|---|---|---|---|---|---|---|
| Right | Neural network | 0.970 (0.961–0.979) | 0.937 (0.945–0.971) | 0.936 (0.918–0.957) | 0.937 (0.906–0.962) | 0.937 (0.906–0.962) |
| Logistic regression | 0.958 (0.949–0.967) | 0.928 (0.938–0.966) | 0.927 (0.908–0.949) | 0.928 (0.895–0.954) | 0.928 (0.895–0.954) | |
| Support vector machine | 0.950 (0.941–0.959) | 0.902 (0.919–0.950) | 0.903 (0.877–0.925) | 0.904 (0.867–0.934) | 0.902 (0.863–0.931) | |
| Gradient boosting | 0.934 (0.925–0.943) | 0.868 (0.894–0.930) | 0.862 (0.840–0.896) | 0.871 (0.828–0.905) | 0.868 (0.825–0.902) | |
| Random forest | 0.865 (0.856–0.874) | 0.813 (0.858–0.900) | 0.798 (0.781–0.848) | 0.821 (0.775–0.862) | 0.813 (0.765–0.853) | |
| Left | Neural network | 0.958 (0.949–0.967) | 0.905 (0.919–0.954) | 0.905 (0.874–0.928) | 0.905 (0.860–0.936) | 0.905 (0.860–0.936) |
| Logistic regression | 0.936 (0.927–0.945) | 0.881 (0.903–0.941) | 0.881 (0.849–0.909) | 0.881 (0.834–0.917) | 0.881 (0.834–0.917) | |
| Support vector machine | 0.934 (0.925–0.943) | 0.877 (0.900–0.939) | 0.877 (0.829–0.914) | 0.877 (0.829–0.914) | 0.877 (0.829–0.914) | |
| Gradient boosting | 0.907 (0.898–0.916) | 0.844 (0.877–0.920) | 0.838 (0.807–0.875) | 0.843 (0.791–0.884) | 0.844 (0.795–0.887) | |
| Random forest | 0.848 (0.839–0.857) | 0.786 (0.837–0.886) | 0.771 (0.745–0.823) | 0.783 (0.729–0.832) | 0.786 (0.732–0.835) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, K.; Kim, J.J.; Im, K.S.; Han, S.C.; Ryu, J.H. Visual and Predictive Assessment of Pneumothorax Recurrence in Adolescents Using Machine Learning on Chest CT. J. Clin. Med. 2025, 14, 5956. https://doi.org/10.3390/jcm14175956
Hyun K, Kim JJ, Im KS, Han SC, Ryu JH. Visual and Predictive Assessment of Pneumothorax Recurrence in Adolescents Using Machine Learning on Chest CT. Journal of Clinical Medicine. 2025; 14(17):5956. https://doi.org/10.3390/jcm14175956
Chicago/Turabian StyleHyun, Kwanyong, Jae Jun Kim, Kyong Shil Im, Sang Chul Han, and Jeong Hwan Ryu. 2025. "Visual and Predictive Assessment of Pneumothorax Recurrence in Adolescents Using Machine Learning on Chest CT" Journal of Clinical Medicine 14, no. 17: 5956. https://doi.org/10.3390/jcm14175956
APA StyleHyun, K., Kim, J. J., Im, K. S., Han, S. C., & Ryu, J. H. (2025). Visual and Predictive Assessment of Pneumothorax Recurrence in Adolescents Using Machine Learning on Chest CT. Journal of Clinical Medicine, 14(17), 5956. https://doi.org/10.3390/jcm14175956







