Cancer Risk in Autoimmune and Immune-Mediated Diseases: A Narrative Review for Practising Clinicians
Abstract
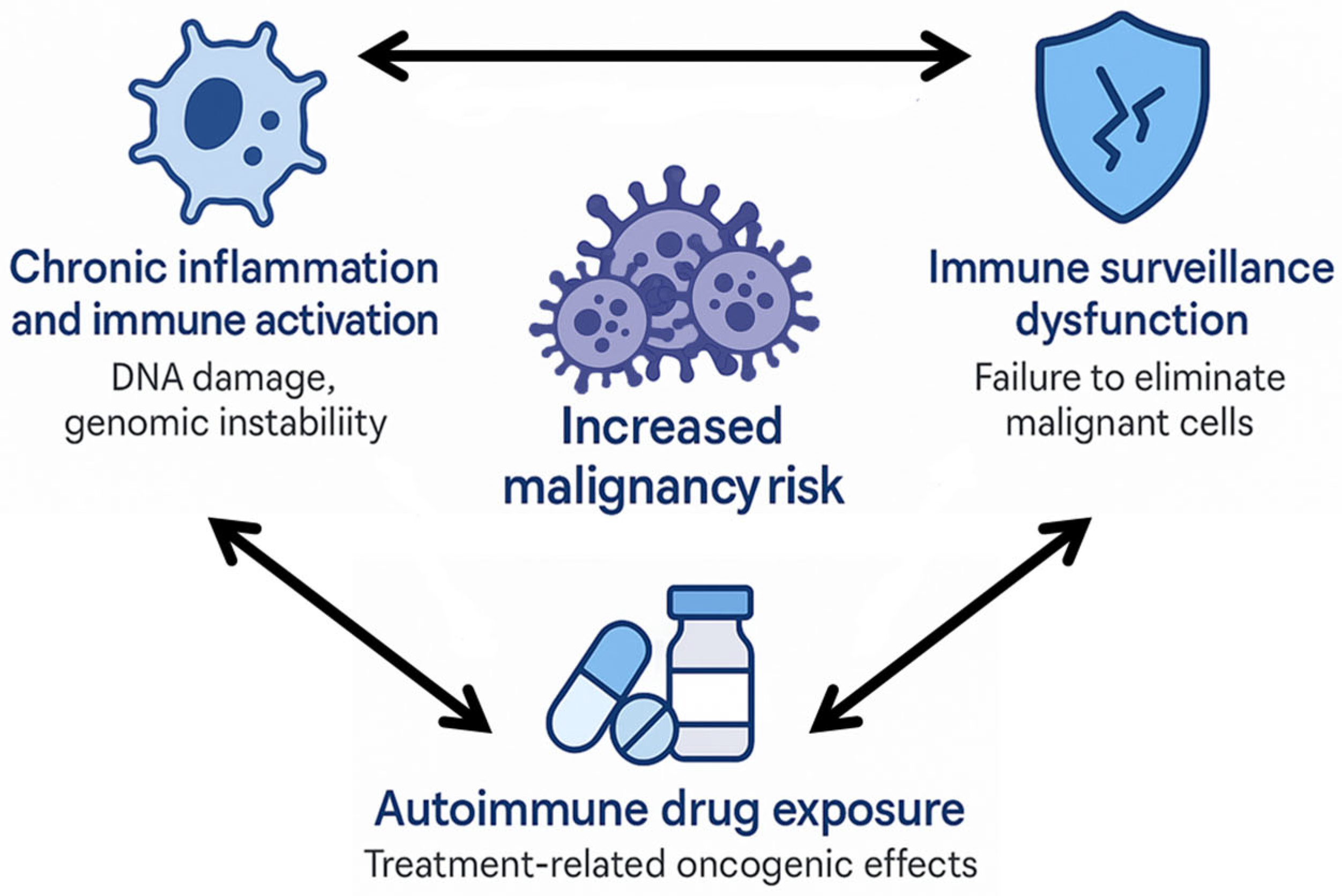
1. Introduction
2. Idiopathic Inflammatory Myopathies
- Idiopathic inflammatory myopathies (IIMs) are autoimmune diseases with a well-established link to cancer, especially dermatomyositis, which shows the highest risk compared to other subtypes.
- Most cancer cases in IIMs appear within the first years after diagnosis, emphasising the importance of early vigilance and systematic screening.
- Recognising clinical predictors like age, sex, disease severity and key autoantibodies is crucial to stratify patients and guide the intensity of cancer surveillance.
- Evidence-based guidelines recommend adjusting screening to each patient’s risk profile, combining basic tests with advanced imaging when needed to improve early detection and prognosis.
3. Sjögren’s Syndrome
- Sjögren’s Syndrome (SS) is one of the autoimmune diseases most strongly associated with non-Hodgkin lymphoma (NHL), illustrating how chronic immune activation can lead to malignancy.
- The risk of lymphoma in SS increases progressively over time, which highlights the need for long-term vigilance.
- Parotid gland enlargement, lymphadenopathy, cryoglobulinaemia, low complement levels and monoclonal gammopathy are important clinical ‘red flags’ that help identify patients who may benefit from closer monitoring.
- Beyond lymphoma, SS has also been linked to an increased risk of certain solid tumours, although this association is less consistent and varies by tumour type.
4. Systemic Sclerosis
- Systemic sclerosis SSc has a recognised increased risk of malignancy, especially lung and haematological cancers.
- A bimodal pattern of cancer occurrence has been described in SSc, with a peak in the early years after disease onset—supporting a possible paraneoplastic link—and a second increase later in the disease course.
- Specific autoantibodies (anti-RNA polymerase III) have been linked to higher malignancy risk, whereas others or negativity for anti-centromere/anti-topoisomerase I may help refine risk stratification.
- Current evidence highlights the need for tailored cancer screening, combining standard population-based tests with additional imaging or targeted work-up in high-risk patients.
5. Systemic Lupus Erythematosus
- Systemic lupus erythematosus (SLE) is associated with a moderately increased malignancy risk, particularly lymphomas and Human Papillomavirus (HPV)-related cancers such as cervical, vulvar, and anal cancer.
- Certain cancers, including breast, prostate, and ovarian cancer, may occur less frequently in SLE, possibly due to hormonal or immunological factors.
- Cyclophosphamide exposure has been linked to a higher malignancy risk, while hydroxychloroquine may offer a protective effect.
- There are no disease-specific cancer screening guidelines for SLE, but annual cervical screening is recommended for women on immunosuppressive therapy.
6. Rheumatoid Arthritis
- Rheumatoid arthritis (RA) is associated with a modestly increased overall cancer risk, especially for lung cancer and lymphomas, while colorectal and breast cancer risks may be slightly reduced.
- Risk factors for malignancy in RA include smoking, high disease activity, ILD, seropositivity, and extra-articular features such as Felty’s syndrome.
- Effective inflammation control reduces cancer risk; methotrexate and TNF inhibitors do not significantly increase overall malignancy rates, though Janus Kinase (JAK) inhibitors require caution in high-risk patients.
- Cancer screening in RA should follow general population guidelines, with particular attention to cervical and skin cancer surveillance in immunosuppressed individuals.
7. Antiphospholipid Syndrome
- Antiphospholipid syndrome (APS) has been associated with malignancy, particularly in patients with unexplained thrombosis or refractory disease.
- Antiphospholipid antibodies positivity is common in patients with solid tumours—especially gastrointestinal, genitourinary, and lung cancers—and is linked to increased thrombotic risk.
- Catastrophic APS may occur in the context of cancer, often triggered by the malignancy or related interventions like surgery.
- While APS may occasionally be paraneoplastic, routine cancer screening beyond standard population guidelines is not currently recommended.
8. ANCA-Associated Vasculitis
- Patients with ANCA-Associated Vasculitis (AAV) have an increased malignancy risk, historically driven by cyclophosphamide exposure.
- Squamous cell carcinomas—most notably those of the lung—are emerging as the most common malignancies, possibly related to chronic inflammation and underlying immune dysregulation.
- Cyclophosphamide remains a key risk factor, with dose-dependent associations, while rituximab appears to have a safer oncologic profile.
- Cancer screening should follow general population guidelines, with focused skin surveillance in those exposed to immunosuppressants.
9. Giant Cell Arteritis and Polymyalgia Rheumatica
- Patients with giant cell arteritis GCA or polymyalgia rheumatica (PMR) have a slightly increased overall malignancy risk, especially within the first year after diagnosis.
- Cutaneous, prostate, lung, kidney, and haematological malignancies are the most frequently reported cancers.
- Atypical clinical presentations may represent paraneoplastic syndromes, highlighting the need for careful differential diagnosis.
- Routine cancer screening beyond standard population-based protocols is not recommended, but targeted vigilance is warranted in high-risk cases.
10. Sarcoidosis
- Sarcoidosis seems associated with an increased overall malignancy risk, particularly for lymphomas.
- Diagnostic challenges include sarcoid-like reactions near tumours or induced by immunotherapy.
- The link with solid tumours, including lung cancer, is inconsistent and may reflect detection bias.
- Clinical vigilance for haematological malignancies might be appropriate, depending on individual patient characteristics
11. Other Conditions
- Mixed connective tissue disease (MCTD) has not been clearly associated with an overall increased malignancy risk, although isolated cases of lymphoma and solid tumours have been reported.
- Chronic immune activation and immunosuppressive therapy may contribute to oncogenesis; atypical or refractory cases should prompt malignancy work-up.
- IgG4-Related Disease (IgG4-RD) is associated with increased risks of lung, pancreatic, and lymphoid cancers, particularly within three years of diagnosis.
- Some forms of IgG4-RD may represent immune paraneoplastic syndromes; cancer screening at diagnosis is considered reasonable.
- VEXAS is strongly linked to haematological malignancies, with up to 55% of patients developing myeloid cancers.
- UBA1 mutations drive clonal haematopoiesis and inflammation, requiring regular monitoring for haematologic cancer.
- Eosinophilic Fasciitis (EF) may precede the diagnosis of lymphoproliferative neoplasms, particularly in older adults.
- Although no biomarkers predict malignancy risk, eosinophilia or atypical features should prompt evaluation for hidden cancer.
12. Practical Summary and Clinical Implications
- Maintain heightened oncological vigilance during the first years following diagnosis, especially in conditions with a strong temporal cancer link (e.g., cancer-associated myositis or SSc with anti-ARA).
- Recognise key clinical and serological features that may warrant intensified cancer work-up (Table 2).
- Implement stratified screening protocols, adjusting intensity and modalities based on each patient’s risk profile (Table 3).
- Be cautious when interpreting new or atypical symptoms, particularly in patients with refractory disease, poor treatment response, or longstanding inflammation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giat, E.; Ehrenfeld, M.; Shoenfeld, Y. Cancer and autoimmune diseases. Autoimmun. Rev. 2017, 16, 1049–1057. [Google Scholar] [CrossRef]
- Shah, A.A.; Casciola-Rosen, L.; Rosen, A. Review: Cancer-induced autoimmunity in the rheumatic diseases. Arthritis Rheum. 2015, 67, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Gomez, I.; Soós, B.; Bodoki, L.; Szamosi, S.; András, C.; Juhász, B.; Váróczy, L.; Antal-Szalmás, P.; Szodoray, P.; et al. Eight pillars of oncorheumatology: Crossroads between malignancies and musculoskeletal diseases. Autoimmun. Rev. 2020, 19, 102658. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, H.; Yang, Y.; Zhou, J.; Zhao, L.; Chen, H.; Fei, Y.; Zhang, W.; Li, M.; Zhao, Y.; et al. The five major autoimmune diseases increase the risk of cancer: Epidemiological data from a large-scale cohort study in China. Cancer Commun. 2022, 42, 435–446. [Google Scholar] [CrossRef]
- Masetti, R.; Tiri, A.; Tignanelli, A.; Turrini, E.; Argentiero, A.; Pession, A.; Esposito, S. Autoimmunity and cancer. Autoimmun. Rev. 2021, 20, 102882. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, L.C.; Shah, A.A. The relationships between cancer and autoimmune rheumatic diseases. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101472. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Huang, W.; Sundquist, J.; Sundquist, K.; Ji, J. Autoimmune diseases and hematological malignancies: Exploring the underlying mechanisms. Semin. Cancer Biol. 2020, 64, 114–121. [Google Scholar] [CrossRef]
- Joseph, C.G.; Darrah, E.; Shah, A.A.; Skora, A.D.; Casciola-Rosen, L.A.; Wigley, F.M.; Boin, F.; Fava, A.; Thoburn, C.; Kinde, I.; et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 2014, 343, 152–157. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Selva-O’Callaghan, A.; Pinal-Fernandez, I.; Trallero-Araguás, E.; Milisenda, J.C.; Grau-Junyent, J.M.; Mammen, A.L. Classification and management of adult inflammatory myopathies. Lancet Neurol. 2018, 17, 816–828. [Google Scholar] [CrossRef]
- Stertz, G. Polymyositis. Berl. Klin. Wochenschr. 1916, 53, 489. [Google Scholar]
- Olazagasti, J.M.; Baez, P.J.; Wetter, D.A.; Ernste, F.C. Cancer risk in dermatomyositis: A meta-analysis of cohort studies. Am. J. Clin. Dermatol. 2015, 16, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, G.; Chen, G.; Wu, B.; Lu, L.; Bao, L. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br. J. Dermatol. 2013, 169, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, A.G.S.; Allard, A.B.; Callen, J.P.; Chinoy, H.; Chung, L.; Fiorentino, D.; George, M.D.; Gordon, P.; Kolstad, K.; Kurtzman, D.J.B.; et al. A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology 2021, 60, 2615–2628. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Z.; Yan, S.; Yang, C.; Wang, B.; Shen, M.; Wang, Z.; Xu, D. Risk, risk factors, and screening of malignancies in dermatomyositis: Current status and future perspectives. Front. Oncol. 2025, 15, 1503140. [Google Scholar] [CrossRef]
- Kang, E.H.; Lee, S.J.; Ascherman, D.P.; Lee, Y.J.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Temporal relationship between cancer and myositis identifies two distinctive subgroups of cancers: Impact on cancer risk and survival in patients with myositis. Rheumatology 2016, 55, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, A.; Sergeant, J.C.; New, P.; McHugh, N.J.; Betteridge, Z.; Lamb, J.A.; Ollier, W.E.; Cooper, R.G.; Chinoy, H.; UKMyoNet. The temporal relationship between cancer and adult onset anti-transcriptional intermediary factor 1 antibody-positive dermatomyositis. Rheumatology 2019, 58, 650–655. [Google Scholar] [CrossRef]
- Campar, A.; Alves, I.; da Silva, A.M.; Farinha, F.; Vasconcelos, C. Idiopathic inflammatory myopathies—The burden of disease: Cohort analysis focusing on damage and comorbidities. Autoimmun. Rev. 2023, 22, 103455. [Google Scholar] [CrossRef]
- Moghadam-Kia, S.; Oddis, C.V.; Ascherman, D.P.; Aggarwal, R. Risk factors and cancer screening in myositis. Rheum. Dis. Clin. North. Am. 2020, 46, 565–576. [Google Scholar] [CrossRef]
- Stockton, D.; Doherty, V.R.; Brewster, D.H. Risk of cancer in patients with dermatomyositis or polymyositis, and follow-up implications: A Scottish population-based cohort study. Br. J. Cancer 2001, 85, 41–45. [Google Scholar] [CrossRef]
- Hsu, J.L.; Liao, M.F.; Chu, C.C.; Kuo, H.C.; Lyu, R.K.; Chang, H.S.; Chen, C.M.; Wu, Y.R.; Chang, K.H.; Weng, Y.C.; et al. Reappraisal of the incidence, various types and risk factors of malignancies in patients with dermatomyositis and polymyositis in Taiwan. Sci. Rep. 2021, 11, 4545. [Google Scholar] [CrossRef]
- Oldroyd, A.G.S.; Callen, J.P.; Chinoy, H.; Chung, L.; Fiorentino, D.; Gordon, P.; Machado, P.M.; McHugh, N.; Selva-O’Callaghan, A.; Schmidt, J.; et al. International guideline for idiopathic inflammatory myopathy–associated cancer screening: An IMACS initiative. Nat. Rev. Rheumatol. 2023, 19, 805–817. [Google Scholar] [CrossRef]
- Fiorentino, D.; Mecoli, C.A.; Igusa, T.; Albayda, J.; Paik, J.J.; Tiniakou, E.; Adler, B.; Mammen, A.L.; Shah, A.A.; Rosen, A.; et al. Association of anti-CCAR1 autoantibodies with decreased cancer risk relative to the general population in patients with anti-transcriptional intermediary factor 1γ-positive dermatomyositis. Arthritis Rheumatol. 2023, 75, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Liu, S.; Wang, Y.; Xu, D.; Li, M.; Zhao, Y.; Zeng, X. Primary Sjögren’s syndrome is associated with increased risk of malignancies besides lymphoma: A systematic review and meta-analysis. Autoimmun. Rev. 2022, 21, 103084. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Z.; Qin, B.; Zhong, R. Primary Sjögren’s syndrome and malignancy risk: A systematic review and meta-analysis. Ann. Rheum. Dis. 2014, 73, 1151–1156. [Google Scholar] [CrossRef]
- Zintzaras, E.; Voulgarelis, M.; Moutsopoulos, H.M. The risk of lymphoma development in autoimmune diseases: A meta-analysis. Arch. Intern. Med. 2005, 165, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Theander, E.; Henriksson, G.; Ljungberg, O.; Mandl, T.; Manthorpe, R.; Jacobsson, L.T. Lymphoma and other malignancies in primary Sjögren’s syndrome: A cohort study on cancer incidence and lymphoma predictors. Ann. Rheum. Dis. 2006, 65, 796–803. [Google Scholar] [CrossRef]
- Lazarus, M.N.; Robinson, D.; Mak, V.; Møller, H.; Isenberg, D.A. Incidence of cancer in a cohort of patients with primary Sjögren’s syndrome. Rheumatology 2006, 45, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Nishishinya, M.B.; Pereda, C.A.; Muñoz-Fernández, S.; Pego-Reigosa, J.M.; Rúa-Figueroa, I.; Andreu, J.L.; Fernández-Castro, M.; Rosas, J.; Loza Santamaría, E. Identification of lymphoma predictors in patients with primary Sjögren’s syndrome: A systematic literature review and meta-analysis. Rheumatol. Int. 2015, 35, 17–26. [Google Scholar] [CrossRef]
- Titsinides, S.; Nikitakis, N.; Piperi, E.; Sklavounou, A. MALT lymphoma of minor salivary glands in a Sjögren’s syndrome patient: A case report and review of literature. J. Oral. Maxillofac. Res. 2017, 8, e5. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zerón, P.; Kostov, B.; Fraile, G.; Caravia-Durán, D.; Maure, B.; Rascón, F.J.; Zamora, M.; Casanovas, A.; Lopez-Dupla, M.; Ripoll, M.; et al. Characterization and risk estimate of cancer in patients with primary Sjögren’s syndrome. J. Hematol. Oncol. 2017, 10, 90. [Google Scholar] [CrossRef]
- Kahraman Denizhan, T.; Oguz Kokoglu, E.; Kızıltepe, M.; Cengiz, C.B.; Tuncez, I.H.; Senel, A.S. Evaluation of the incidence of malignancy in Sjögren’s syndrome: A single-center study from Turkey. Cureus 2025, 17, e81219. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yao, P.; Li, J.; Wei, X.; Liu, X.; Wu, H.; Wang, W.; Feng, C.; Li, C.; Zhang, Y.; et al. Causal associations of Sjögren’s syndrome with cancers: A two-sample Mendelian randomization study. Arthritis Res. Ther. 2023, 25, 171. [Google Scholar] [CrossRef]
- Deng, J.; Liu, M.; Xiao, R.; Wang, J.; Liao, X.; Ye, Z.; Sun, Z. Risk, incidence, and mortality of breast cancer in primary Sjögren’s syndrome: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 904682. [Google Scholar] [CrossRef] [PubMed]
- Goulabchand, R.; Malafaye, N.; Jacot, W.; Witkowski Durand Viel, P.; Morel, J.; Lukas, C.; Rozier, P.; Lamure, S.; Noel, D.; Molinari, N.; et al. Cancer incidence in primary Sjögren’s syndrome: Data from the French hospitalization database. Autoimmun. Rev. 2021, 20, 102987. [Google Scholar] [CrossRef]
- Dong, L.; Chen, Y.; Masaki, Y.; Okazaki, T.; Umehara, H. Possible mechanisms of lymphoma development in Sjögren’s syndrome. Curr. Immunol. Rev. 2013, 9, 13–22. [Google Scholar] [CrossRef]
- Ruiz-Ordoñez, I.; Piedrahita, J.M.; Arévalo, J.A.; Agualimpia, A.; Tobón, G.J. Lymphomagenesis predictors and related pathogenesis. J. Transl. Autoimmun. 2021, 4, 100098. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; De Vita, S.; Tzioufas, A.G. Hepatitis C virus, Sjögren’s syndrome and B-cell lymphoma: Linking infection, autoimmunity and cancer. Autoimmun. Rev. 2005, 4, 8–15. [Google Scholar] [CrossRef]
- Nocturne, G.; Mariette, X. Sjögren syndrome-associated lymphomas: An update on pathogenesis and management. Br. J. Haematol. 2015, 168, 317–327. [Google Scholar] [CrossRef]
- Fragkioudaki, S.; Mavragani, C.P.; Moutsopoulos, H.M. Predicting the risk for lymphoma development in Sjögren syndrome: An easy tool for clinical use. Medicine 2016, 95, e3766. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; Bombardieri, S.; Bootsma, H.; De Vita, S.; Dörner, T.; Fisher, B.A.; Gottenberg, J.E.; Hernandez-Molina, G.; Kocher, A.; et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann. Rheum. Dis. 2020, 79, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Carubbi, F.; Cipriani, P.; Marrelli, A.; Benedetto, P.; Ruscitti, P.; Berardicurti, O.; Pantano, I.; Liakouli, V.; Alvaro, S.; Alunno, A.; et al. Efficacy and safety of rituximab treatment in early primary Sjögren’s syndrome: A prospective, multi-center, follow-up study. Arthritis Res. Ther. 2013, 15, R172. [Google Scholar] [CrossRef]
- Emery, P.; Furst, D.E.; Kirchner, P.; Melega, S.; Lacey, S.; Lehane, P.B. Risk of malignancies in patients with rheumatoid arthritis treated with rituximab: Analyses of global postmarketing safety data and long-term clinical trial data. Rheumatol. Ther. 2020, 7, 121–131. [Google Scholar] [CrossRef]
- Berardicurti, O.; Pavlych, V.; Cola, I.D.; Ruscitti, P.; Di Benedetto, P.; Navarini, L.; Marino, A.; Cipriani, P.; Giacomelli, R. Long-term safety of rituximab in primary Sjögren’s syndrome: The experience of a single center. J. Rheumatol. 2022, 49, 171–175. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Andréasson, K.; Smith, V. Systemic sclerosis. Lancet 2023, 401, 304–318. [Google Scholar] [CrossRef]
- Morrisroe, K.; Nikpour, M. Cancer and scleroderma: Recent insights. Curr. Opin. Rheumatol. 2020, 32, 479–487. [Google Scholar] [CrossRef]
- Pauling, J.D.; McHugh, N.J.; McGrogan, A. Systemic sclerosis and cancer in the UK: An epidemiological analysis using the Clinical Practice Research Datalink. Rheumatology 2025, 64, 1959–1965. [Google Scholar] [CrossRef]
- Hill, C.L.; Nguyen, A.M.; Roder, D.; Roberts-Thomson, P. Risk of cancer in patients with scleroderma: A population-based cohort study. Ann. Rheum. Dis. 2003, 62, 728–731. [Google Scholar] [CrossRef]
- Varga, J.; Denton, C.P. Systemic sclerosis and malignancy. Curr. Opin. Rheumatol. 2015, 27, 563–568. [Google Scholar]
- Colaci, M.; Giuggioli, D.; Sebastiani, M.; Manfredi, A.; Vacchi, C.; Spagnolo, P.; Cerri, S.; Luppi, F.; Richeldi, L.; Ferri, C. Lung cancer in scleroderma: Results from an Italian rheumatologic center and review of the literature. Autoimmun. Rev. 2013, 12, 374–379. [Google Scholar] [CrossRef]
- Onishi, A.; Sugiyama, D.; Kumagai, S.; Morinobu, A. Cancer incidence in systemic sclerosis: Meta-analysis of population-based cohort studies. Arthritis Rheum. 2013, 65, 1913–1921. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Wan, Y.-N.; Peng, W.-J.; Yan, J.W.; Li, B.Z.; Mei, B.; Chen, B.; Yao, H.; Yang, G.J.; Tao, J.H.; et al. The risk of cancer development in systemic sclerosis: A meta-analysis. Cancer Epidemiol. 2013, 37, 523–527. [Google Scholar] [CrossRef]
- Bonifazi, M.; Tramacere, I.; Pomponio, G.; Gabrielli, B.; Avvedimento, E.V.; La Vecchia, C.; Negri, E.; Gabrielli, A. Systemic sclerosis (scleroderma) and cancer risk: Systematic review and meta-analysis of observational studies. Rheumatology 2013, 52, 143–154. [Google Scholar] [CrossRef]
- Partouche, L.; Goulabchand, R.; Maria, A.T.J.; Rivière, S.; Jorgensen, C.; Rigau, V.; Bourgier, C.; Bessis, D.; Le Quellec, A.; Quere, I.; et al. Biphasic temporal relationship between cancers and systemic sclerosis: A clinical series from Montpellier University Hospital and review of the literature. J. Clin. Med. 2020, 9, 853. [Google Scholar] [CrossRef]
- Nemes-Tömöri, D.; Jász, D.K.; Tari, D.; Bói, B.; Ágoston-Szabó, Á.; Szűcs, G.; Majai, G.E. Malignancy in systemic sclerosis: A multicenter retrospective study. Biomedicines 2025, 13, 993. [Google Scholar] [CrossRef]
- Siau, K.; Laversuch, C.J.; Singh, S. Scleroderma and malignancy: A review of the current evidence. Ann. Rheum. Dis. 2010, 69, 445–448. [Google Scholar]
- Chatterjee, S.; Dombi, G.W.; Severson, R.K.; Mayes, M.D. Risk of lung cancer in scleroderma: A population-based cohort study. Arthritis Rheum. 2005, 52, 3945–3950. [Google Scholar] [CrossRef]
- Colaci, M.; Giuggioli, D.; Vacchi, C.; Ferri, C. Haematological malignancies in systemic sclerosis patients: Case reports and review of the world literature. Case Rep. Rheumatol. 2017, 2017, 6230138. [Google Scholar] [CrossRef] [PubMed]
- Dutt, A.; Arcinas, L.; Chen, J.; Shaikh, R.; Jun, S.; Kooner, A.; Gandhi, D.; Dolehide, C. Characterizing the links between systemic sclerosis and breast cancer. Cureus 2024, 16, e66653. [Google Scholar] [CrossRef]
- Shah, A.A.; Casciola-Rosen, L. Cancer and scleroderma: A paraneoplastic disease with implications for malignancy screening. Curr. Opin. Rheumatol. 2015, 27, 563–570. [Google Scholar] [CrossRef]
- Lazzaroni, M.G.; Cavazzana, I.; Colombo, E.; Dobrota, R.; Hernandez, J.; Hesselstrand, R.; Varju, C.; Nagy, G.; Smith, V.; Caramaschi, P.; et al. Malignancies in patients with anti-RNA polymerase III antibodies and systemic sclerosis: Analysis of the EULAR scleroderma trials and research cohort and possible recommendations for screening. J. Rheumatol. 2017, 44, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Bello, D.; de Tena, J.G.; Guillén-Del Castillo, A.; Selva-O’Callaghan, A.; Callejas-Moraga, E.L.; Marín-Sánchez, A.M.; Fonollosa-Pla, V.; Simeón-Aznar, C.P. Novel risk factors related to cancer in scleroderma. Autoimmun. Rev. 2017, 16, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, C.; Marcos, M.; Guillén-Del Castillo, A.; Rubio-Rivas, M.; Argibay, A.; Marín-Ballvé, A.; Rodríguez-Pintó, I.; Baldà-Masmiquel, M.; Callejas-Moraga, E.; Colunga, D.; et al. Standardized incidence ratios and risk factors for cancer in patients with systemic sclerosis: Data from the Spanish Scleroderma Registry (RESCLE). Autoimmun. Rev. 2022, 21, 103167. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, M.; Huizinga, T.W.J.; de Vries-Bouwstra, J.K. Auto-antibodies and cancer in systemic sclerosis. Autoimmun. Rev. 2017, 16, 883–884. [Google Scholar] [CrossRef]
- Shah, A.A.; Xu, G.; Rosen, A.; Hummers, L.K.; Wigley, F.M.; Elledge, S.J.; Casciola-Rosen, L. Anti-RNPC-3 antibodies as a marker of cancer-associated scleroderma. Arthritis Rheumatol. 2017, 69, 1306–1312. [Google Scholar] [CrossRef]
- Perurena-Prieto, J.; Viñas-Giménez, L.; Sanz-Martínez, M.T.; Selva-O’Callaghan, A.; Callejas-Moraga, E.L.; Colobran, R.; Guillén-Del-Castillo, A.; Simeón-Aznar, C.P. Anti-nuclear valosin-containing protein-like autoantibody is associated with calcinosis and higher risk of cancer in systemic sclerosis. Rheumatology 2024, 63, 2278–2283. [Google Scholar] [CrossRef]
- Igusa, T.; Hummers, L.K.; Visvanathan, K.; Richardson, C.; Wigley, F.M.; Casciola-Rosen, L.; Rosen, A.; Shah, A.A. Autoantibodies and scleroderma phenotype define subgroups at high-risk and low-risk for cancer. Ann. Rheum. Dis. 2018, 77, 1179–1186. [Google Scholar] [CrossRef]
- Shah, A.A.; Laiho, M.; Rosen, A.; Casciola-Rosen, L. Protective effect against cancer of antibodies to the large subunits of both RNA polymerases I and III in scleroderma. Arthritis Rheumatol. 2019, 71, 1571–1579. [Google Scholar] [CrossRef]
- Tonutti, A.; Motta, F.; Isailovic, N.; Ceribelli, A.; Ragusa, R.; Nappi, E.; Bonovas, S.; Selmi, C.; De Santis, M. Autoantibodies, cutaneous subset and immunosuppressants contribute to the cancer risk in systemic sclerosis. RMD Open 2024, 10, e004492. [Google Scholar] [CrossRef] [PubMed]
- Lepri, G.; Catalano, M.; Bellando-Randone, S.; Pillozzi, S.; Giommoni, E.; Giorgione, R.; Botteri, C.; Matucci-Cerinic, M.; Antonuzzo, L.; Guiducci, S. Systemic sclerosis association with malignancy. Clin. Rev. Allergy Immunol. 2022, 63, 398–416. [Google Scholar] [CrossRef] [PubMed]
- Morrisroe, K.; Hansen, D.; Huq, M.; Stevens, W.; Sahhar, J.; Ngian, G.S.; Ferdowsi, N.; Hill, C.; Roddy, J.; Walker, J.; et al. Incidence, risk factors, and outcomes of cancer in systemic sclerosis. Arthritis Care Res. 2020, 72, 1625–1635. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Daoussis, D.; Pagkopoulou, E.; Garyfallos, A.; Kitas, G.D.; Dimitroulas, T. Cancer risk in systemic sclerosis: Identifying risk and managing high-risk patients. Expert Rev. Clin. Immunol. 2020, 16, 1105–1113. [Google Scholar] [CrossRef]
- Bernatsky, S.; Ramsey-Goldman, R.; Labrecque, J.; Joseph, L.; Boivin, J.F.; Petri, M.; Zoma, A.; Manzi, S.; Urowitz, M.B.; Gladman, D.; et al. Cancer risk in systemic lupus: An updated international multi-centre cohort study. J. Autoimmun. 2013, 42, 130–135. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chang, Y.T.; Wang, C.B.; Wu, C. Malignancy in systemic lupus erythematosus: A nationwide cohort study in Taiwan. Am. J. Med. 2010, 123, 1150.e1–1150.e6. [Google Scholar] [CrossRef]
- Parikh-Patel, A.; White, R.H.; Allen, M.; Cress, R. Cancer risk in a cohort of patients with systemic lupus erythematosus (SLE) in California. Cancer Causes Control. 2008, 19, 887–894. [Google Scholar] [CrossRef]
- Dreyer, L.; Faurschou, M.; Mogensen, M.; Jacobsen, S. High incidence of potentially virus-induced malignancies in systemic lupus erythematosus: A long-term follow-up study in a Danish cohort. Arthritis Rheum. 2011, 63, 3032–3037. [Google Scholar] [CrossRef]
- Tallbacka, K.R.; Pettersson, T.; Pukkala, E. Increased incidence of cancer in systemic lupus erythematosus: A Finnish cohort study with more than 25 years of follow-up. Scand. J. Rheumatol. 2018, 47, 461–464. [Google Scholar] [CrossRef]
- Westermann, R.; Zobbe, K.; Cordtz, R.; Haugaard, J.H.; Dreyer, L. Increased cancer risk in patients with cutaneous lupus erythematosus and systemic lupus erythematosus compared with the general population: A Danish nationwide cohort study. Lupus 2021, 30, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Qiu, L.J.; Hu, L.F.; Cen, H.; Zhang, M.; Wen, P.F.; Wang, X.S.; Pan, H.F.; Ye, D.Q. Lung, liver, prostate, bladder malignancies risk in systemic lupus erythematosus: Evidence from a meta-analysis. Lupus 2014, 23, 284–292. [Google Scholar] [CrossRef]
- Cao, L.; Tong, H.; Xu, G.; Liu, P.; Meng, H.; Wang, J.; Zhao, X.; Tang, Y.; Jin, J. Systemic lupus erythematosus and malignancy risk: A meta-analysis. PLoS ONE 2015, 10, e0122964. [Google Scholar]
- Clarke, A.E.; Pooley, N.; Marjenberg, Z.; Langham, J.; Nicholson, L.; Langham, S.; Embleton, N.; Wang, X.; Desta, B.; Barut, V.; et al. Risk of malignancy in patients with systemic lupus erythematosus: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2021, 51, 1230–1241. [Google Scholar] [CrossRef]
- Mao, S.; Shen, H.; Zhang, J. Systemic lupus erythematosus and malignancies risk. J. Cancer Res. Clin. Oncol. 2016, 142, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Wang, Y.; Bai, Y.; Gu, D. Association between systemic lupus erythematosus and cancer morbidity and mortality: Findings from cohort studies. Front. Oncol. 2022, 12, 860794. [Google Scholar] [CrossRef]
- Mellemkjær, L.; Andersen, V.; Linet, M.S.; Gridley, G.; Hoover, R.; Olsen, J.H. Non-Hodgkin’s lymphoma and other cancers among a cohort of patients with systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 761–768. [Google Scholar] [CrossRef]
- Björnådal, L.; Löfström, B.; Yin, L.; Lundberg, I.E.; Ekbom, A. Increased cancer incidence in a Swedish cohort of patients with systemic lupus erythematosus. Scand. J. Rheumatol. 2002, 31, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Kovacs, L.; Szodoray, P. Malignancies in systemic lupus erythematosus. Autoimmun. Rev. 2010, 9, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Goobie, G.C.; Bernatsky, S.; Ramsey-Goldman, R.; Clarke, A.E. Malignancies in systemic lupus erythematosus: A 2015 update. Curr. Opin. Rheumatol. 2015, 27, 454–460. [Google Scholar] [CrossRef]
- Ali, Y.M.; Urowitz, M.B.; Ibanez, D.; Gladman, D.D. Monoclonal gammopathy in systemic lupus erythematosus. Lupus 2007, 16, 426–429. [Google Scholar] [CrossRef]
- Song, L.; Wang, Y.; Zhang, J.; Song, N.; Xu, X.; Lu, Y. The risks of cancer development in systemic lupus erythematosus (SLE) patients: A systematic review and meta-analysis. Arthritis Res. Ther. 2018, 20, 270. [Google Scholar] [CrossRef] [PubMed]
- Zard, E.; Arnaud, L.; Mathian, A.; Chakhtoura, Z.; Hie, M.; Touraine, P.; Heard, I.; Amoura, Z. Increased risk of high grade cervical squamous intraepithelial lesions in systemic lupus erythematosus: A meta-analysis of the literature. Autoimmun. Rev. 2014, 13, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Georges, D.; Shiels, M.S.; Engels, E.A.; Albuquerque, A.; Poynten, I.M.; de Pokomandy, A.; Easson, A.M.; Stier, E.A. A meta-analysis of anal cancer incidence by risk group: Toward a unified anal cancer risk scale. Int. J. Cancer 2021, 148, 38–47. [Google Scholar] [CrossRef]
- Kosałka-Węgiel, J.; Pacholczak-Madej, R.; Dziedzic, R.; Siwiec-Koźlik, A.; Spałkowska, M.; Milewski, M.; Zaręba, L.; Bazan-Socha, S.; Korkosz, M. Malignancy in systemic lupus erythematosus: Relation to disease characteristics in 92 patients—A single center retrospective study. Rheumatol. Int. 2024, 44, 1701–1713. [Google Scholar] [CrossRef]
- Tessier-Cloutier, B.; Clarke, A.E.; Ramsey-Goldman, R.; Gordon, C.; Hansen, J.E.; Bernatsky, S. Systemic lupus erythematosus and malignancies: A review article. Rheum. Dis. Clin. North. Am. 2014, 40, 497–506. [Google Scholar] [CrossRef]
- Chang, S.L.; Hsu, H.T.; Weng, S.F.; Lin, Y.S. Impact of head and neck malignancies on risk factors and survival in systemic lupus erythematosus. Acta Otolaryngol. 2013, 133, 1088–1095. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.M.; Wang, G.S.; Qian, L.; Tao, J.H.; Ma, Y.; Li, X.P. Thyroid cancer in systemic lupus erythematosus: A meta-analysis. Int. J. Clin. Exp. Pathol. 2014, 7, 6270–6273. [Google Scholar] [PubMed]
- Castro, F.A.; Liu, X.; Försti, A.; Ji, J.; Sundquist, J.; Sundquist, K.; Koshiol, J.; Hemminki, K. Increased risk of hepatobiliary cancers after hospitalization for autoimmune disease. Clin. Gastroenterol. Hepatol. 2014, 12, 1038–1045.e7. [Google Scholar] [CrossRef]
- Seo, M.S.; Yeo, J.; Hwang, I.C.; Shim, J.Y. Risk of pancreatic cancer in patients with systemic lupus erythematosus: A meta-analysis. Clin. Rheumatol. 2019, 38, 3109–3116. [Google Scholar] [CrossRef]
- Dey, D.; Kenu, E.; Isenberg, D.A. Cancer complicating systemic lupus erythematosus—A dichotomy emerging from a nested case-control study. Lupus 2013, 22, 919–927. [Google Scholar] [CrossRef]
- Ladouceur, A.; Tessier-Cloutier, B.; Clarke, A.E.; Ramsey-Goldman, R.; Gordon, C.; Hansen, J.E.; Bernatsky, S. Cancer and systemic lupus erythematosus. Rheum. Dis. Clin. North. Am. 2020, 46, 533–550. [Google Scholar] [CrossRef]
- Noble, P.W.; Bernatsky, S.; Clarke, A.E.; Isenberg, D.A.; Ramsey-Goldman, R.; Hansen, J.E. DNA-damaging autoantibodies and cancer: The lupus butterfly theory. Nat. Rev. Rheumatol. 2016, 12, 429–434. [Google Scholar] [CrossRef]
- Weisbart, R.H.; Chan, G.; Jordaan, G.; Noble, P.W.; Liu, Y.; Glazer, P.M.; Nishimura, R.N.; Hansen, J.E. DNA-dependent targeting of cell nuclei by a lupus autoantibody. Sci. Rep. 2015, 5, 12022. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Chauhan, S.K.; Singh, V.V.; Rai, M.; Rai, G. Heat shock protein 27 and its regulatory molecules express differentially in SLE patients with distinct autoantibody profiles. Immunol. Lett. 2015, 164, 25–32. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, M.; Chang, C.; Chan, V.; Lu, Q.; Wu, H. The complex role of AIM2 in autoimmune diseases and cancers. Immun. Inflamm. Dis. 2021, 9, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Han, J.Y.; Jeon, Y.; You, S.H.; Jung, S.Y.; Jang, E.J.; Sung, Y.K. Cancer incidence and the influence of immunosuppressive agents in Korean patients with systemic lupus erythematosus: A retrospective cohort study. Arthritis Res. Ther. 2025, 27, 14. [Google Scholar] [CrossRef]
- Bernatsky, S.; Ramsey-Goldman, R.; Joseph, L.; Boivin, J.F.; Costenbader, K.H.; Urowitz, M.B.; Gladman, D.D.; Fortin, P.R.; Nived, O.; Petri, M.A.; et al. Lymphoma risk in systemic lupus: Effects of disease activity versus treatment. Ann. Rheum. Dis. 2014, 73, 138–142. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Lin, M.S.; Su, Y.J.; Cheng, T.T.; Lin, Y.S.; Chen, Y.C.; Chiu, W.C.; Chen, T.H. Cumulative immunosuppressant exposure is associated with diversified cancer risk among 14,832 patients with systemic lupus erythematosus: A nested case-control study. Rheumatology 2017, 56, 620–628. [Google Scholar]
- Ruiz-Irastorza, G.; Ugarte, A.; Egurbide, M.V.; Garmendia, M.; Pijoan, J.I.; Martinez-Berriotxoa, A.; Aguirre, C. Antilarials may influence the risk of malignancy in systemic lupus erythematosus. Ann. Rheum. Dis. 2007, 66, 815–817. [Google Scholar] [CrossRef]
- Guo, J.; Ren, Z.; Li, J.; Li, T.; Liu, S.; Yu, Z. The relationship between cancer and medication exposure in patients with systemic lupus erythematosus: A nested case-control study. Arthritis Res. Ther. 2020, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Cao, N.W.; Chu, X.J.; Zhou, H.Y.; Wang, H.; Yu, S.J.; Ye, D.Q.; Li, B.Z. Antimalarials may reduce cancer risk in patients with systemic lupus erythematosus: A systematic review and meta-analysis of prospective studies. Ann. Med. 2021, 53, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Bernatsky, S.; Ramsey-Goldman, R.; Clarke, A.E. Malignancy in systemic lupus erythematosus: What have we learned? Best Pract. Res. Clin. Rheumatol. 2009, 23, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Tessier-Cloutier, B.; Clarke, A.E.; Pineau, C.A.; Keeling, S.; Bissonauth, A.; Ramsey-Goldman, R.; Lee, J.; Bernatsky, S. What investigations are needed to optimally monitor for malignancies in SLE? Lupus 2015, 24, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.A.; Thompson, A.; Gandhi, K.K.; Hochberg, M.C.; Suissa, S. Incidence of malignancy in adult patients with rheumatoid arthritis: A meta-analysis. Arthritis Res. Ther. 2015, 17, 212. [Google Scholar] [CrossRef]
- Smitten, A.L.; Simon, T.A.; Hochberg, M.C.; Suissa, S. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R45. [Google Scholar] [CrossRef]
- Mercer, L.K.; Lunt, M.; Low, A.L.; Dixon, W.G.; Watson, K.D.; Symmons, D.P.; Hyrich, K.L.; BSRBR Control Centre Consortium. Risk of solid cancer in patients exposed to anti-tumour necrosis factor therapy: Results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann. Rheum. Dis. 2015, 74, 1087–1093. [Google Scholar] [CrossRef]
- Baecklund, E.; Iliadou, A.; Askling, J.; Ekbom, A.; Backlin, C.; Granath, F.; Catrina, A.I.; Rosenquist, R.; Feltelius, N.; Sundström, C.; et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006, 54, 692–701. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Chatzidionysiou, K. Lung cancer in rheumatoid arthritis: Is there a need for better risk assessment and screening? Clin. Rheumatol. 2020, 39, 957–961. [Google Scholar] [CrossRef]
- Moosic, K.B.; Ananth, K.; Andrade, F.; Feith, D.J.; Darrah, E.; Loughran, T.P., Jr. Intersection between large granular lymphocyte leukemia and rheumatoid arthritis. Front. Oncol. 2022, 12, 869205. [Google Scholar] [CrossRef]
- Hong, L.E.; Wechalekar, M.D.; Kutyna, M.; Small, A.; Lim, K.; Thompson-Peach, C.; Li, J.J.; Chhetri, R.; Scott, H.S.; Brown, A.; et al. IDH-mutant myeloid neoplasms are associated with seronegative rheumatoid arthritis and innate immune activation. Blood 2024, 143, 1873–1877. [Google Scholar] [CrossRef]
- Kauppi, M.; Pukkala, E.; Isomäki, H. Low incidence of colorectal cancer in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 1996, 14, 551–553. [Google Scholar]
- Tian, G.; Liang, J.N.; Wang, Z.Y.; Zhou, D. Breast cancer risk in rheumatoid arthritis: An updated meta-analysis. Biomed. Res. Int. 2014, 2014, 453012. [Google Scholar] [CrossRef] [PubMed]
- Cibere, J.; Sibley, J.; Haga, M. Rheumatoid arthritis and the risk of malignancy. Arthritis Rheum. 1997, 40, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Wadström, H.; Frisell, T.; Sparén, P.; Askling, J.; ARTIS Study Group. Do RA or TNF inhibitors increase the risk of cervical neoplasia or of recurrence of previous neoplasia? A nationwide study from Sweden. Ann. Rheum. Dis. 2016, 75, 1272–1278. [Google Scholar] [CrossRef]
- Llorca, J.; Lopez-Diaz, M.J.; Gonzalez-Juanatey, C.; Ollier, W.E.; Martin, J.; Gonzalez-Gay, M.A. Persistent chronic inflammation contributes to the development of cancer in patients with rheumatoid arthritis from a defined population of northwestern Spain. Semin. Arthritis Rheum. 2007, 37, 31–38. [Google Scholar] [CrossRef]
- Gridley, G.; Klippel, J.H.; Hoover, R.N.; Fraumeni, J.F., Jr. Incidence of cancer among men with the Felty syndrome. Ann. Intern. Med. 1994, 120, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Baecklund, E.; Smedby, K.E.; Sutton, L.A.; Askling, J.; Rosenquist, R. Lymphoma development in patients with autoimmune and inflammatory disorders—What are the driving forces? Semin. Cancer Biol. 2014, 24, 61–70. [Google Scholar] [CrossRef]
- Kamel, O.W.; van de Rijn, M.; Weiss, L.M.; Del Zoppo, G.J.; Hench, P.K.; Robbins, B.A.; Montgomery, P.G.; Warnke, R.A.; Dorfman, R.F. Reversible lymphomas associated with Epstein–Barr virus occurring during methotrexate therapy for rheumatoid arthritis and dermatomyositis. N. Engl. J. Med. 1993, 328, 1317–1321. [Google Scholar] [CrossRef]
- Salloum, E.; Cooper, D.L.; Howe, G.; Lacy, J.; Tallini, G.; Crouch, J.; Schultz, M.; Murren, J. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J. Clin. Oncol. 1996, 14, 1943–1949. [Google Scholar] [CrossRef]
- Kreher, M.A.; Konda, S.; Noland, M.M.B.; Longo, M.I.; Valdes-Rodriguez, R. Risk of melanoma and nonmelanoma skin cancer with immunosuppressants, part II: Methotrexate, alkylating agents, biologics, and small molecule inhibitors. J. Am. Acad. Dermatol. 2023, 88, 534–542. [Google Scholar] [CrossRef]
- Wolfe, F.; Michaud, K. Lymphoma in rheumatoid arthritis: The effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 2004, 50, 1740–1751. [Google Scholar] [CrossRef]
- Bongartz, T.; Sutton, A.J.; Sweeting, M.J.; Buchan, I.; Matteson, E.L.; Montori, V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006, 296, 2201–2210. [Google Scholar] [CrossRef]
- Lopez-Olivo, M.A.; Tayar, J.H.; Martinez-Lopez, J.A.; Pollono, E.N.; Cueto, J.P.; Gonzales-Crespo, M.R.; Fulton, S.; Suarez-Almazor, M.E. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: A meta-analysis. JAMA 2012, 308, 898–908. [Google Scholar] [CrossRef]
- D’Arcy, M.E.; Beachler, D.C.; Pfeiffer, R.M.; Curtis, J.R.; Mariette, X.; Seror, R.; Mahale, P.; Rivera, D.R.; Yanik, E.L.; Engels, E.A. Tumor necrosis factor inhibitors and the risk of cancer among older Americans with rheumatoid arthritis. Cancer Epidemiol. Biomark. Prev. 2021, 30, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Gris, J.C.; Mousty, É.; Bouvier, S.; Ripart, S.; Cochery-Nouvellon, É.; Fabbro-Peray, P.; Broner, J.; Letouzey, V.; Pérez-Martin, A. Increased incidence of cancer in the follow-up of obstetric antiphospholipid syndrome within the NOH-APS cohort. Haematologica 2020, 105, 490–497. [Google Scholar] [CrossRef]
- Nipu, M.A.I.; Kundu, S.; Alam, S.S.; Dina, A.N.; Hasan, M.A.; Khan, M.; Khalil, M.I.; Hossan, T.; Islam, M.A. Anticardiolipin antibodies in patients with cancer: A case-control study. Cancers 2023, 15, 2087. [Google Scholar] [CrossRef]
- Cervera, R.; Serrano, R.; Pons-Estel, G.J.; Ceberio-Hualde, L.; Shoenfeld, Y.; de Ramón, E.; Buonaiuto, V.; Jacobsen, S.; Zeher, M.M.; Tarr, T.; et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann. Rheum. Dis. 2015, 74, 1011–1018. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Tayar, J.H.; Fa’ak, F.; Sharma, G.; Lopez-Olivo, M.A.; Yousif, A.; Shagroni, T.; Al-Hawamdeh, S.; Rojas-Hernandez, C.M.; Suarez-Almazor, M.E. Systematic review of observational studies reporting antiphospholipid antibodies in patients with solid tumors. Blood Adv. 2020, 4, 3326–3332. [Google Scholar] [CrossRef]
- Font, C.; Vidal, L.; Espinosa, G.; Tàssies, D.; Monteagudo, J.; Farrús, B.; Visa, L.; Cervera, R.; Gascon, P.; Reverter, J.C. Solid cancer, antiphospholipid antibodies, and venous thromboembolism. Autoimmun. Rev. 2011, 10, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A. Antiphospholipid antibodies and antiphospholipid syndrome in cancer: Uninvited guests in troubled times. Semin. Cancer Biol. 2020, 64, 108–113. [Google Scholar] [CrossRef]
- Yoon, K.H.; Wong, A.; Shakespeare, T.; Sivalingam, P. High prevalence of antiphospholipid antibodies in Asian cancer patients with thrombosis. Lupus 2003, 12, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Vassalo, J.; Spector, N.; de Meis, E.E.; Rabello, L.S.; Rosolem, M.M.; do Brasil, P.E.; Salluh, J.I.; Soares, M. Antiphospholipid antibodies in critically ill patients with cancer: A prospective cohort study. J. Crit. Care 2014, 29, 533–538. [Google Scholar] [CrossRef]
- Miesbach, W.; Asherson, R.A.; Cervera, R.; Shoenfeld, Y.; Gomez Puerta, J.; Bucciarelli, S.; Espinoza, G.; Font, J.; Members of CAPS Registry Group. The catastrophic antiphospholipid (Asherson’s) syndrome and malignancies. Autoimmun. Rev. 2006, 6, 94–97. [Google Scholar] [CrossRef]
- Carpintieri, S.; Uyar, E.; Anand, C.; Buryk, Y. Cancer history, antiphospholipid syndrome, and lupus anticoagulant: A perfect storm for thrombosis. Cureus 2024, 16, e76481. [Google Scholar] [CrossRef]
- Miller, G.J.; Bauer, K.A.; Howarth, D.J.; Cooper, J.A.; Humphries, S.E.; Rosenberg, R.D. Increased incidence of neoplasia of the digestive tract in men with persistent activation of the coagulant pathway. J. Thromb. Haemost. 2004, 2, 2107–2114. [Google Scholar] [CrossRef]
- Han, X.Y.; Li, Z.Y.; Zhao, M.H.; Little, M.A.; Chen, M. Malignancy is increased in patients with antineutrophil cytoplasmic antibody-associated vasculitis in China. Arthritis Res. Ther. 2024, 26, 113. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, E.; Yıldırım, T.D.; Ulusoy, B.O.; Öğüt, T.S.; Karabacak, M.; Sadioğlu Çağdaş, Ö.; Yıldırım, R.; Güven, D.C.; Akleylek, C.; Ediboğlu, E.; et al. Unveiling cancer risk in ANCA-associated vasculitis: Result from the Turkish Vasculitis Study Group (TRVaS). Intern. Emerg. Med. 2024, 19, 1025–1034. [Google Scholar] [CrossRef]
- Heijl, C.; Westman, K.; Höglund, P.; Mohammad, A.J. Malignancies in patients with antineutrophil cytoplasmic antibody-associated vasculitis: A population-based cohort study. J. Rheumatol. 2020, 47, 1229–1237. [Google Scholar] [CrossRef]
- Rahmattulla, C.; Berden, A.E.; Wakker, S.C.; Reinders, M.E.; Hagen, E.C.; Wolterbeek, R.; Bruijn, J.A.; Bajema, I.M. Incidence of malignancies in patients with antineutrophil cytoplasmic antibody-associated vasculitis diagnosed between 1991 and 2013. Arthritis Rheumatol. 2015, 67, 3270–3278. [Google Scholar] [CrossRef]
- Faurschou, M.; Sorensen, I.J.; Mellemkjaer, L.; Loft, A.G.; Thomsen, B.S.; Tvede, N.; Baslund, B. Malignancies in Wegener’s granulomatosis: Incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J. Rheumatol. 2008, 35, 100–105. [Google Scholar]
- Li, L.; Teng, J.; Kou, N.; Yue, Y.; Wang, H. ANCA-associated vasculitis and lung cancer: An immunological perspective. Clin. Exp. Med. 2024, 24, 208. [Google Scholar] [CrossRef]
- Crespo, J.; Sun, H.; Welling, T.H.; Tian, Z.; Zou, W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 2013, 25, 214–221. [Google Scholar] [CrossRef]
- Tatsis, E.; Reinhold-Keller, E.; Steindorf, K.; Feller, A.C.; Gross, W.L. Wegener’s granulomatosis associated with renal cell carcinoma. Arthritis Rheum. 1999, 42, 751–756. [Google Scholar] [CrossRef]
- Pankhurst, T.; Savage, C.O.; Gordon, C.; Harper, L. Malignancy is increased in ANCA-associated vasculitis. Rheumatology 2004, 43, 1532–1535. [Google Scholar] [CrossRef]
- Faurschou, M.; Mellemkjaer, L.; Sorensen, I.J.; Thomsen, B.S.; Dreyer, L.; Baslund, B. Cancer preceding Wegener’s granulomatosis: A case-control study. Rheumatology 2009, 48, 421–424. [Google Scholar] [CrossRef]
- Wester Trejo, M.A.C.; Bajema, I.M.; van Daalen, E.E. Antineutrophil cytoplasmic antibody-associated vasculitis and malignancy. Curr. Opin. Rheumatol. 2018, 30, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, B.; Sanchez-Alamo, B.; Schirmer, J.H.; Berti, A.; Blockmans, D.; Cid, M.C.; Holle, J.U.; Hollinger, N.; Karadag, O.; Kronbichler, A.; et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann. Rheum. Dis. 2024, 83, 30–47. [Google Scholar] [CrossRef] [PubMed]
- van Daalen, E.E.; Rizzo, R.; Kronbichler, A.; Wolterbeek, R.; Bruijn, J.A.; Jayne, D.R.; Bajema, I.M.; Rahmattulla, C. Effect of rituximab on malignancy risk in patients with ANCA-associated vasculitis. Ann. Rheum. Dis. 2017, 76, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, F.; Matteson, E.L.; Dejaco, C. Polymyalgia rheumatica and giant cell arteritis. JAMA 2020, 324, 993–994. [Google Scholar] [CrossRef]
- van der Geest, K.S.M.; Sandovici, M.; Bley, T.A.; Stone, J.R.; Slart, R.H.J.A.; Brouwer, E. Large vessel giant cell arteritis. Lancet Rheumatol. 2024, 6, e397–e408. [Google Scholar] [CrossRef]
- Ungprasert, P.; Sanguankeo, A.; Upala, S.; Knight, E.L. Risk of malignancy in patients with giant cell arteritis and polymyalgia rheumatica: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2014, 44, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Sundquist, J.; Sundquist, K.; Hemminki, K. Cancer risk in patients hospitalised with polymyalgia rheumatica and giant cell arteritis: A follow-up study in Sweden. Rheumatology 2010, 49, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Greigert, H.; Mounier, M.; Arnould, L.; Creuzot-Garcher, C.; Ramon, A.; Martin, L.; Tarris, G.; Ponnelle, T.; Audia, S.; Bonnotte, B.; et al. Haematological malignancies in giant cell arteritis: A French population-based study. Rheumatology 2021, 60, 5408–5412. [Google Scholar] [CrossRef]
- Dar, L.; Ben-Shabat, N.; Tiosano, S.; Watad, A.; McGonagle, D.; Komaneshter, D.; Cohen, A.; Bragazzi, N.L.; Amital, H. The incidence and predictors of solid- and hematological malignancies in patients with giant cell arteritis: A large real-world database study. Int. J. Environ. Res. Public Health 2021, 18, 7595. [Google Scholar] [CrossRef]
- Muller, S.; Hider, S.; Helliwell, T.; Partington, R.; Mallen, C. The real evidence for polymyalgia rheumatica as a paraneoplastic syndrome. Reumatismo 2018, 70, 23–34. [Google Scholar] [CrossRef]
- Muller, S.; Hider, S.L.; Belcher, J.; Helliwell, T.; Mallen, C.D. Is cancer associated with polymyalgia rheumatica? A cohort study in the General Practice Research Database. Ann. Rheum. Dis. 2014, 73, 1769–1773. [Google Scholar] [CrossRef]
- Manzo, C.; Natale, M. Polymyalgia rheumatica and cancer: A critical review of the literature. Open Access Rheumatol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Gorlen, T.F.; Brittain, J.M.; Østergaard, M.; Fischer, B.M.; Døhn, U.M.; Terslev, L. Low incidence of malignancy in patients with suspected polymyalgia rheumatica or giant cell arteritis, examined with FDG-PET/CT. Front. Med. 2024, 11, 1309905. [Google Scholar] [CrossRef] [PubMed]
- Manzo, C.; Nune, A. Polymyalgia rheumatica and cancer: Surveillance duration and other points to ponder. Reumatologia 2023, 61, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Brincker, H.; Wilbek, E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br. J. Cancer 1974, 29, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Brincker, H. The sarcoidosis–lymphoma syndrome. Br. J. Cancer 1986, 54, 467–473. [Google Scholar] [CrossRef]
- Patt, Y.S.; Ben-Shabat, N.; Sharif, K.; Patt, C.; Elizur, Y.; Arow, M.; Cohen, A.D.; Watad, A.; McGonagle, D.; Amital, H.; et al. The association between sarcoidosis and malignancy: A comprehensive population-based cohort study. J. Clin. Med. 2024, 13, 7045. [Google Scholar] [CrossRef]
- Smedby, K.E.; Vajdic, C.M.; Falster, M.; Engels, E.A.; Martínez-Maza, O.; Turner, J.; Hjalgrim, H.; Vineis, P.; Seniori Costantini, A.; Bracci, P.M.; et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: A pooled analysis within the InterLymph Consortium. Blood 2008, 111, 4029–4038. [Google Scholar] [CrossRef]
- Rømer, F.K.; Hommelgaard, P.; Schou, G. Sarcoidosis and cancer revisited: A long-term follow-up study of 555 Danish sarcoidosis patients. Eur. Respir. J. 1998, 12, 906–912. [Google Scholar] [CrossRef]
- Ungprasert, P.; Crowson, C.S.; Matteson, E.L. Risk of malignancy among patients with sarcoidosis: A population-based cohort study. Arthritis Care Res. 2017, 69, 46–50. [Google Scholar] [CrossRef]
- Seersholm, N.; Vestbo, J.; Viskum, K. Risk of malignant neoplasms in patients with pulmonary sarcoidosis. Thorax 1997, 52, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.P.; Lower, E.E.; du Bois, R.M. Sarcoidosis. Lancet 2003, 361, 1111–1118. [Google Scholar] [CrossRef]
- Cohen Aubart, F.; Lhote, R.; Amoura, A.; Valeyre, D.; Haroche, J.; Amoura, Z.; Lebrun-Vignes, B. Drug-induced sarcoidosis: An overview of the WHO pharmacovigilance database. J. Intern. Med. 2020, 288, 356–362. [Google Scholar] [CrossRef]
- Bonifazi, M.; Bravi, F.; Gasparini, S.; La Vecchia, C.; Gabrielli, A.; Wells, A.U.; Renzoni, E.A. Sarcoidosis and cancer risk: Systematic review and meta-analysis of observational studies. Chest 2015, 147, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Faurschou, M.; Omland, L.H.; Obel, N.; Lindhardsen, J.; Baslund, B. Risk of cancer among sarcoidosis patients with biopsy-verified nonnecrotizing granulomatous inflammation: Population-based cohort study. J. Rheumatol. 2022, 49, 186–191. [Google Scholar] [CrossRef]
- Gunnarsson, R.; Hetlevik, S.O.; Lilleby, V.; Molberg, Ø. Mixed connective tissue disease. Best Pract. Res. Clin. Rheumatol. 2016, 30, 95–111. [Google Scholar] [CrossRef]
- Chevalier, K.; Thoreau, B.; Michel, M.; Godeau, B.; Agard, C.; Papo, T.; Sacre, K.; Seror, R.; Mariette, X.; Cacoub, P.; et al. Clinical presentation, course, and prognosis of patients with mixed connective tissue disease: A multicenter retrospective cohort. J. Intern. Med. 2024, 295, 532–543. [Google Scholar] [CrossRef]
- Kudsi, M.; Khalayli, N.; Hola, L.; Aldeeb, M.; Aziz, A. Mixed connective tissue and ovarian cancer: A case report. Ann. Med. Surg. 2023, 86, 467–471. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wu, C.C.; Ou, T.T.; Yen, J.H.; Liu, H.W.; Tsai, W.C. Malignant thymoma associated with mixed connective tissue disease: A case report. Clin. Rheumatol. 2006, 25, 262–264. [Google Scholar] [CrossRef]
- Gracia-Cazaña, T.; Delgado-Beltrán, C.; Concellón, M.A.; Fuertes, M.A. Mixed connective tissue disease in a patient with Castleman disease and Hodgkin lymphoma: Excellent clinical response to rituximab. Actas Dermo-Sifiliogr. 2015, 106, 843–846. [Google Scholar] [CrossRef]
- Monoe, K.; Kanno, Y.; Saito, H.; Abe, K.; Takahashi, A.; Takiguchi, J.; Rai, T.; Hikichi, T.; Ohira, H. A case of hepatocellular carcinoma associated with mixed connective tissue disease. Nihon Shokakibyo Gakkai Zasshi 2007, 104, 568–572. [Google Scholar] [PubMed]
- Wallace, Z.S.; Naden, R.P.; Chari, S.; Choi, H.K.; Della-Torre, E.; Dicaire, J.F.; Hart, P.A.; Inoue, D.; Kawano, M.; Khosroshahi, A.; et al. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann. Rheum. Dis. 2020, 79, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, J.; Ning, X.; Li, H.; Ma, X.; Wang, K.; Bian, W.; Zhang, Y.; Yu, G.; Li, Z. Malignancy risk of immunoglobulin G4-related disease: Evidence from a large cohort multicenter retrospective study. Rheumatol. Ther. 2021, 8, 1207–1221. [Google Scholar] [CrossRef]
- Hirano, K.; Tada, M.; Sasahira, N.; Isayama, H.; Mizuno, S.; Takagi, K.; Watanabe, T.; Saito, T.; Kawahata, S.; Uchino, R.; et al. Incidence of malignancies in patients with IgG4-related disease. Intern. Med. 2014, 53, 171–176. [Google Scholar] [CrossRef]
- Song, M.; Latorre, G.; Ivanovic-Zuvic, D.; Camargo, M.C.; Rabkin, C.S. Autoimmune diseases and gastric cancer risk: A systematic review and meta-analysis. Cancer Res. Treat. 2019, 51, 841–850. [Google Scholar] [CrossRef]
- Tang, H.; Yang, H.; Zhang, P.; Wu, D.; Zhang, S.; Zhao, J.; Peng, L.; Chen, H.; Fei, Y.; Zhang, X.; et al. Malignancy and IgG4-related disease: The incidence, related factors and prognosis from a prospective cohort study in China. Sci. Rep. 2020, 10, 4910. [Google Scholar] [CrossRef]
- Sumimoto, K.; Uchida, K.; Ikeura, T.; Hirano, K.; Yamamoto, M.; Takahashi, H.; Nishino, T.; Mizushima, I.; Kawano, M.; Kamisawa, T.; et al. Nationwide epidemiological survey of immunoglobulin G4-related disease with malignancy in Japan. J. Gastroenterol. Hepatol. 2022, 37, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Haghbin, H.; Chuang, J.; Fatima, R.; Zakirkhodjaev, N.; Lee-Smith, W.; Aziz, M. Correlation of autoimmune pancreatitis and malignancy: Systematic review and meta-analysis. Dig. Dis. Sci. 2022, 67, 3252–3264. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wu, Y.; Liu, J.; Zhuang, Y.; Jin, X.; Wang, L. The risk of malignancy in patients with IgG4-related disease: A systematic review and meta-analysis. Arthritis Res. Ther. 2022, 24, 14. [Google Scholar] [CrossRef]
- Wallace, Z.S.; Wallace, C.J.; Lu, N.; Choi, H.K.; Stone, J.H. Association of IgG4-related disease with history of malignancy. Arthritis Rheumatol. 2016, 68, 2283–2289. [Google Scholar] [CrossRef]
- Shiokawa, M.; Kodama, Y.; Yoshimura, K.; Kawanami, C.; Mimura, J.; Yamashita, Y.; Asada, M.; Kikuyama, M.; Okabe, Y.; Inokuma, T.; et al. Risk of cancer in patients with autoimmune pancreatitis. Am. J. Gastroenterol. 2013, 108, 610–617. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi, H.; Tabeya, T.; Suzuki, C.; Naishiro, Y.; Ishigami, K.; Yajima, H.; Shimizu, Y.; Obara, M.; Yamamoto, H.; et al. Risk of malignancies in IgG4-related disease. Mod. Rheumatol. 2012, 22, 414–418. [Google Scholar] [CrossRef]
- Loeza-Uribe, M.P.; Hinojosa-Azaola, A.; Sánchez-Hernández, B.E.; Crispín, J.C.; Apodaca-Chávez, E.; Ferrada, M.A.; Martín-Nares, E. VEXAS syndrome: Clinical manifestations, diagnosis, and treatment. Reumatol. Clin. (Engl. Ed.) 2024, 20, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Grayson, P.C.; Patel, B.A.; Young, N.S. VEXAS syndrome. Blood 2021, 137, 3591–3594. [Google Scholar] [CrossRef]
- Beck, D.B.; Ferrada, M.A.; Sikora, K.A.; Ombrello, A.K.; Collins, J.C.; Pei, W.; Balanda, N.; Ross, D.L.; Ospina Cardona, D.; Wu, Z.; et al. Somatic mutations in UBA1 and severe adult-onset autoinflammatory disease. N. Engl. J. Med. 2020, 383, 2628–2638. [Google Scholar] [CrossRef]
- Poulter, J.A.; Collins, J.C.; Cargo, C.; De Tute, R.M.; Evans, P.; Ospina Cardona, D.; Bowen, D.T.; Cunnington, J.R.; Baguley, E.; Quinn, M.; et al. Novel somatic mutations in UBA1 as a cause of VEXAS syndrome. Blood 2021, 137, 3676–3681. [Google Scholar] [CrossRef]
- Bourbon, E.; Heiblig, M.; Gerfaud-Valentin, M.; Barba, T.; Durel, C.A.; Lega, J.C.; Barraco, F.; Sève, P.; Jamilloux, Y.; Sujobert, P. Therapeutic options in VEXAS syndrome: Insights from a retrospective series. Blood 2021, 137, 3682–3684. [Google Scholar] [CrossRef]
- Sun, L.; Babushok, D.V. Secondary myelodysplastic syndrome and leukemia in acquired aplastic anemia and paroxysmal nocturnal hemoglobinuria. Blood 2020, 136, 36–49. [Google Scholar] [CrossRef]
- Shulman, L.E. Diffuse fasciitis with eosinophilia: A new syndrome? Trans. Assoc. Am. Physicians 1974, 87, 70–86. [Google Scholar]
- Pinal-Fernandez, I.; Selva-O’Callaghan, A.; Grau, J.M. Diagnosis and classification of eosinophilic fasciitis. Autoimmun. Rev. 2014, 13, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Haddad, H.; Sundaram, S.; Magro, C.; Gergis, U. Eosinophilic fasciitis as a paraneoplastic syndrome: A case report and review of the literature. Hematol. Oncol. Stem Cell Ther. 2014, 7, 90–92. [Google Scholar] [CrossRef]
- Lakhanpal, S.; Ginsburg, W.W.; Michet, C.J.; Doyle, J.A.; Moore, S.B. Eosinophilic fasciitis: Clinical spectrum and therapeutic response in 52 cases. Semin. Arthritis Rheum. 1988, 17, 221–231. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ito, T.; Asano, Y.; Sato, S.; Motegi, S.I.; Ishikawa, O.; Matsushita, T.; Takehara, K.; Makino, T.; Okiyama, N.; et al. Characteristics of Japanese patients with eosinophilic fasciitis: A brief multicenter study. J. Dermatol. 2020, 47, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.S.; Hanson, C.A.; Cooper, K.D. Concurrent eosinophilic fasciitis and cutaneous T-cell lymphoma. Eosinophilic fasciitis as a paraneoplastic syndrome of T-cell malignant neoplasms? Arch. Dermatol. 1991, 127, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Satomi, H.; Ishikawa, R.; Shishido, T.; Sato, K.; Ueki, T.; Sumi, M.; Kobayashi, H. Eosinophilic fasciitis with hypereosinophilia as the initial clinical manifestation of peripheral T-cell lymphoma, not otherwise specified. Intern. Med. 2022, 61, 3425–3429. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.O.; Ahn, M.J.; Lee, Y.Y.; Jung, T.J.; Choi, I.Y.; Kim, I.S.; Park, C.K. Eosinophilic fasciitis preceding relapse of peripheral T-cell lymphoma. J. Kor. Med. Sci. 2000, 15, 346–350. [Google Scholar] [CrossRef]
- Chohan, S.; Wong, N.; Hanson, J.; Osto, M.; Daveluy, S. Eosinophilic fasciitis may present as a paraneoplastic syndrome of hematological malignancies—A systematic review. JAAD Int. 2023, 11, 85–87. [Google Scholar] [CrossRef]
- Philpott, H.; Hissaria, P.; Warren, L.; Singhal, N.; Brown, M.; Proudman, S.; Cleland, L.; Gillis, D. Eosinophilic fasciitis as a paraneoplastic phenomenon associated with metastatic colorectal carcinoma. Australas. J. Dermatol. 2008, 49, 27–29. [Google Scholar] [CrossRef]
- Maione, V.; Miccio, L.; Rovaris, S.; Zardo, D.; Bighetti, S.; Bettolini, L.; Naldi, L. Eosinophilic fasciitis and breast cancer: A case report highlighting recurrence signals. Dermatol. Rep. 2024, 16, 9928. [Google Scholar] [CrossRef] [PubMed]
- Rea, A.; Anderson, A.; Moshiri, A.; Paulson, K.; Thompson, J.A.; Kalus, A. Eosinophilic fasciitis as a paraneoplastic syndrome in melanoma. JAAD Case Rep. 2021, 17, 49–51. [Google Scholar] [CrossRef] [PubMed]
| High Risk | Intermediate Risk | Low Risk |
|---|---|---|
| DM Age over 40 at diagnosis Treatment refractoriness or relapses Dysphagia (moderate/severe) Cutaneous necrosis or ulceration TIF1γ NXP2 | Amyopathic DM Polymyositis Immune-mediated necrotizing myopathy Male sex SAE1 HMGCR Mi-2 MDA-5 | ASyS Overlap syndromes Raynaud’s phenomenon Arthritis Interstitial lung disease Anti-SRP Anti-Jo1 Non–Jo-1 ASyS Anti–PM-Scl, Ku, anti-RNP, anti-Ro/La |
| Disease | Age, Sex | Clinical Findings | Serum Findings |
|---|---|---|---|
| IIMs * | >40 at diagnosis Male | Dysphagia Skin ulceration or necrosis Refractory or aggressive disease | TIF1γ, NXP2 |
| Sjögren’s Syndrome | Persistent parotid enlargement Lymphadenopathy | Low C4 complement levels Monoclonal gammopathy Cryoglobulins, rheumatoid factor | |
| Systemic Sclerosis | Onset > 65 | Aggressive, atypical, or treatment-refractory disease course. Significant weight loss or other constitutional symptoms (disproportionate to the severity of the autoimmune disease). ILD (for lung cancer). | ARA Negative for ACA and ATA in SSc-diffuse disease |
| SLE | Significant weight loss or other constitutional symptoms (disproportionate to the severity of the autoimmune disease). Persistent HPV infection Prior cyclophosphamide exposure or long-term immunosuppression | ||
| Rheumatoid Arthritis | Older age Male | Persistent inflammation or high disease activity ILD (for lung cancer) Felty’s syndrome (for large granular lymphocyte leukaemia) | Seropositivity |
| Antiphospholipid Syndrome | Unexplained or recurrent thrombosis despite therapy Refractory disease | ||
| AAV | Older age Male | High cumulative exposure to cyclophosphamide | Anti-PR3 positivity |
| GCA/PMR | Older age Male | Atypical (e.g., absence of morning stiffness) or disproportionate symptoms Poor response to steroids | |
| Sarcoidosis | Disproportionate symptoms, atypical clinical or radiological course Refractory disease | ||
| MCTD | Refractory or atypical clinical course Treatment-refractory disease | ||
| IgG4-RD | Pancreatobiliary involvement Multi-organ disease High inflammatory activity | ||
| VEXAS Syndrome | Bone marrow dysplasia at diagnosis. | Persistent cytopenias Somatic mutation UBA1 | |
| Eosinophilic Fasciitis | Atypical systemic features Refractory or unusual clinical course | Marked eosinophilia |
| Disease | Stratified Screening Protocol |
|---|---|
| IIMs | Risk-stratified approach: basic tests for standard risk; CT scans, tumour markers, PET-CT for high-risk (e.g., TIF1γ+). |
| Sjögren’s Syndrome | Follow general guidelines, monitoring potential warning features (e.g., parotid enlargement). |
| Systemic Sclerosis | Tailored based on autoantibodies (e.g., ARA+ or ACA/ATA−) and clinical features; standard plus extended study in high-risk patients during first 3–5 years. |
| SLE | General population guidelines plus annual cervical screening for immunosuppressed women; urine cytology considered post-cyclophosphamide. |
| Rheumatoid Arthritis | Follow general guidelines; emphasise cervical/skin cancer checks in immunosuppressed; no added screening unless warning features are present. |
| Antiphospholipid Syndrome | No systematic screening beyond standard population protocols; consider cancer in refractory or atypical cases. |
| AAV | General population guidelines; focus on skin exams for those exposed to immunosuppressants. |
| GCA/PMR | Standard guidelines; assess atypical/refractory cases for paraneoplastic syndromes. |
| Sarcoidosis | General vigilance; screen for haematologic malignancies in high-risk or atypical presentations. |
| MCTD | No intensified screening recommended; evaluate for malignancy in atypical or treatment-refractory cases. |
| IgG4-RD | In addition to general population recommendations, cancer screening should be guided by the presence of warning features. |
| VEXAS Syndrome | Regular monitoring for haematologic malignancy |
| Eosinophilic Fasciitis | In addition to general population recommendations, cancer screening should be guided by the presence of warning features. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal-Bello, D.; Frutos-Pérez, B.; Duarte-Millán, M.Á.; Toledano-Macías, M.; Jaenes-Barrios, B.; Morales-Ortega, A. Cancer Risk in Autoimmune and Immune-Mediated Diseases: A Narrative Review for Practising Clinicians. J. Clin. Med. 2025, 14, 5954. https://doi.org/10.3390/jcm14175954
Bernal-Bello D, Frutos-Pérez B, Duarte-Millán MÁ, Toledano-Macías M, Jaenes-Barrios B, Morales-Ortega A. Cancer Risk in Autoimmune and Immune-Mediated Diseases: A Narrative Review for Practising Clinicians. Journal of Clinical Medicine. 2025; 14(17):5954. https://doi.org/10.3390/jcm14175954
Chicago/Turabian StyleBernal-Bello, David, Begoña Frutos-Pérez, Miguel Ángel Duarte-Millán, María Toledano-Macías, Beatriz Jaenes-Barrios, and Alejandro Morales-Ortega. 2025. "Cancer Risk in Autoimmune and Immune-Mediated Diseases: A Narrative Review for Practising Clinicians" Journal of Clinical Medicine 14, no. 17: 5954. https://doi.org/10.3390/jcm14175954
APA StyleBernal-Bello, D., Frutos-Pérez, B., Duarte-Millán, M. Á., Toledano-Macías, M., Jaenes-Barrios, B., & Morales-Ortega, A. (2025). Cancer Risk in Autoimmune and Immune-Mediated Diseases: A Narrative Review for Practising Clinicians. Journal of Clinical Medicine, 14(17), 5954. https://doi.org/10.3390/jcm14175954





