Prediction of Distal Dural Ring Location in Internal Carotid Paraclinoid Aneurysms Using the Tuberculum Sellae–Anterior Clinoid Process Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
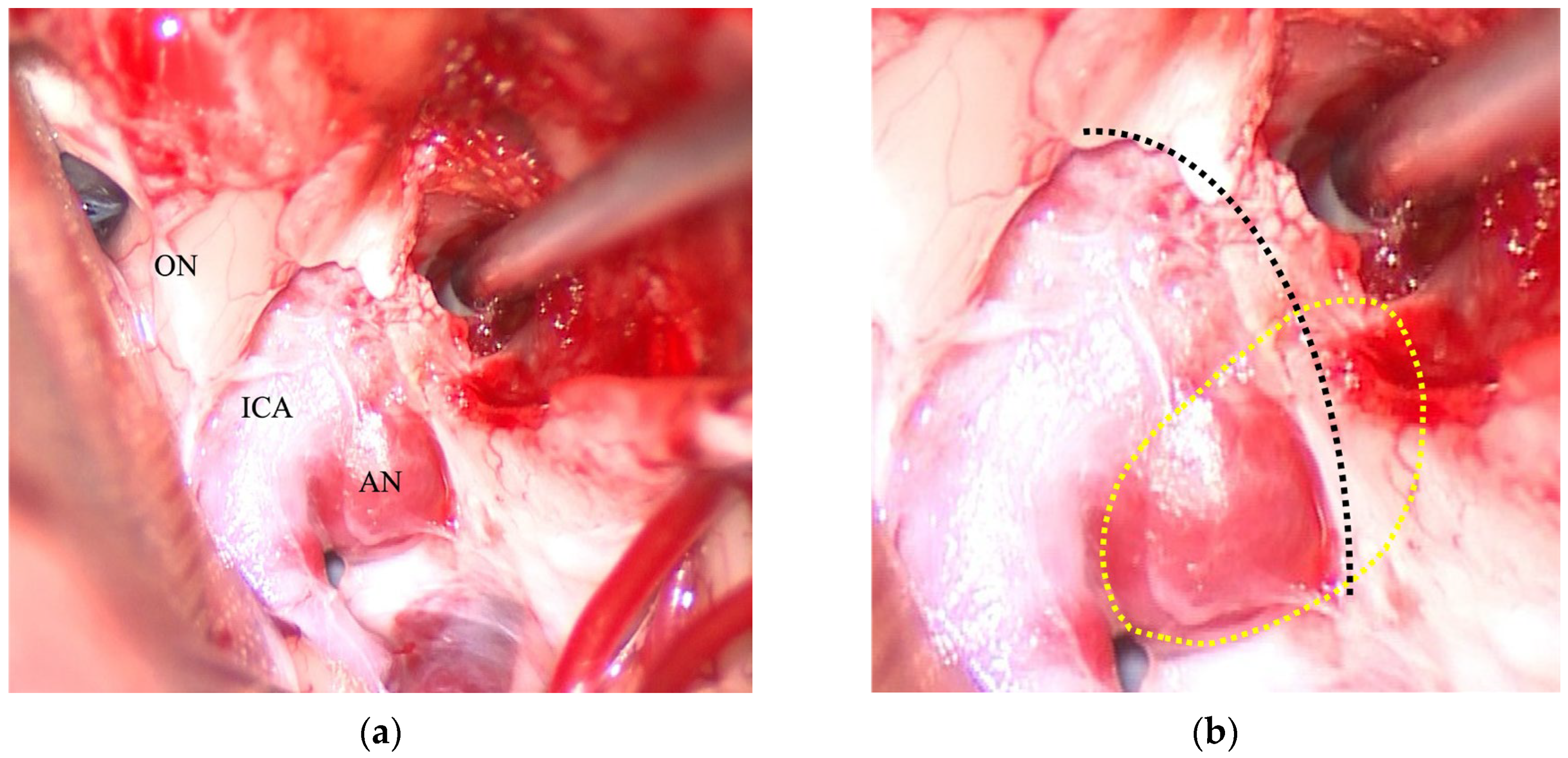
2.2. Surgical Procedure
2.3. 3D-CTA Acquisition
- SOMATOM Sensation: Collimation: 0.6 mm, tube voltage: 120 kVp, tube current: 240 mA, rotation time: 0.5 s.
- SOMATOM Force: Collimation: 0.6 mm, tube voltage: 90 kVp, quality reference mAs: 230 mA, pitch factor: 1.2, rotation time: 0.5 s.
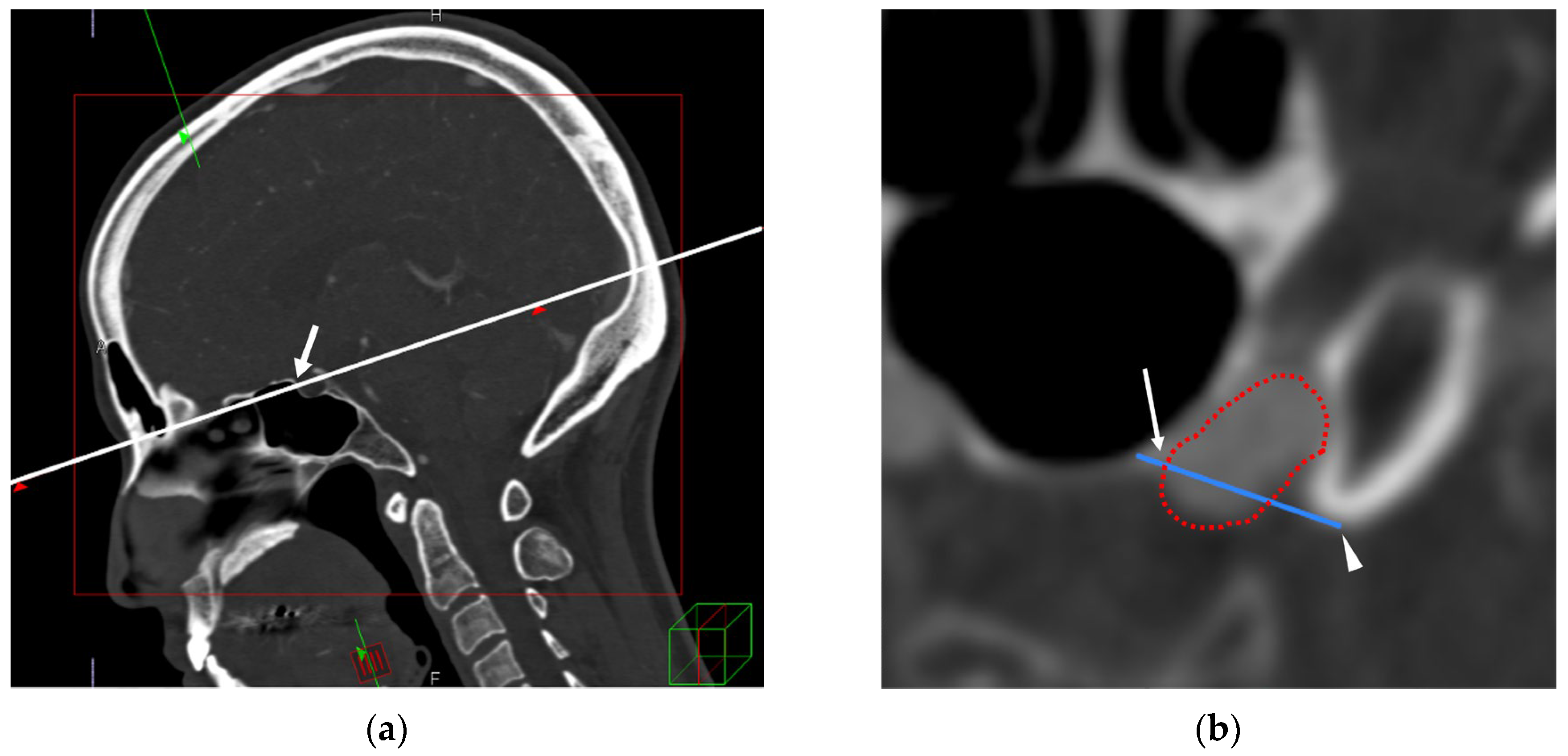
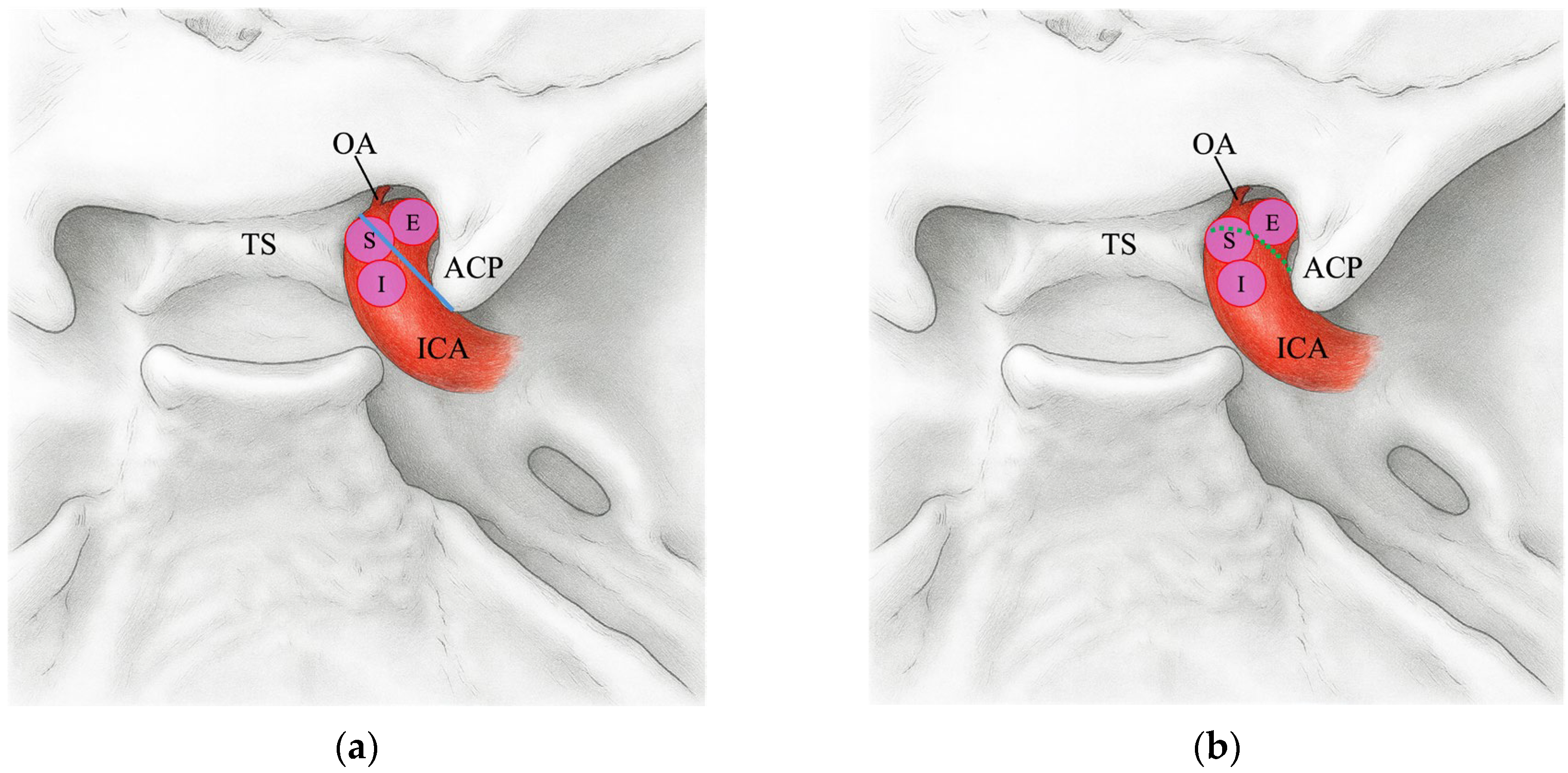
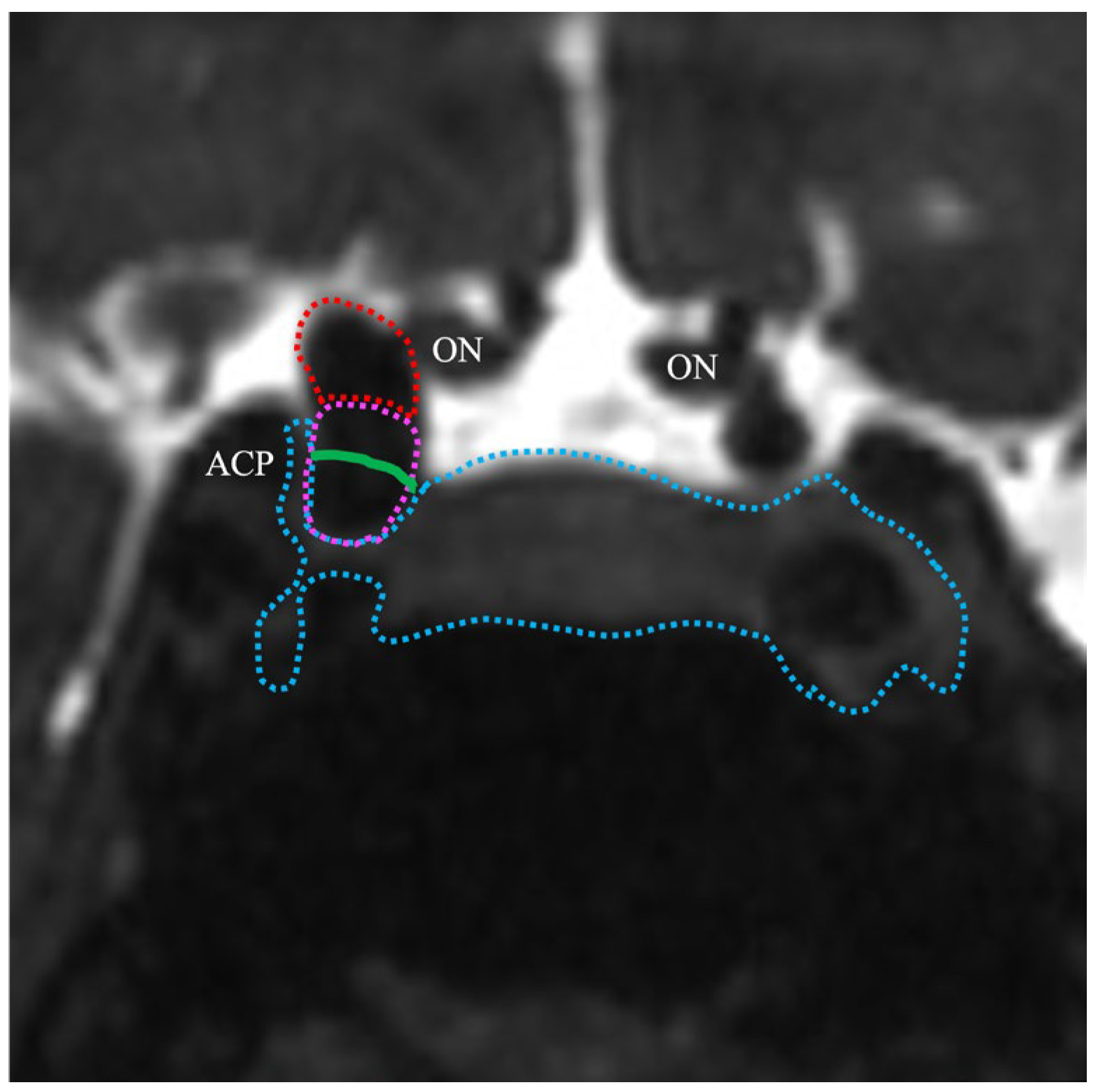
2.4. TS–ACP Line Method
2.5. Image Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Anatomical Basis and Technical Considerations
4.2. Clinical Implications
4.3. Comparison with Alternative Methods
4.4. Limitations
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICA | Internal carotid artery |
| DDR | Distal dural ring |
| TS–ACP | Tuberculum sellae–anterior clinoid process |
| 3D-CTA | 3D-computed tomography angiography |
| SAH | Subarachnoid hemorrhage |
| MRA | Magnetic resonance angiography |
| TS | Tuberculum sellae |
| ACP | Anterior clinoid process |
| MRI | Magnetic resonance imaging |
| CISS | Constructive interference in steady state |
| AI | Artificial intelligence |
| CI | Confidence interval |
References
- Thines, L.; Gauvrit, J.Y.; Leclerc, X.; Le Gars, D.; Delmaire, C.; Pruvo, J.P.; Lejeune, J.P. Usefulness of MR imaging for the assessment of nonophthalmic paraclinoid aneurysms. AJNR Am. J. Neuroradiol. 2008, 29, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.F.; Walker, M.T.; Zabramski, J.M.; Partovi, S.; Wallace, R.C.; Spetzler, R.F. Distinction between paraclinoid and cavernous sinus aneurysms with computed tomographic angiography. Neurosurgery 2003, 52, 1131–1137, discussion 1138. [Google Scholar] [CrossRef]
- Thines, L.; Delmaire, C.; Le Gars, D.; Pruvo, J.-P.; Lejeune, J.-P.; Lehmann, P.; Francke, J.-P. MRI location of the distal dural ring plane: Anatomoradiological study and application to paracilonoid carotid artery aneurysms. Eur. Radiol. 2006, 16, 479–488. [Google Scholar] [CrossRef]
- Scerbak, J.; Lapteva, O.; Sahin, O.S.; Ksanas, U.; Barkauskiene, A.; Lengvenis, G.; Ozaydin, B.; Cikla, U.; Baskaya, M.K. Identification of the distal dural ring and definition of paraclinoid aneurysms according to bony landmarks on 3-dimensional computed tomography angiography: A cadaveric and radiological study. Oper. Neurosurg. 2020, 19, 319–329. [Google Scholar] [CrossRef]
- Liao, C.H.; Lin, C.-J.; Lin, C.-F.; Huang, H.-Y.; Chen, M.-H.; Hsu, S.P.C.; Shih, Y.-H. Comparison of the effectiveness of using the optic strut and tuberculum sellae as radiological landmarks in diagnosing paraclinoid aneurysms with CT angiography. J. Neurosurg. 2016, 125, 275–282. [Google Scholar] [CrossRef]
- Hashimoto, K.; Nozaki, K.; Hashimoto, N. Optic strut as a radiographic landmark in evaluating neck location of a paraclinoid aneurysm. Neurosurgery 2006, 59, 880–895, discussion 896. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, A.; Hafez, A.; Jahromi, B.R.; Kivisaari, R.; Canato, B.; Choque, J.; Colasanti, R.; Fransua, S.; Lehto, H.; Andrade-Barazarte, H.; et al. Anatomic features of paraclinoid aneurysms: Computed tomography angiography study of 144 aneurysms in 136 consecutive patients. Neurosurgery 2017, 81, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Beretta, F.; Sepahi, A.N.; Zuccarello, M.; Tomsick, T.A.; Keller, J.T. Radiographic imaging of the distal dural ring for determining the intradural or extradural location of aneurysms. Skull Base 2005, 15, 253–262. [Google Scholar] [CrossRef]
- Tanaka, Y.; Matsuda, Y.; Tsumoto, T.; Terada, T. Visual symptoms after flow-diverter stenting of internal carotid artery aneurysms: A retrospective cohort study. Surg. Neurol. Int. 2025, 16, 250. [Google Scholar] [CrossRef]
- Kanemaru, K.; Yoshioka, H.; Hashimoto, K.; Senbokuya, N.; Arai, H.; Fujimura, M.; Suzuki, K.; Matsuda, K.; Sakai, N.; Nishikawa, R.; et al. Treatment of unruptured large and giant paraclinoid aneurysms in Japan at the time of flow diverter introduction: A nationwide, multicenter survey by the Japanese Society on Surgery for Cerebral Stroke. World Neurosurg. 2025, 195, 123571. [Google Scholar] [CrossRef]
- Gaub, M.; Murtha, G.; Lafuente, M.; Webb, M.; Luo, A.; Birnbaum, L.A.; Mascitelli, J.R.; Al Saiegh, F. Flow diversion for endovascular treatment of intracranial aneurysms: Past, present, and future directions. J. Clin. Med. 2024, 13, 4167. [Google Scholar] [CrossRef]
- Han, H.J.; Park, K.Y.; Kim, B.M.; Kim, Y.B.; Jeon, H.J.; Suh, S.H.; Suh, S.; Kim, J.J.; Kim, J.H.; Jang, C.K.; et al. FloWise flow diverter for treatment of unruptured wide-neck intracranial aneurysms: A prospective, multicenter, single-arm, open-label, pivotal study. Korean J. Radiol. 2025, 26, 716–726. [Google Scholar] [CrossRef]
- Oikawa, S.; Kyoshima, K.; Kobayashi, S. Surgical anatomy of the juxta-dural ring area. J. Neurosurg. 1998, 89, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nunez, M.A.; Mohyeldin, A.; Vigo, V.; Mao, Y.; Cohen-Gadol, A.A.; Fernandez-Miranda, J.C. Microsurgical anatomy of the dorsal clinoid space: Implications for endoscopic endonasal parasellar surgery. J. Neurol. Surg. 2022, 137, 1418–1430. [Google Scholar] [CrossRef]
- Andaluz, N.; Beretta, F.; Bernucci, C.; Keller, J.T.; Zuccarello, M. Evidence for the improved exposure of the ophthalmic segment of the internal carotid artery after anterior clinoidectomy: Morphometric analysis. Acta Neurochir. 2006, 148, 971–975, discussion 975. [Google Scholar] [CrossRef]
- Fernandes, S.T.; Doria-Netto, H.L.; Alves, R.V.; Lapate, R.L.; Ferreira, N.P.F.D.; Teixeira, M.J.; Solla, D.J.F.; Yamaki, V.N.; Figueiredo, E.G. The diagnostic accuracy of MRI in determining the relations between paraclinoid aneurysms and the cavernous sinus. Neuroradiology 2022, 64, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Kai, Y.; Morioka, M.; Yano, S.; Kitajima, M.; Fukuoka, H.; Sasao, A.; Murakami, R.; Nakayama, Y.; Awai, K.; et al. Differentiation between paraclinoid and cavernous sinus aneurysms with contrast-enhanced 3D constructive interference in steady state MR imaging. AJNR Am. J. Neuroradiol. 2008, 29, 130–133. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, C.B.; Wang, J.-Y.; Jiang, B.; Zhang, L.-B.; Zeng, M.; Chen, Y.-B.; Zhang, H.-F.; Chen, F.-H. Application of 3-dimensional computerized tomography angiography for defining cavernous sinus aneurysms and intradural aneurysms involving the internal carotid artery around the anterior clinoid process. World Neurosurg. 2017, 106, 785–789. [Google Scholar] [CrossRef]
- Kim, J.M.; Romano, A.; Sanan, A.; van Loveren, H.R.; Keller, J.T. Microsurgical anatomic features and nomenclature of the paraclinoid region. Neurosurgery 2000, 46, 670–680, discussion 680. [Google Scholar] [CrossRef]
- Matsumoto, M.; Mizutani, T.; Sugiyama, T.; Sumi, K.; Nakajo, T.; Arai, S. Distance between the falciform ligament and distal dural ring as a surgical landmark for the treatment of paraclinoid aneurysms. World Neurosurg. 2019, 124, e498–e502. [Google Scholar] [CrossRef]
- Thines, L.; Lee, S.K.; Dehdashti, A.R.; Agid, R.; Willinsky, R.A.; Wallace, C.M.; Terbrugge, K.G. Direct imaging of the distal dural ring and paraclinoid internal carotid artery aneurysms with high-resolution T2 turbo-spin echo technique at 3-T magnetic resonance imaging. Neurosurgery 2009, 64, 1059–1064, discussion 1064. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Nakazawa, T.; Yamada, N.; Higashi, M.; Hishikawa, T.; Miyamoto, S.; Naito, H. Identification of the distal dural ring with use of fusion images with 3D-MR cisternography and MR angiography: Application to paraclinoid aneurysms. AJNR Am. J. Neuroradiol. 2009, 30, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Oki, M.; Oki, T.; Ito, R.; Roberts, N.; Watanabe, Y. Identification of the distal dural ring using three-dimensional motion-sensitised driven-equilibrium prepared T1-weighted fast spin-echo imaging: Application to paraclinoid aneurysms. Magn. Reson. Med. Sci. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Lee, N.; Jung, J.Y.; Huh, S.K.; Kim, D.J.; Kim, D.I.; Kim, J. Distinction between intradural and extradural aneurysms involving the paraclinoid internal carotid artery with T2-weighted three-dimensional fast spin-echo MRI. J. Korean Neurosurg. Soc. 2010, 47, 437–441. [Google Scholar] [CrossRef]
- Watanabe, Y.; Makidono, A.; Nakamura, M.; Saida, Y. 3D MR cisternography to identify distal dural rings: Comparison of 3D-CISS and 3D-SPACE sequences. Magn. Reson. Med. Sci. 2011, 10, 29–32. [Google Scholar] [CrossRef]
- Zhou, Z.; Jin, Y.; Ye, H.; Zhang, X.; Liu, J.; Zhang, W. Classification, detection, and segmentation performance of image-based AI in intracranial aneurysm: A systematic review. BMC Med. Imaging 2024, 24, 164. [Google Scholar] [CrossRef]
- Shi, Z.; Hu, B.; Lu, M.; Chen, Z.; Zhang, M.; Yu, Y.; Zhou, C.; Zhong, J.; Wu, B.; Zhang, X.; et al. Assessing the impact of an artificial intelligence-based model for intracranial aneurysm detection in CT angiography on patient diagnosis and outcomes (IDEAL Study)—A protocol for a multicenter, double- blinded randomized controlled trial. Trials BMC 2024, 25, 358. [Google Scholar] [CrossRef]
- Goelz, L.; Laudani, A.; Genske, U.; Scheel, M.; Bohner, G.; Bauknecht, H.-C.; Mutze, S.; Hamm, B.; Jahnke, P. Inconsistency of AI in intracranial aneurysm detection with varying dose and image reconstruction. Sci. Rep. 2025, 15, 19921. [Google Scholar] [CrossRef]
| DDR–E | DDR–I | DDR–S | |
|---|---|---|---|
| TS–ACP line–E | 1 | 0 | 0 |
| TS–ACP line–I | 0 | 52 | 4 |
| TS–ACP line–S | 0 | 5 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, M.; Mizutani, T.; Sugiyama, T.; Sumi, K.; Arai, S.; Morofuji, Y. Prediction of Distal Dural Ring Location in Internal Carotid Paraclinoid Aneurysms Using the Tuberculum Sellae–Anterior Clinoid Process Line. J. Clin. Med. 2025, 14, 5951. https://doi.org/10.3390/jcm14175951
Matsumoto M, Mizutani T, Sugiyama T, Sumi K, Arai S, Morofuji Y. Prediction of Distal Dural Ring Location in Internal Carotid Paraclinoid Aneurysms Using the Tuberculum Sellae–Anterior Clinoid Process Line. Journal of Clinical Medicine. 2025; 14(17):5951. https://doi.org/10.3390/jcm14175951
Chicago/Turabian StyleMatsumoto, Masaki, Tohru Mizutani, Tatsuya Sugiyama, Kenji Sumi, Shintaro Arai, and Yoichi Morofuji. 2025. "Prediction of Distal Dural Ring Location in Internal Carotid Paraclinoid Aneurysms Using the Tuberculum Sellae–Anterior Clinoid Process Line" Journal of Clinical Medicine 14, no. 17: 5951. https://doi.org/10.3390/jcm14175951
APA StyleMatsumoto, M., Mizutani, T., Sugiyama, T., Sumi, K., Arai, S., & Morofuji, Y. (2025). Prediction of Distal Dural Ring Location in Internal Carotid Paraclinoid Aneurysms Using the Tuberculum Sellae–Anterior Clinoid Process Line. Journal of Clinical Medicine, 14(17), 5951. https://doi.org/10.3390/jcm14175951









