Decreased Endogenous Nitric Oxide Production in Patients with Acute Decompensated Heart Failure with Preserved Ejection Fraction
Abstract
1. Introduction
2. Methods
2.1. Study Design and Protocol
2.2. Aims and Hypotheses
2.3. Study Measures
2.4. Statistical Analysis
3. Results
3.1. Nitric Oxide Analysis in Chronic and Acute Phases of HFpEF
3.2. Comparison Between HF Phenotypes
4. Discussion
Comparison of NOA Findings with Previous Literature
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nair, N. Epidemiology and pathogenesis of heart failure with preserved ejection fraction. Rev. Cardiovasc. Med. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Fernandes, S.L.; Carvalho, R.R.; Santos, L.G.; Sa, F.M.; Ruivo, C.; Mendes, S.L.; Martins, H.; Morais, J.A. Pathophysiology and Treatment of Heart Failure with Preserved Ejection Fraction: State of the Art and Prospects for the Future. Arq. Bras. Cardiol. 2020, 114, 120–129. [Google Scholar] [CrossRef]
- Oktay, A.A.; Rich, J.D.; Shah, S.J. The emerging epidemic of heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 2013, 10, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, S.J.; Cuijpers, I.; Heymans, S.; Jones, E.A.V. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved Pathological Understanding. Cells 2020, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Peikert, A.; Martinez, F.A.; Vaduganathan, M.; Claggett, B.L.; Kulac, I.J.; Desai, A.S.; Jhund, P.S.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; et al. Efficacy and Safety of Dapagliflozin in Heart Failure With Mildly Reduced or Preserved Ejection Fraction According to Age: The DELIVER Trial. Circ. Heart Fail. 2022, 15, e010080. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef]
- Gevaert, A.B.; Beckers, P.J.; Van Craenenbroeck, A.H.; Lemmens, K.; Van De Heyning, C.M.; Heidbuchel, H.; Vrints, C.J.; Van Craenenbroeck, E.M. Endothelial dysfunction and cellular repair in heart failure with preserved ejection fraction: Response to a single maximal exercise bout. Eur. J. Heart Fail. 2019, 21, 125–127. [Google Scholar] [CrossRef]
- Yang, J.H.; Obokata, M.; Reddy, Y.N.V.; Redfield, M.M.; Lerman, A.; Borlaug, B.A. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 432–441. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Hadi, H.A.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Cuijpers, I.; Simmonds, S.J.; van Bilsen, M.; Czarnowska, E.; Gonzalez Miqueo, A.; Heymans, S.; Kuhn, A.R.; Mulder, P.; Ratajska, A.; Jones, E.A.V.; et al. Microvascular and lymphatic dysfunction in HFpEF and its associated comorbidities. Basic. Res. Cardiol. 2020, 115, 39. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Kelm, M.; Preik-Steinhoff, H.; Preik, M.; Strauer, B.E. Serum nitrite sensitively reflects endothelial NO formation in human forearm vasculature: Evidence for biochemical assessment of the endothelial L-arginine-NO pathway. Cardiovasc. Res. 1999, 41, 765–772. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Rassaf, T.; Schindler, A.; Picker, O.; Scheeren, T.; Godecke, A.; Schrader, J.; Schulz, R.; et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic. Biol. Med. 2003, 35, 790–796. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Jax, T.; Kerber, S.; Gharini, P.; Balzer, J.; Zotz, R.B.; Scharf, R.E.; Willers, R.; et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic. Biol. Med. 2006, 40, 295–302. [Google Scholar] [CrossRef]
- Pinder, A.G.; Rogers, S.C.; Khalatbari, A.; Ingram, T.E.; James, P.E. The measurement of nitric oxide and its metabolites in biological samples by ozone-based chemiluminescence. Methods Mol. Biol. 2008, 476, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Rifkind, J.M. Measurement of plasma nitrite by chemiluminescence. Methods Mol. Biol. 2010, 610, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Osanai, T.; Kamada, T.; Matsunaga, T.; Ishizaka, H.; Hanada, H.; Okumura, K. High plasma level of asymmetric dimethylarginine in patients with acutely exacerbated congestive heart failure: Role in reduction of plasma nitric oxide level. Heart Vessel 2003, 18, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Falls, R.; Wang, B.H.; Vogrin, S.; Neil, C.J. Decreased endogenous nitric oxide production in acute decompensated heart failure with a reduced ejection fraction. ESC Heart Fail. 2025, 12, 2278–2286. [Google Scholar] [CrossRef]
- Miller, G.D.; Marsh, A.P.; Dove, R.W.; Beavers, D.; Presley, T.; Helms, C.; Bechtold, E.; King, S.B.; Kim-Shapiro, D. Plasma nitrate and nitrite are increased by a high-nitrate supplement but not by high-nitrate foods in older adults. Nutr. Res. 2012, 32, 160–168. [Google Scholar] [CrossRef]
- Lauer, T.; Preik, M.; Rassaf, T.; Strauer, B.E.; Deussen, A.; Feelisch, M.; Kelm, M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc. Natl. Acad. Sci. USA 2001, 98, 12814–12819. [Google Scholar] [CrossRef]
- Ratchford, S.M.; Clifton, H.L.; Gifford, J.R.; La Salle, D.T.; Thurston, T.S.; Bunsawat, K.; Alpenglow, J.K.; Richardson, R.S.; Wright, J.B.; Ryan, J.J.; et al. Impact of Acute Antioxidant Administration on Inflammation and Vascular Function in Heart Failure with Preserved Ejection Fraction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R607–R614. [Google Scholar] [CrossRef]
- Eggebeen, J.; Kim-Shapiro, D.B.; Haykowsky, M.; Morgan, T.M.; Basu, S.; Brubaker, P.; Rejeski, J.; Kitzman, D.W. One Week of Daily Dosing With Beetroot Juice Improves Submaximal Endurance and Blood Pressure in Older Patients With Heart Failure and Preserved Ejection Fraction. JACC Heart Fail. 2016, 4, 428–437. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Koepp, K.E.; Melenovsky, V. Sodium Nitrite Improves Exercise Hemodynamics and Ventricular Performance in Heart Failure With Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2015, 66, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Rix, P.J.; Vick, A.; Attkins, N.J.; Barker, G.E.; Bott, A.W.; Alcorn, H., Jr.; Gladwin, M.T.; Shiva, S.; Bradley, S.; Hussaini, A.; et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of nebulized sodium nitrite (AIR001) following repeat-dose inhalation in healthy subjects. Clin. Pharmacokinet. 2015, 54, 261–272. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Melenovsky, V.; Koepp, K.E. Inhaled Sodium Nitrite Improves Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection Fraction. Circ. Res. 2016, 119, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Anstrom, K.J.; Lewis, G.D.; Shah, S.J.; Levine, J.A.; Koepp, G.A.; Givertz, M.M.; Felker, G.M.; LeWinter, M.M.; Mann, D.L.; et al. Effect of Inorganic Nitrite vs Placebo on Exercise Capacity Among Patients With Heart Failure With Preserved Ejection Fraction: The INDIE-HFpEF Randomized Clinical Trial. JAMA 2018, 320, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Babes, E.E.; Babes, V.V. ADMA and prognosis in patients with heart failure and preserved ejection fraction. Eur. Heart J. 2013, 34, P1491. [Google Scholar] [CrossRef]
- Hage, C.; Michaelsson, E.; Kull, B.; Miliotis, T.; Svedlund, S.; Linde, C.; Donal, E.; Daubert, J.C.; Gan, L.M.; Lund, L.H. Myeloperoxidase and related biomarkers are suggestive footprints of endothelial microvascular inflammation in HFpEF patients. ESC Heart Fail. 2020, 7, 1534–1546. [Google Scholar] [CrossRef]
- Janner, J.H.; Godtfredsen, N.S.; Ladelund, S.; Vestbo, J.; Prescott, E. Aortic augmentation index: Reference values in a large unselected population by means of the SphygmoCor device. Am. J. Hypertens. 2010, 23, 180–185. [Google Scholar] [CrossRef]
- Dudenbostel, T.; Glasser, S.P. Effects of antihypertensive drugs on arterial stiffness. Cardiol. Rev. 2012, 20, 259–263. [Google Scholar] [CrossRef]
- Jatic, Z.; Skopljak, A.; Hebibovic, S.; Sukalo, A.; Rustempasic, E.; Valjevac, A. Effects of Different Antihypertensive Drug Combinations on Blood Pressure and Arterial Stiffness. Med. Arch. 2019, 73, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Janic, M.; Lunder, M.; Sabovic, M. Arterial stiffness and cardiovascular therapy. Biomed. Res. Int. 2014, 2014, 621437. [Google Scholar] [CrossRef] [PubMed]
- Pauca, A.L.; Kon, N.D.; O’Rourke, M.F. Benefit of glyceryl trinitrate on arterial stiffness is directly due to effects on peripheral arteries. Heart 2005, 91, 1428–1432. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Chronic HFpEF group |
|
|
| Acute decompensated HFpEF group |
|
|
| Variable | Chronic HFpEF (n = 19) | Acute HFpEF (n = 16) | p-Value |
|---|---|---|---|
| Male | 8 (42) | 10 (63) | 0.23 |
| Age | 69 ± 15 | 68 ± 11 | 0.87 |
| Co-morbidities | |||
| Hypercholesterolaemia | 12 (63) | 8 (50) | 0.43 |
| Hypertension | 13 (68) | 13 (81) | 0.39 |
| IHD | 9 (47) | 8 (50) | 0.88 |
| Stroke | 1 (5) | 3 (19) | 0.31 |
| AF | 8 (42) | 11 (69) | 0.12 |
| Diabetes mellitus | 8 (42) | 11 (69) | 0.12 |
| Smoker | 10 (53) | 4 (25) | 0.096 |
| Ex-Smoker | 6 (32) | 10 (63) | 0.067 |
| Current | 3 (16) | 2 (13) | 1.0 |
| Alcohol abuse | 1 (5) | 0 (0) | 1.0 |
| Substance abuse | 1 (5) | 0 (0) | 1.0 |
| Thyroid disease | 2 (11) | 5 (31) | 0.21 |
| Iron deficiency/Anaemia | 3 (16) | 4 (25) | 0.66 |
| OSA | 4 (21) | 7 (44) | 0.27 |
| Gout | 2 (11) | 2 (13) | 1.0 |
| COPD/Asthma | 5 (26) | 4 (25) | 1.0 |
| GORD | 4 (21) | 3 (19) | 1.0 |
| Cancer | 0 (0) | 2 (13) | 0.2 |
| CKD | 4 (21) | 4 (25) | 1.0 |
| Medications | |||
| Beta Blocker | 16 (84) | 13 (81) | 0.82 |
| ACEi | 9 (47) | 8 (50) | 0.88 |
| ARB | 6 (32) | 0 (0) | 0.022 * |
| Organic nitrate | 6 (32) | 2 (13) | 0.24 |
| CCB | 2 (11) | 2 (13) | 1.0 |
| Lipid lowering agents | 15 (79) | 10 (63) | 0.28 |
| OHA | 8 (42) | 8 (50) | 0.64 |
| Insulin | 3 (16) | 7 (44) | 0.13 |
| Loop diuretic | 14 (74) | 16 (100) | 0.027 * |
| Thiazide diuretic | 2 (11) | 2 (13) | 1.0 |
| MRA | 9 (47) | 11 (69) | 0.2 |
| Digoxin | 2 (11) | 1 (6) | 1.0 |
| PPI | 5 (26) | 6 (38) | 0.48 |
| Aspirin | 9 (47) | 8 (50) | 0.87 |
| Other Anti-platelets | 4 (21) | 3 (19) | 1.0 |
| Anti-coagulant | 8 (42) | 11 (69) | 0.12 |
| Sacubitril/Valsartan | 1 (5) | 0 (0) | 1.0 |
| Echocardiographic and haemodynamic variables | |||
| LVEF (%) | 55 ± 5 | 58 ± 8 | 0.16 |
| RAP (mmHg) | 5.5 ± 3.5 | 6.9 ± 4.4 | 0.25 |
| RVSP (mmHg) | 33.1 ± 14.1 | 40.6 ± 8.8 | 0.063 |
| LAVI (ml/m2) | 42.5 ± 13.6 | 43.2 ± 15.8 | 0.92 |
| E/e′ | 16 (12.5–21.5) | 20 (17–27) | 0.04 * |
| PCWP (mmHg) # | 21.74 (17.4–28.6) | 26.7 (23.0–35.4) | 0.04 * |
| sysBP (mmHg) | 147.0 ± 18.3 | 132.2 ± 18.1 | 0.026 * |
| diaBP (mmHg) | 79.1 ± 13.3 | 75.2 ± 13.8 | 0.42 |
| ADP (mmHg) | 85.5 ±16.5 | 76.7 ± 13.8 | 0.11 |
| MAP (mmHg) | 100.1 ± 13.9 | 93.8 ± 14.0 | 0.21 |
| HR (bpm) | 65.4 ±10.8 | 71.8 ± 10.7 | 0.1 |
| ASP (mmHg) | 133.1 ± 15.1 | 120.0 ± 14.9 | 0.018 * |
| APP (mmHg) | 52.8 ± 14.6 | 43.3 ± 13.7 | 0.066 |
| BNP | 2105 (1155–3055) | 1603 (1080–3546) | 0.65 |
| Variable | Chronic HFpEF | Acute HFpEF | p-Value 1 | OR (95% CI) | p-Value 2 |
|---|---|---|---|---|---|
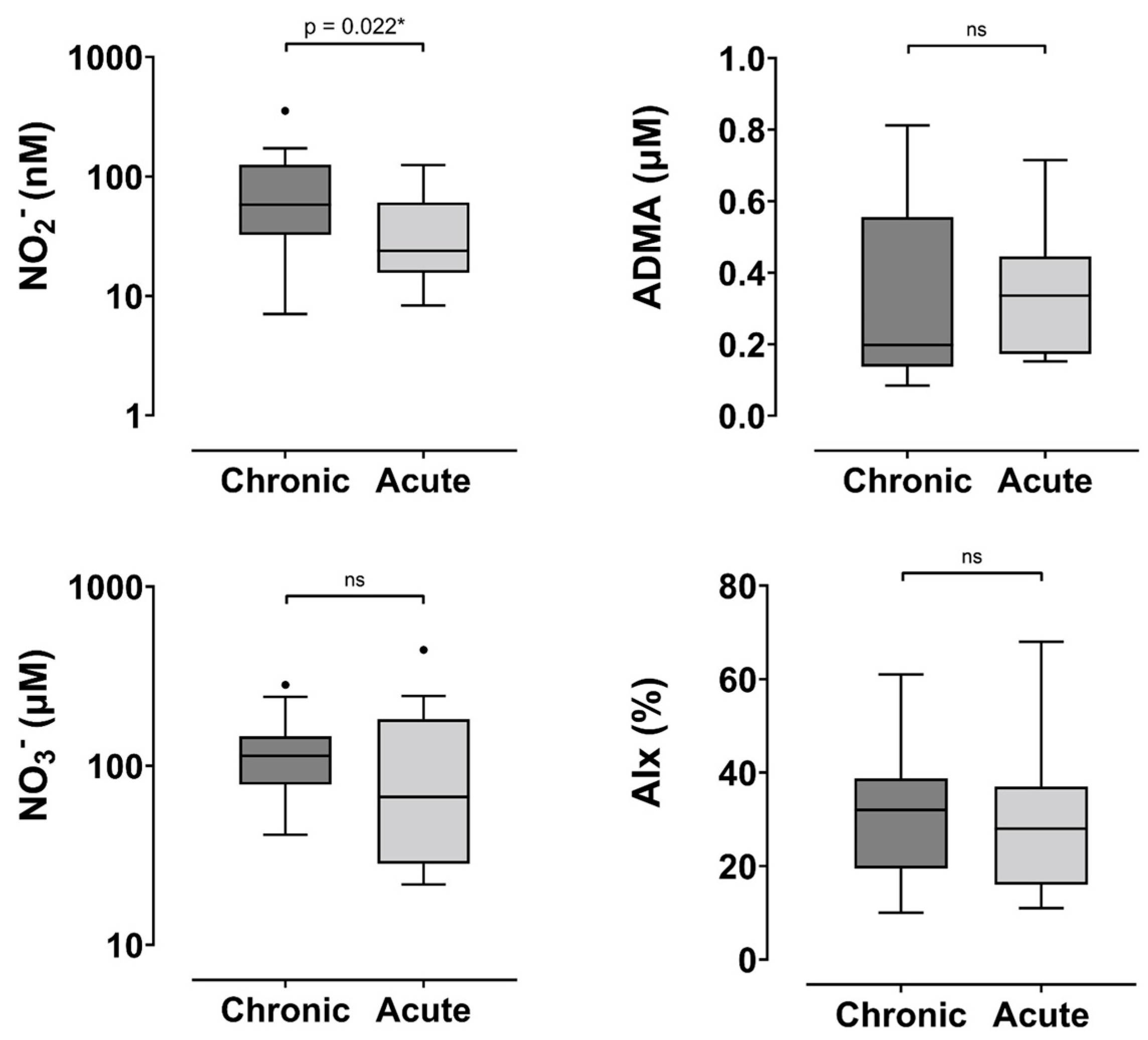
| NO2− (nM) | 58.1 (32.5–125.4) | 23.9 (15.7–60.4) | 0.022 * | 0.98 (0.96–1.0) | 0.077 |
| NO3− (µM) | 113 (78.4–145.9) | 66.9 (28.4–181.7) | 0.23 | 1.0 (0.99–1.0) | 0.80 |
| ADMA (µM) | 0.19 (0.14–0.55) | 0.37 (0.17–0.45) | 0.34 | 0.93 (0.63–1.36) | 0.68 3 |
| AIx † | 30.6 ± 13.4 | 29 ± 15 | 0.75 | 0.99 (0.94–1.05) | 0.77 |
| Variable | HFpEF | HFrEF | p-Value |
|---|---|---|---|
| Chronic Phase | |||
| NO2− (nM) | 58.1 (32.5–125.4) | 60 (30.4–209.3) | 0.99 |
| NO3− (µM) | 113 (78.4–145.9) | 96.7 (82.9–153.2) | 0.89 |
| ADMA (µM) | 0.19 (0.14–0.55) | 0.16 (0.13–0.24) | 0.16 |
| AIx (%) † | 30.6 +/− 13.4 | 25.6 (12.0) | 0.25 |
| Acute Phase | |||
| NO2− (nM) | 23.9 (15.7–60.4) | 30.7 (19.1–52.4) | 0.76 |
| NO3− (µM) | 66.9 (28.4–181.7) | 63.3 (36.9–161.8) | 0.89 |
| ADMA (µM) | 0.37 (0.17–0.45) | 0.4 (0.27–0.56) | 0.20 |
| AIx (%) † | 29 ± 15 | 26.7 (23.3) | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falls, R.; Wang, B.H.; Vogrin, S.; Neil, C.J. Decreased Endogenous Nitric Oxide Production in Patients with Acute Decompensated Heart Failure with Preserved Ejection Fraction. J. Clin. Med. 2025, 14, 5928. https://doi.org/10.3390/jcm14175928
Falls R, Wang BH, Vogrin S, Neil CJ. Decreased Endogenous Nitric Oxide Production in Patients with Acute Decompensated Heart Failure with Preserved Ejection Fraction. Journal of Clinical Medicine. 2025; 14(17):5928. https://doi.org/10.3390/jcm14175928
Chicago/Turabian StyleFalls, Roman, Bing H. Wang, Sara Vogrin, and Christopher J. Neil. 2025. "Decreased Endogenous Nitric Oxide Production in Patients with Acute Decompensated Heart Failure with Preserved Ejection Fraction" Journal of Clinical Medicine 14, no. 17: 5928. https://doi.org/10.3390/jcm14175928
APA StyleFalls, R., Wang, B. H., Vogrin, S., & Neil, C. J. (2025). Decreased Endogenous Nitric Oxide Production in Patients with Acute Decompensated Heart Failure with Preserved Ejection Fraction. Journal of Clinical Medicine, 14(17), 5928. https://doi.org/10.3390/jcm14175928






