Diagnostic Accuracy of Dual-Energy CT Parameters for Discrimination of Hypodense Liver Lesions in Patients Affected by Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. DECT Scan Protocol
2.3. CT Image Reconstruction
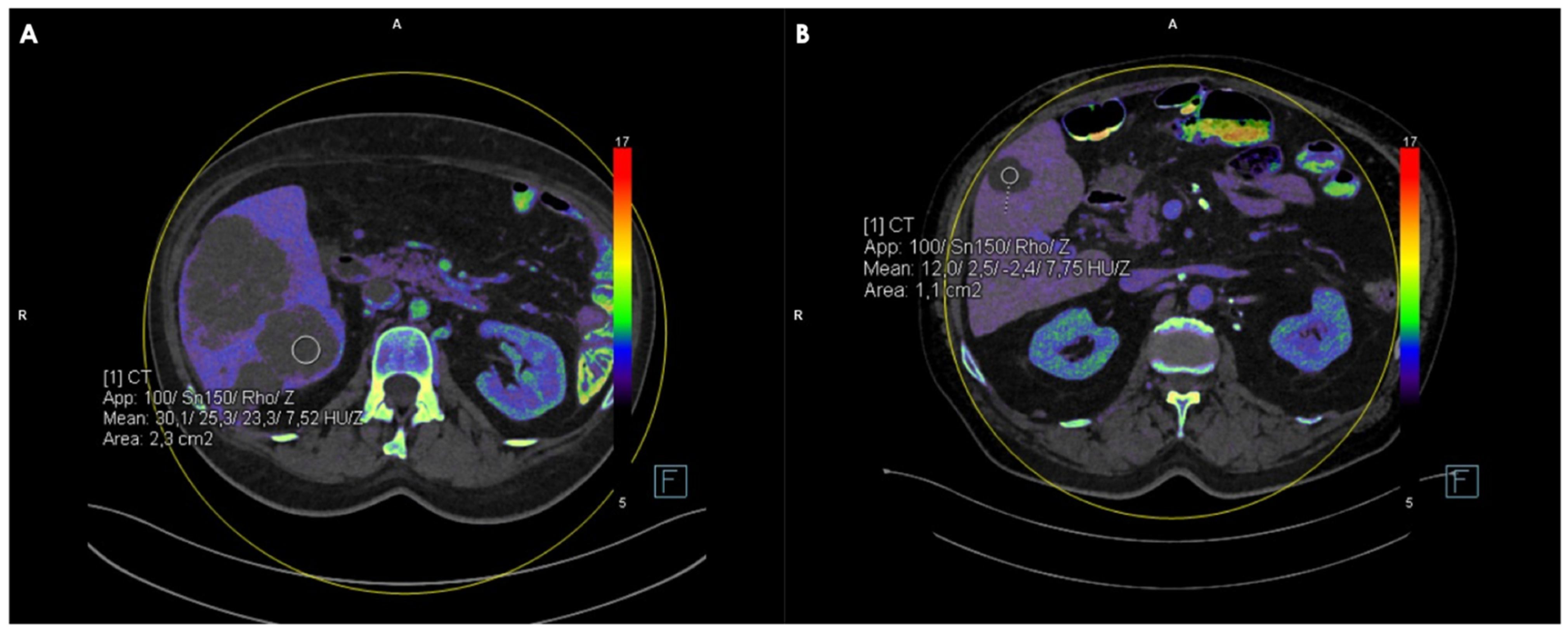
2.4. Measurements of CT, FF, Zeff and IC Values
2.5. Reference Standard
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| CRC | colorectal cancer |
| CT | computed tomography |
| DECT | dual-energy computed tomography |
| FF | fat fraction |
| GRE | gradient echo |
| HASTE | half-Fourier acquisition single shot turbo spin echo |
| IC | iodine concentration |
| IQR | interquartile range |
| MRI | magnetic resonance |
| PACS | picture archiving and communication system |
| ROC | receiver-operating characteristic curve |
| ROI | region of interest |
| SD | standard deviation |
| TNC | true non-contrast |
| VIBE | volumetric interpolated breath-hold examination |
| VNC | virtual non-contrast |
| Zeff | atomic number |
References
- Ciardiello, F.; Ciardiello, D.; Martini, G.; Napolitano, S.; Tabernero, J.; Cervantes, A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J. Clin. 2022, 72, 372–401. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; De Gramont, A.; Figueras, J.; Guthrie, A.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; et al. The oncosurgery approach to managing liver metastases from colorectal cancer: A multidisciplinary international consensus. Oncologist 2012, 17, 1225–1239. [Google Scholar] [CrossRef]
- Baker, M.E.; Pelley, R. Hepatic metastases: Basic principles and implications for radiologists. Radiology 1995, 197, 329–337. [Google Scholar] [CrossRef]
- Sica, G.T.; Ji, H.; Ros, P.R. CT and MR imaging of hepatic metastases. Am. J. Roentgenol. 2000, 174, 691–698. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. ESMO Guidelines Committee Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef]
- Cunningham, C.; Leong, K.; Clark, S.; Plumb, A.; Taylor, S.; Geh, I.; Karandikar, S.; Moran, B. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the Management of Cancer of the Colon, Rectum and Anus (2017)—Diagnosis, Investigations and Screening. Color. Dis. 2017, 19, 9–17. [Google Scholar] [CrossRef]
- Renzulli, M.; Clemente, A.; Ierardi, A.M.; Pettinari, I.; Tovoli, F.; Brocchi, S.; Peta, G.; Cappabianca, S.; Carrafiello, G.; Golfieri, R. Imaging of Colorectal Liver Metastases: New Developments and Pending Issues. Cancers 2020, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.D.; Pinho, D.F.; Kulkarni, N.M.; Hahn, P.F.; Guimaraes, A.R.; Sahani, D.V. Oncologic applications of dual-energy CT in the abdomen. Radiographics 2014, 34, 589–612. [Google Scholar] [CrossRef]
- Tatsugami, F.; Higaki, T.; Nakamura, Y.; Honda, Y.; Awai, K. Dual-energy CT: Minimal essentials for radiologists. Jpn. J. Radiol. 2022, 40, 547–559. [Google Scholar] [CrossRef]
- Patino, M.; Prochowski, A.; Agrawal, M.D.; Simeone, F.J.; Gupta, R.; Hahn, P.F.; Sahani, D.V. Material Separation Using Dual-Energy CT: Current and Emerging Applications. Radiographics 2016, 36, 1087–1105. [Google Scholar] [CrossRef]
- Lenga, L.; Czwikla, R.; Wichmann, J.L.; Leithner, D.; Albrecht, M.H.; Booz, C.; Arendt, C.T.; Yel, I.; D’Angelo, T.; Vogl, T.J.; et al. Dual-energy CT in patients with colorectal cancer: Improved assessment of hypoattenuating liver metastases using noise-optimized virtual monoenergetic imaging. Eur. J. Radiol. 2018, 106, 184–191. [Google Scholar] [CrossRef]
- Lenga, L.; Lange, M.; Arendt, C.T.; Booz, C.; Yel, I.; Bodelle, B.; D’ANgelo, T.; Hammerstingl, R.M.; Huizinga, N.A.; Vogl, T.J.; et al. Measurement Reliability and Diagnostic Accuracy of Virtual Monoenergetic Dual-Energy CT in Patients with Colorectal Liver Metastases. Acad. Radiol. 2020, 27, e168–e175. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Darnell, A.; Rengo, M.; Muscogiuri, G.; Bellini, D.; Ayuso, C.; Laghi, A. Dual-energy CT: Oncologic applications. Am. J. Roentgenol. 2012, 199 (Suppl. S5), S98–S105. [Google Scholar] [CrossRef]
- Mileto, A.; Allen, B.C.; Pietryga, J.A.; Farjat, A.E.; Zarzour, J.G.; Bellini, D.; Ebner, L.; Desiree, E. Characterization of Incidental Renal Mass with Dual-Energy CT: Diagnostic Accuracy of Effective Atomic Number Maps for Discriminating Nonenhancing Cysts from Enhancing Masses. Am. J. Roentgenol. 2017, 209, W221–W230. [Google Scholar] [CrossRef]
- Yel, I.; D’angelo, T.; Gruenewald, L.D.; Koch, V.; Golbach, R.; Mahmoudi, S.; Ascenti, G.; Blandino, A.; Vogl, T.J.; Booz, C.; et al. Dual-Energy CT Material Decomposition: The Value in the Detection of Lymph Node Metastasis from Breast Cancer. Diagnostics 2024, 14, 466. [Google Scholar] [CrossRef]
- Winkelmann, M.T.; Gassenmaier, S.; Walter, S.S.; Artzner, C.; Nikolaou, K.; Bongers, M.N. Differentiation of Hamartomas and Malignant Lung Tumors in Single-Phased Dual-Energy Computed Tomography. Tomography 2024, 10, 255–265. [Google Scholar] [CrossRef]
- Yel, I.; Koch, V.; Gruenewald, L.D.; Mahmoudi, S.; Alizadeh, L.S.; Goekduman, A.; Eichler, K.; Vogl, T.J.; Dimitrova, M.; Booz, C. Advancing Differentiation of Hepatic Metastases in Malignant Melanoma Through Dual-Energy Computed Tomography Rho/Z Maps. Diagnostics 2024, 14, 742. [Google Scholar] [CrossRef]
- Ivey, G.D.; Johnston, F.M.; Azad, N.S.; Christenson, E.S.; Lafaro, K.J.; Shubert, C.R. Current Surgical Management Strategies for Colorectal Cancer Liver Metastases. Cancers 2022, 14, 1063. [Google Scholar] [CrossRef]
- Chow, F.C.; Chok, K.S. Colorectal liver metastases: An update on multidisciplinary approach. World J. Hepatol. 2019, 11, 150–172. [Google Scholar] [CrossRef]
- Gallinger, S.; Biagi, J.J.; Fletcher, G.G.; Nhan, C.; Ruo, L.; McLeod, R.S. Liver resection for colorectal cancer metastases. Curr. Oncol. 2013, 20, e255–e265. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 34, 10–32. [Google Scholar] [CrossRef]
- Hazhirkarzar, B.; Khoshpouri, P.; Shaghaghi, M.; Ghasabeh, M.A.; Pawlik, T.M.; Kamel, I.R. Current state of the art imaging approaches for colorectal liver metastasis. HepatoBiliary Surg. Nutr. 2020, 9, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Arizono, S.; Isoda, H.; Togashi, K. Imaging Characteristics of Liver Metastases Overlooked at Contrast-Enhanced CT. Am. J. Roentgenol. 2019, 212, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Yu, J.S.; Chung, J.J.; Kim, J.H.; Kim, K.W. Diagnosing small hepatic cysts on multidetector CT: An additional merit of thinner coronal reformations. Korean J. Radiol. 2011, 12, 341–350. [Google Scholar] [CrossRef][Green Version]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Bucolo, G.M.; Ascenti, V.; Barbera, S.; Fontana, F.; Aricò, F.M.; Piacentino, F.; Coppola, A.; Cicero, G.; Marino, M.A.; Booz, C.; et al. Virtual Non-Contrast Spectral CT in Renal Masses: Is It Time to Discard Conventional Unenhanced Phase? J. Clin. Med. 2023, 12, 4718. [Google Scholar] [CrossRef]
- Connolly, M.J.; McInnes, M.D.F.; El-Khodary, M.; McGrath, T.A.; Schieda, N. Diagnostic accuracy of virtual non-contrast enhanced dual-energy CT for diagnosis of adrenal adenoma: A systematic review and meta-analysis. Eur. Radiol. 2017, 27, 4324–4335. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Chen, B.; Wang, H.; Zhang, Y.; Yi, X.; Liao, W.; Zhao, L. Diagnostic performance of magnetic resonance imaging for colorectal liver metastasis: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 1969. [Google Scholar] [CrossRef] [PubMed]

| Population | Age | Metastases | Cysts | |
|---|---|---|---|---|
| Men | 71 | 65 ± 12 | 79 | 46 |
| Women | 49 | 65 ± 13 | 68 | 30 |
| Total | 120 | 65 ± 12 | 147 | 76 |
| Cysts | Metastases | p-Value | |
|---|---|---|---|
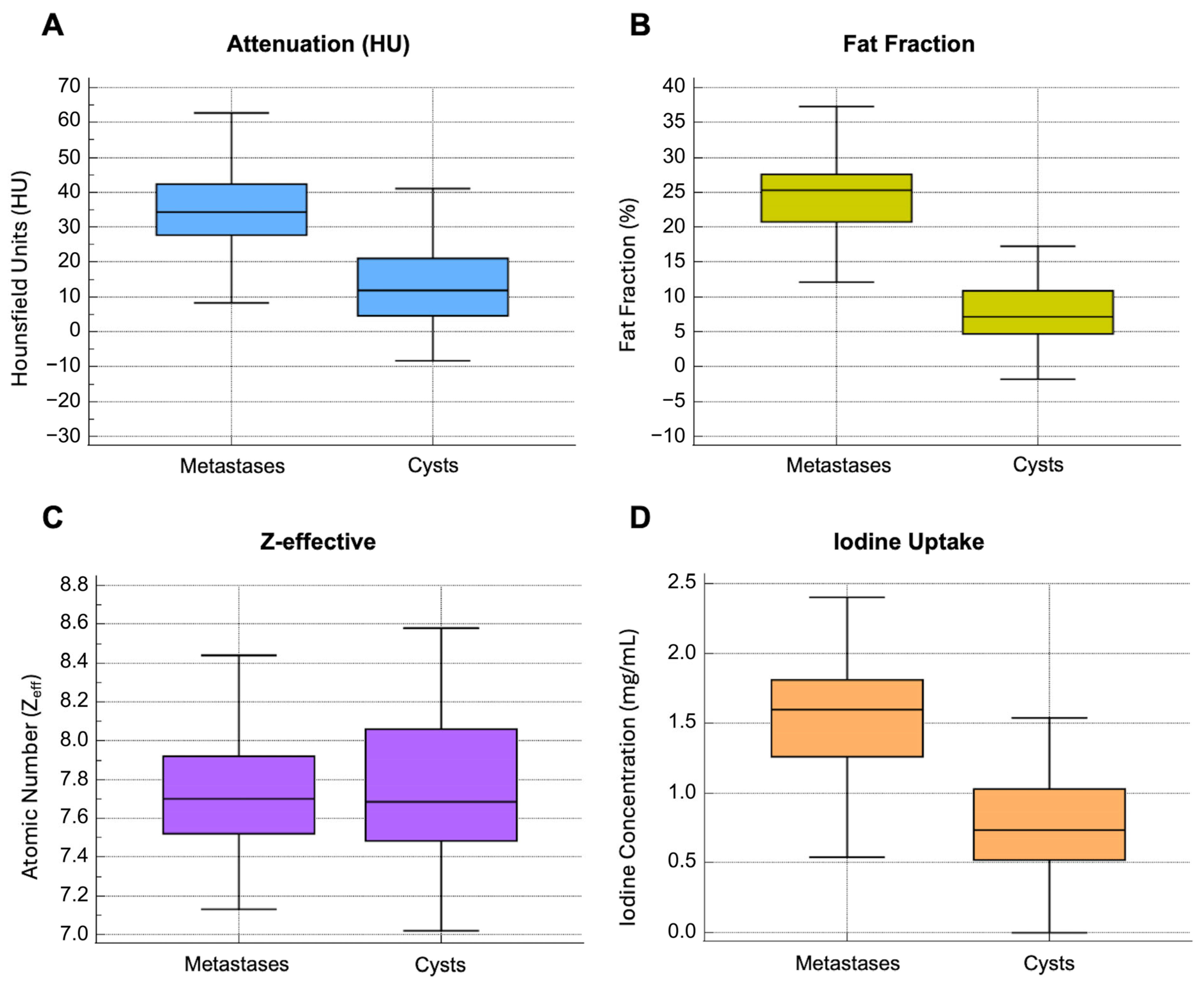
| Attenuation (HU) | 11.9 [9.4–16.2] | 34.3 [27.8–42.5] | <0.0001 |
| Fat Fraction (%) | 7.2 [4.7–10.9] | 25.3 [20.7–27.6] | <0.0001 |
| Atomic Number (Zeff) | 7.7 [7.5–8.1] | 7.7 [7.6–7.9] | 0.781 |
| Iodine Concentration (mg/mL) | 0.7 [0.5–1] | 1.6 [1.3–1.8] | <0.0001 |
| Intra-Reader Agreement Observer 1 | Intra-Reader Agreement Observer 2 | Inter-Reader Agreement | |
|---|---|---|---|
| Attenuation | 0.94 (0.93–0.96) | 0.93 (0.91–0.95) | 0.93 (0.91–0.94) |
| Fat Fraction | 0.86 (0.82–0.89) | 0.86 (0.82–0.89) | 0.85 (0.81–0.88) |
| Z-eff | 0.88 (0.85–0.91) | 0.89 (0.86–0.92) | 0.90 (0.87–0.92) |
| Iodine Concentration | 0.87 (0.83–0.90) | 0.88 (0.85–0.91) | 0.88 (0.85–0.91) |
| AUC (95% CI) | Cut-Off | Sensitivity (95% CI) | Specificity (95% CI) | Comparison (p-Value) | |||
|---|---|---|---|---|---|---|---|
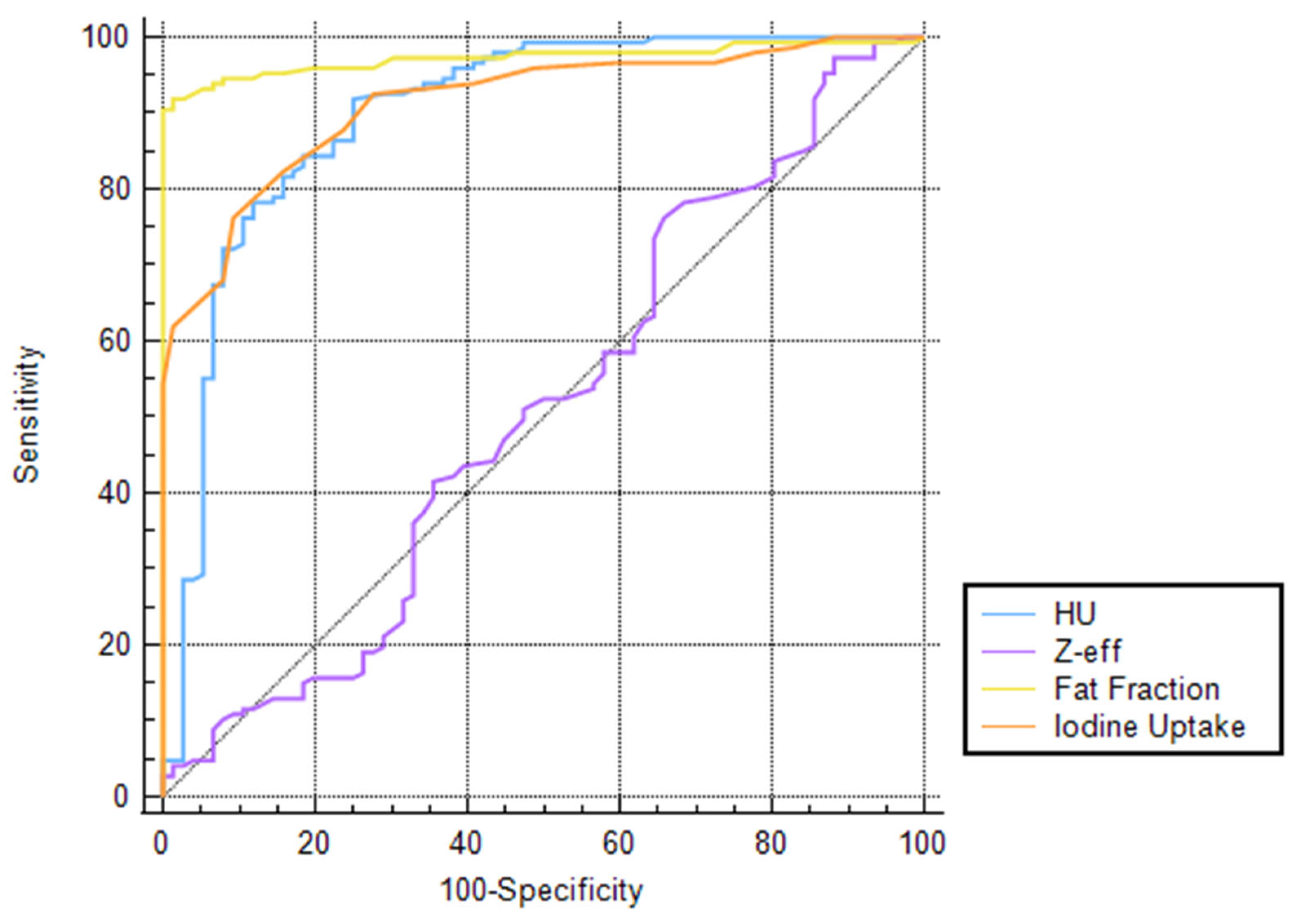
| Attenuation (HU) | 0.90 (0.85–0.94) | >19.8 | 91.8 (86.4–95.7) | 75 (63.7–84.2) | FF 0.0074 | Zeff <0.0001 | IC 0.6419 |
| Fat Fraction (%) | 0.97 (0.94–0.99) | >15.9 | 91.8 (86.2–95.7) | 98.7 (92.9–100) | HU 0.0074 | Zeff <0.0001 | IC 0.0018 |
| Atomic Number (Zeff) | 0.51 (0.44–0.58) | >7.51 | 76.2 (68.5–82.8) | 34.2 (23.7–46.0) | HU <0.0001 | FF <0.0001 | IC <0.0001 |
| Iodine Concentration (mg/mL) | 0.91 (0.87–0.95) | >1.2 | 76.2 (68.5–82.8) | 90.8 (81.9–96.2) | HU 0.6419 | FF 0.0018 | Zeff <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Angelo, T.; Lanzafame, L.R.M.; Steinert, T.; Mazziotti, S.; França, M.; Othman, A.E.; Dimitrova, M.; Mahmoudi, S.; Yel, I.; Alizadeh, L.S.; et al. Diagnostic Accuracy of Dual-Energy CT Parameters for Discrimination of Hypodense Liver Lesions in Patients Affected by Colorectal Cancer. J. Clin. Med. 2025, 14, 5929. https://doi.org/10.3390/jcm14175929
D’Angelo T, Lanzafame LRM, Steinert T, Mazziotti S, França M, Othman AE, Dimitrova M, Mahmoudi S, Yel I, Alizadeh LS, et al. Diagnostic Accuracy of Dual-Energy CT Parameters for Discrimination of Hypodense Liver Lesions in Patients Affected by Colorectal Cancer. Journal of Clinical Medicine. 2025; 14(17):5929. https://doi.org/10.3390/jcm14175929
Chicago/Turabian StyleD’Angelo, Tommaso, Ludovica R. M. Lanzafame, Timo Steinert, Silvio Mazziotti, Manuela França, Ahmed E. Othman, Mirela Dimitrova, Scherwin Mahmoudi, Ibrahim Yel, Leona S. Alizadeh, and et al. 2025. "Diagnostic Accuracy of Dual-Energy CT Parameters for Discrimination of Hypodense Liver Lesions in Patients Affected by Colorectal Cancer" Journal of Clinical Medicine 14, no. 17: 5929. https://doi.org/10.3390/jcm14175929
APA StyleD’Angelo, T., Lanzafame, L. R. M., Steinert, T., Mazziotti, S., França, M., Othman, A. E., Dimitrova, M., Mahmoudi, S., Yel, I., Alizadeh, L. S., Grünewald, L. D., Koch, V., Martin, S. S., Vogl, T. J., & Booz, C. (2025). Diagnostic Accuracy of Dual-Energy CT Parameters for Discrimination of Hypodense Liver Lesions in Patients Affected by Colorectal Cancer. Journal of Clinical Medicine, 14(17), 5929. https://doi.org/10.3390/jcm14175929







