Systematic Review and Meta-Analysis of Human Papillomavirus Prevalence and Genotypic Disparities Among Human Immunodeficiency Virus-Positive Women in Africa
Abstract
1. Introduction
Objective
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction and Quality Assessment
2.4. Study Inclusion and Exclusion
2.5. Risk of Bias Assessment and Its Implications
2.6. Data Analysis
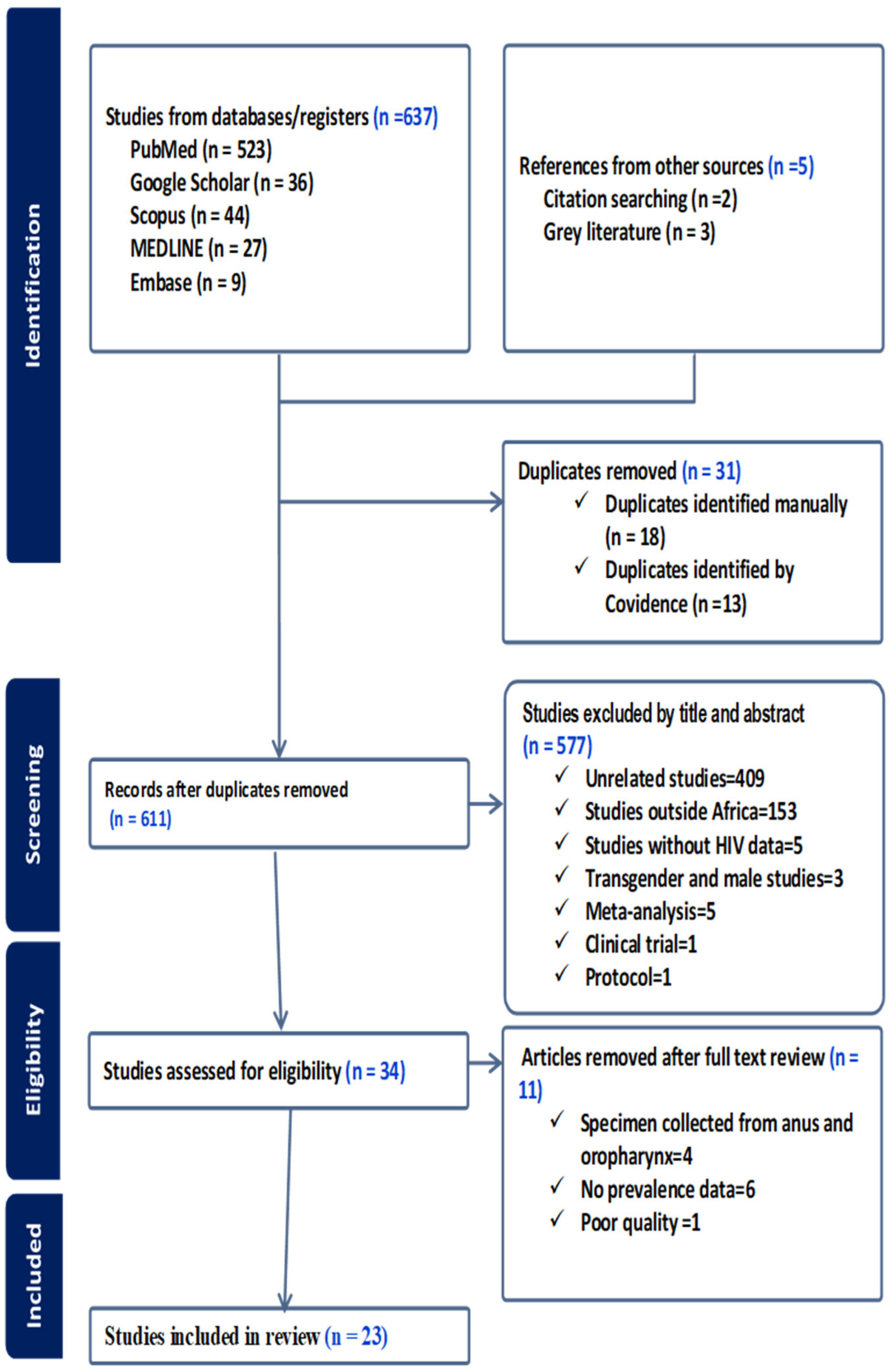
3. Results
3.1. Study Characteristics
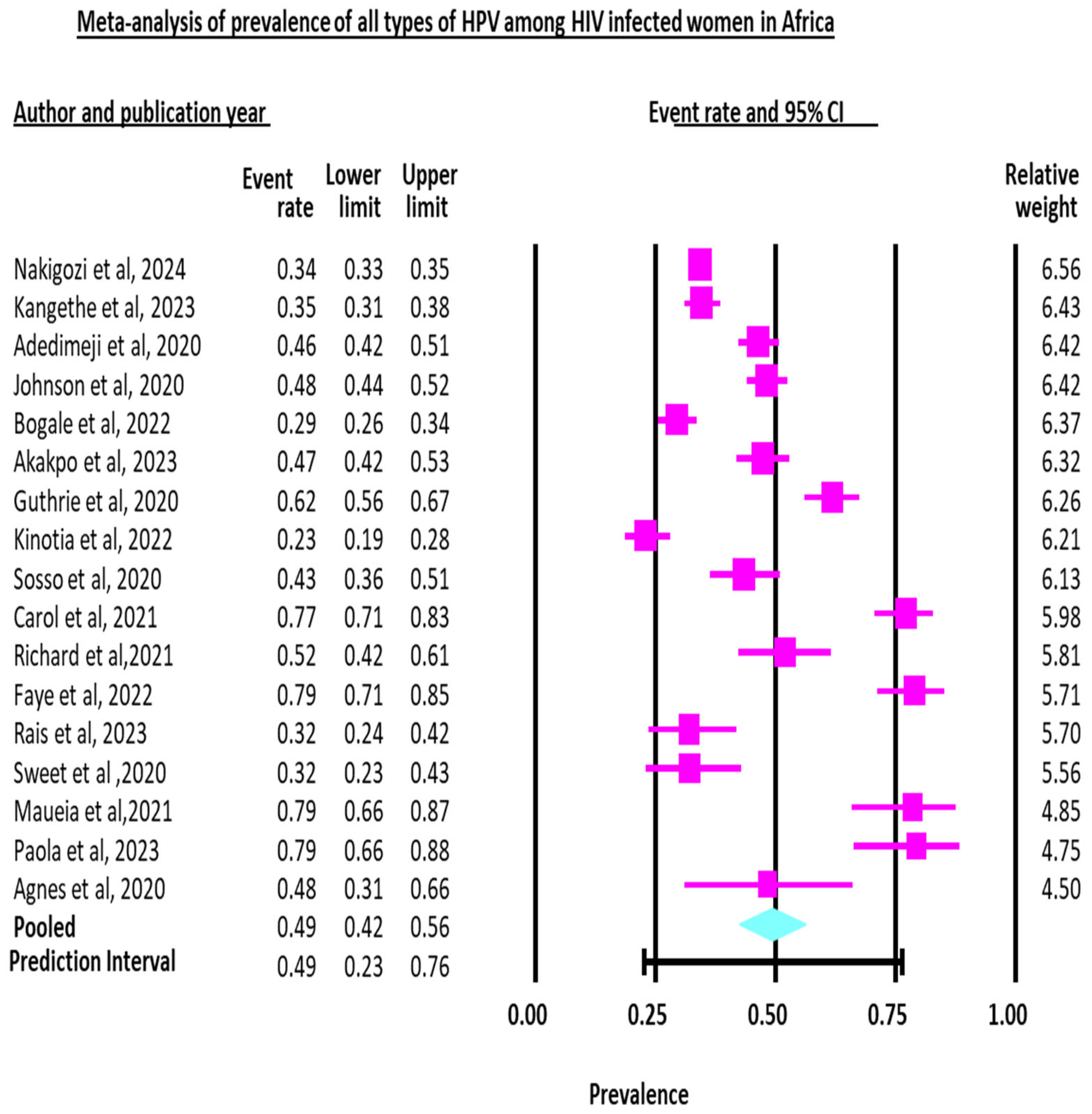
3.2. Pooled Prevalence of HPV
3.2.1. Pooled Prevalence of All Types of Human Papillomavirus (HPV) Infection
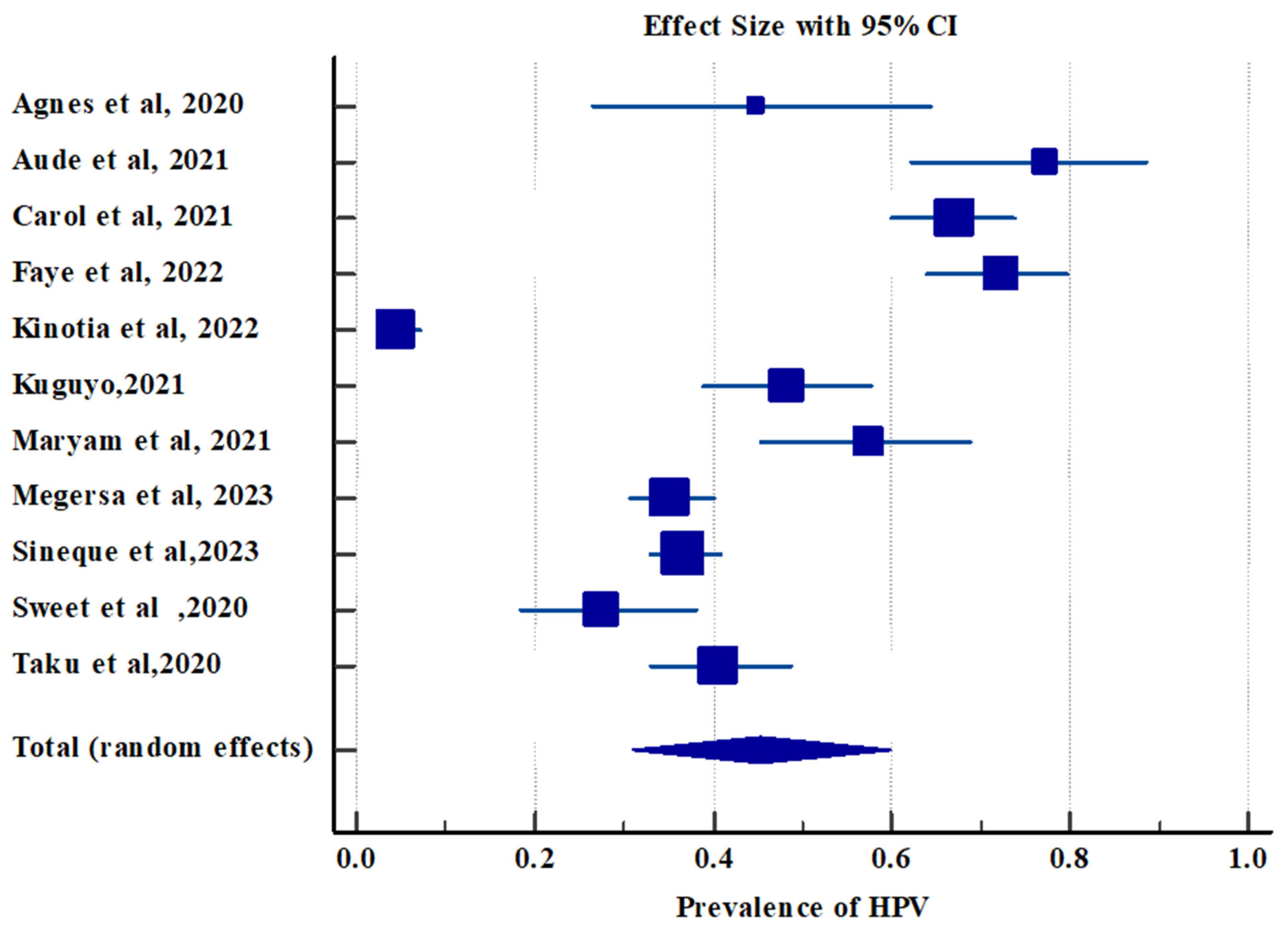
3.2.2. Pooled Prevalence of High-Risk Human Papillomavirus Infection
3.2.3. Pooled Prevalence of Low-Risk Human Papillomavirus Infection
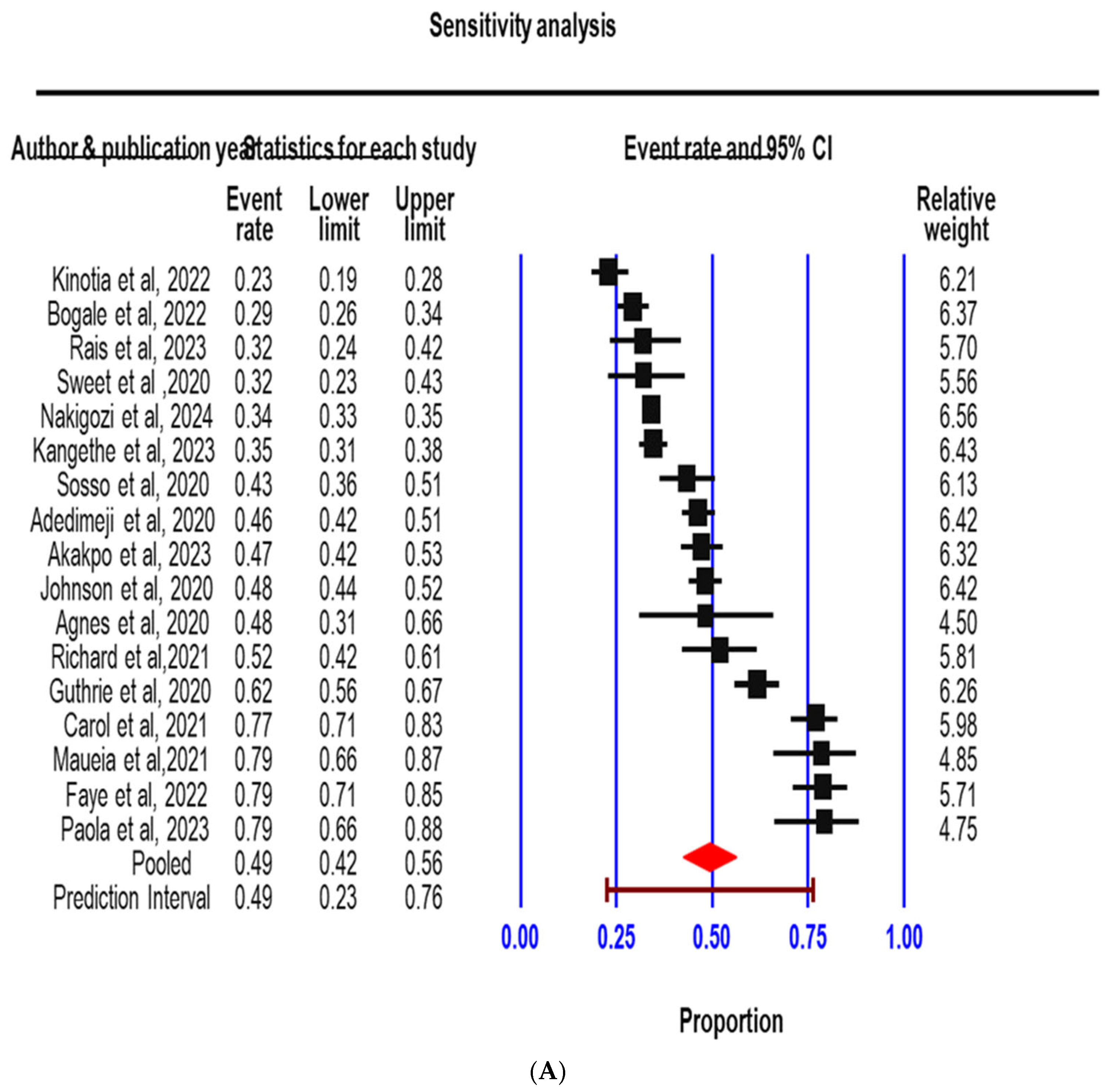
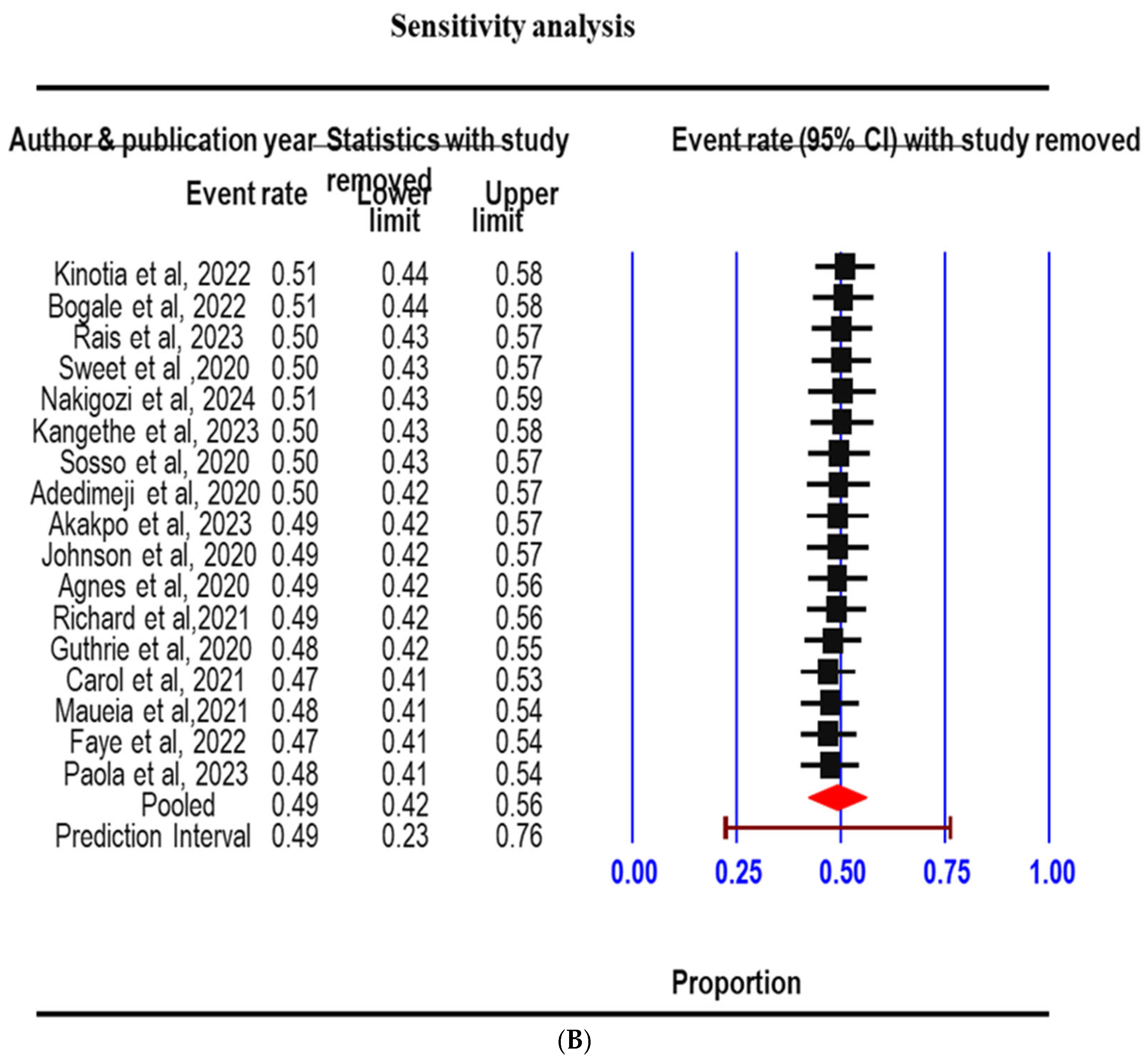
3.3. Sensitivity Analysis
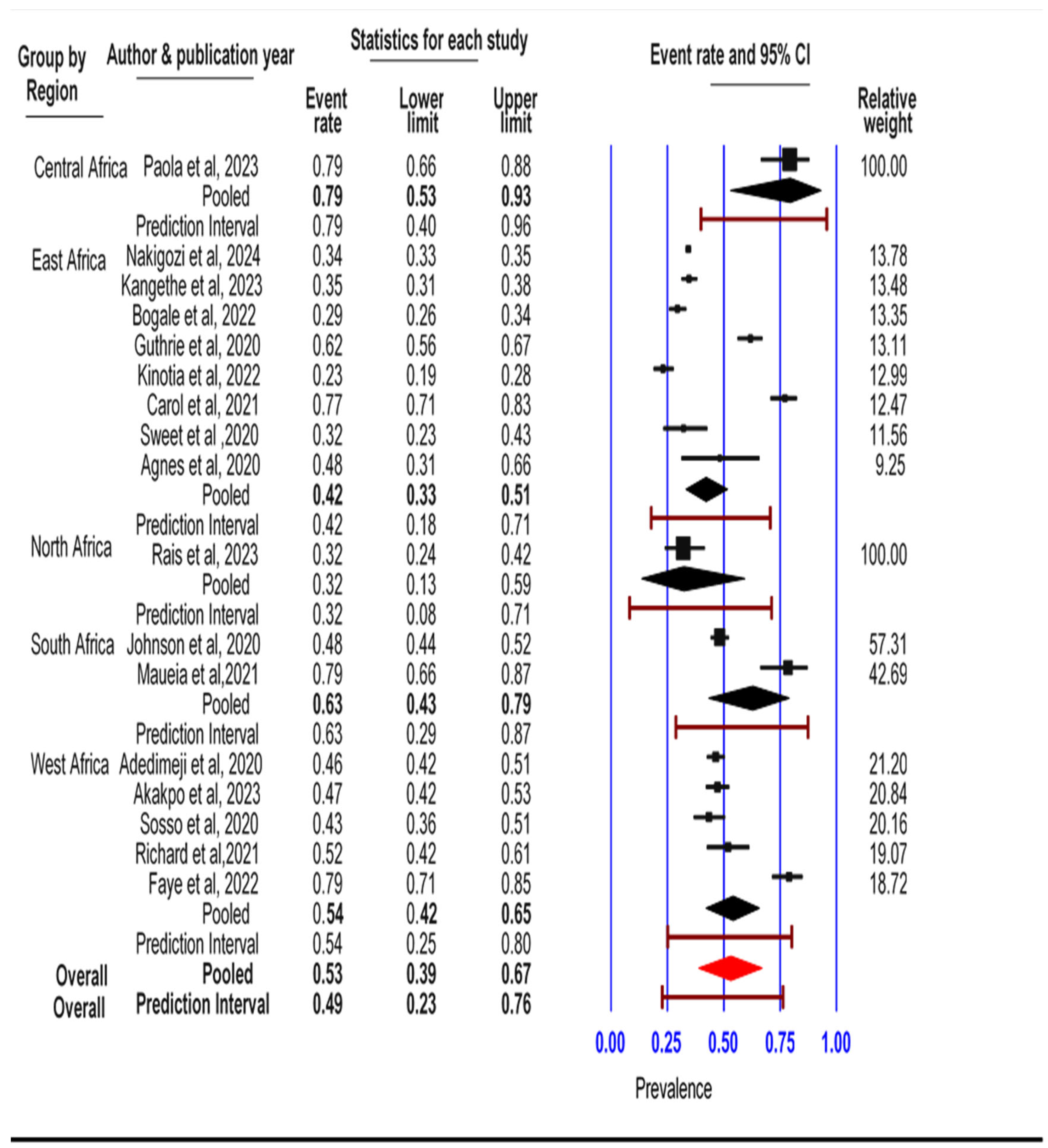
3.4. Subgroup Analysis
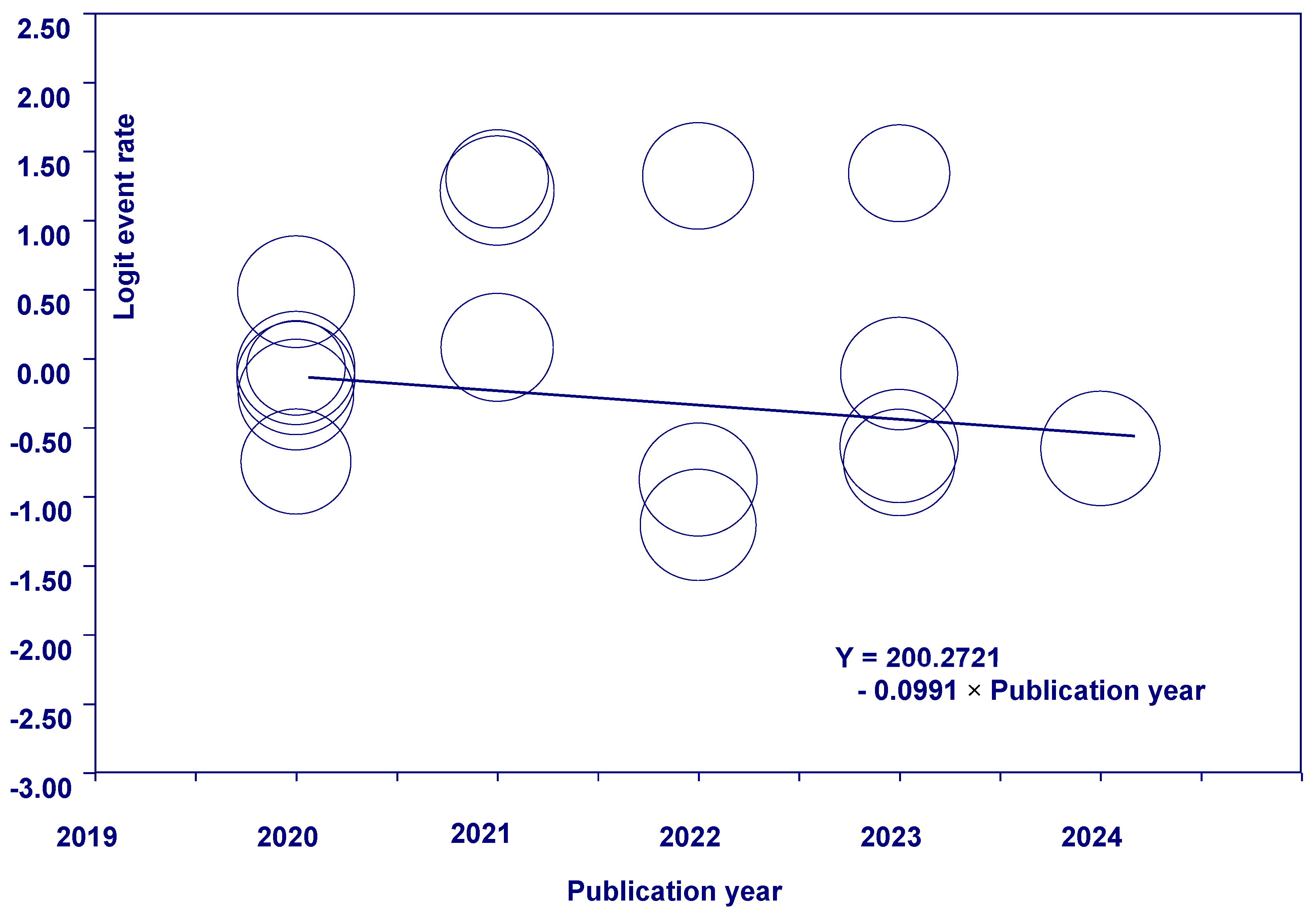
3.5. Meta-Regression
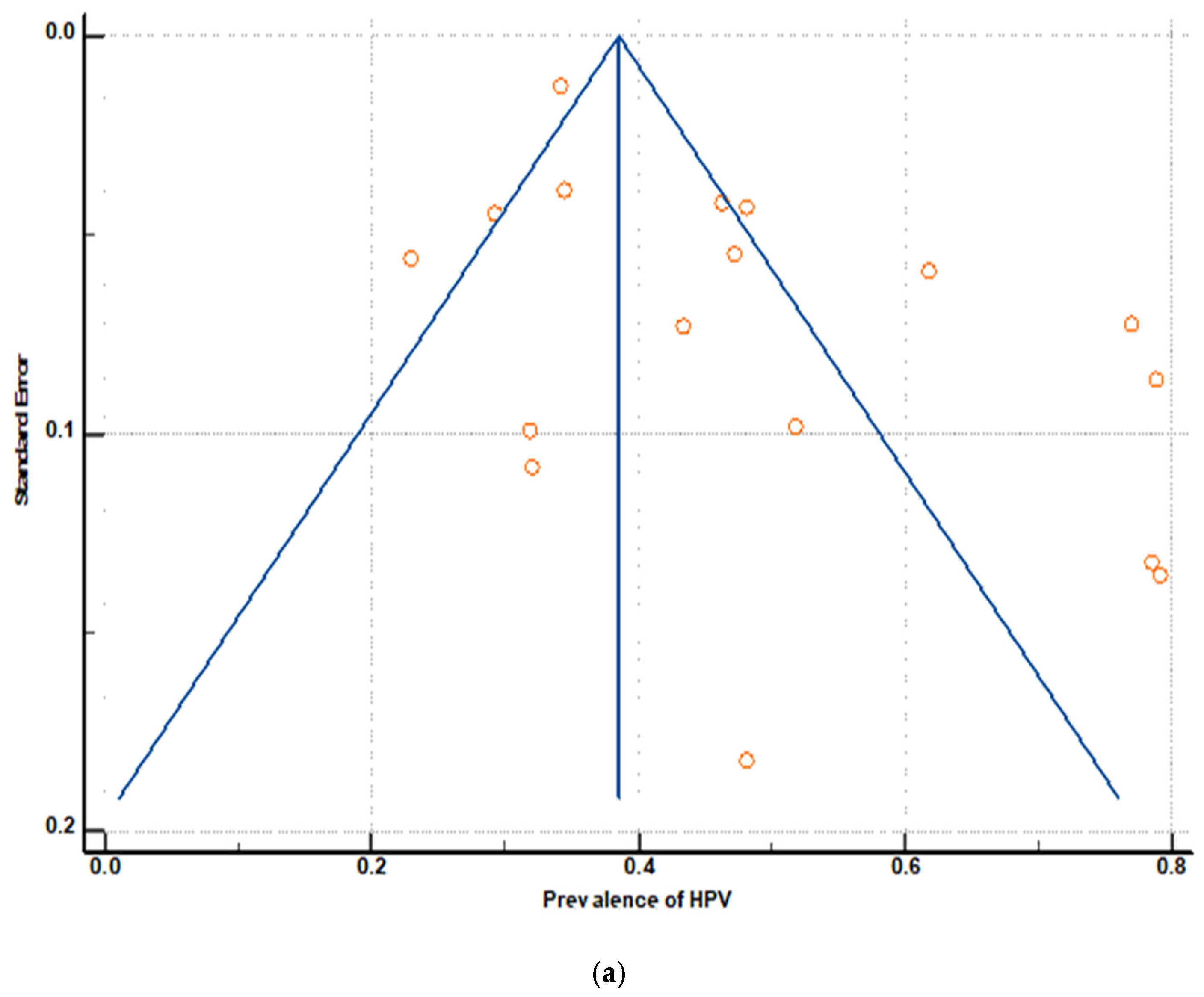
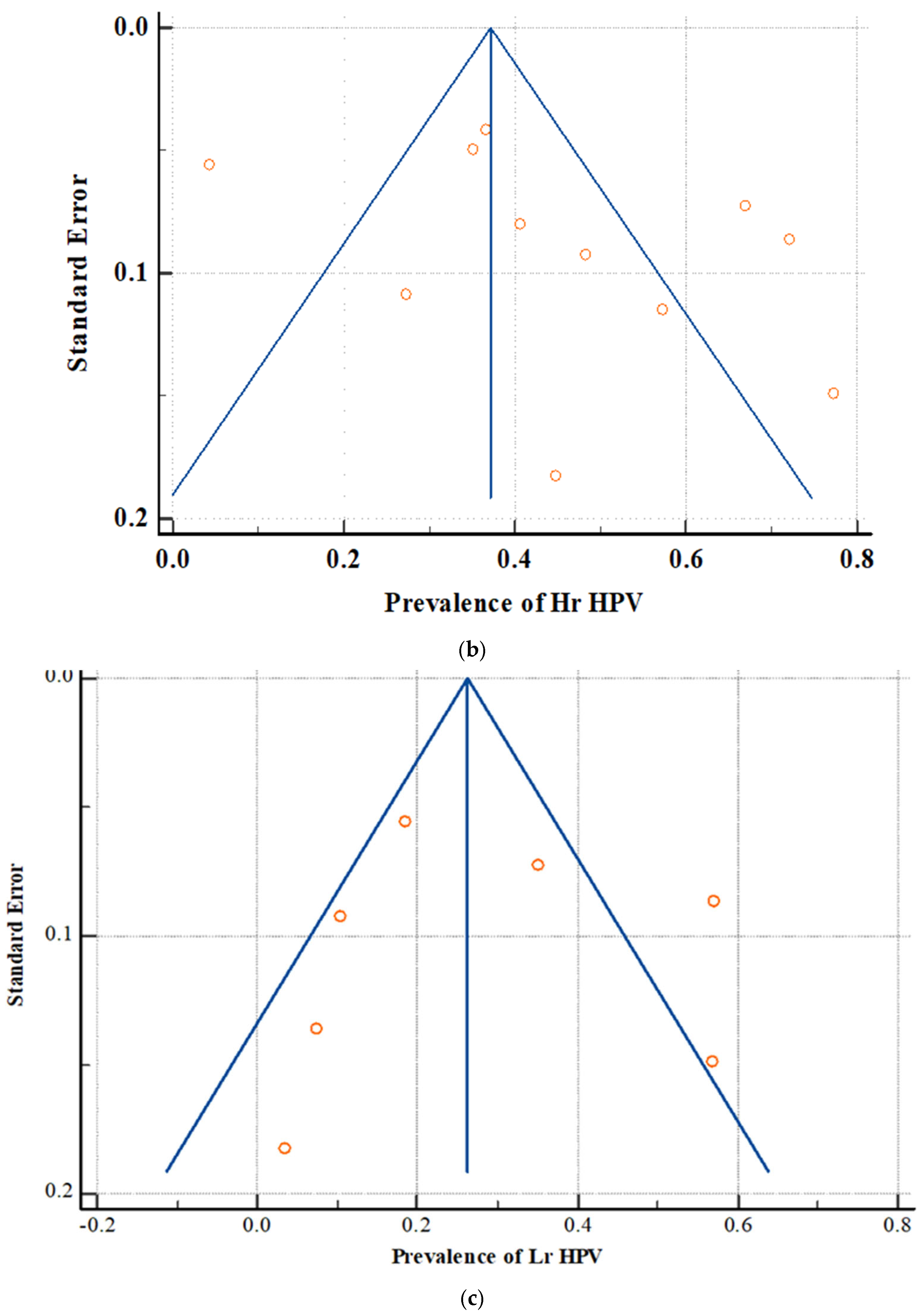
3.6. Publication Bias
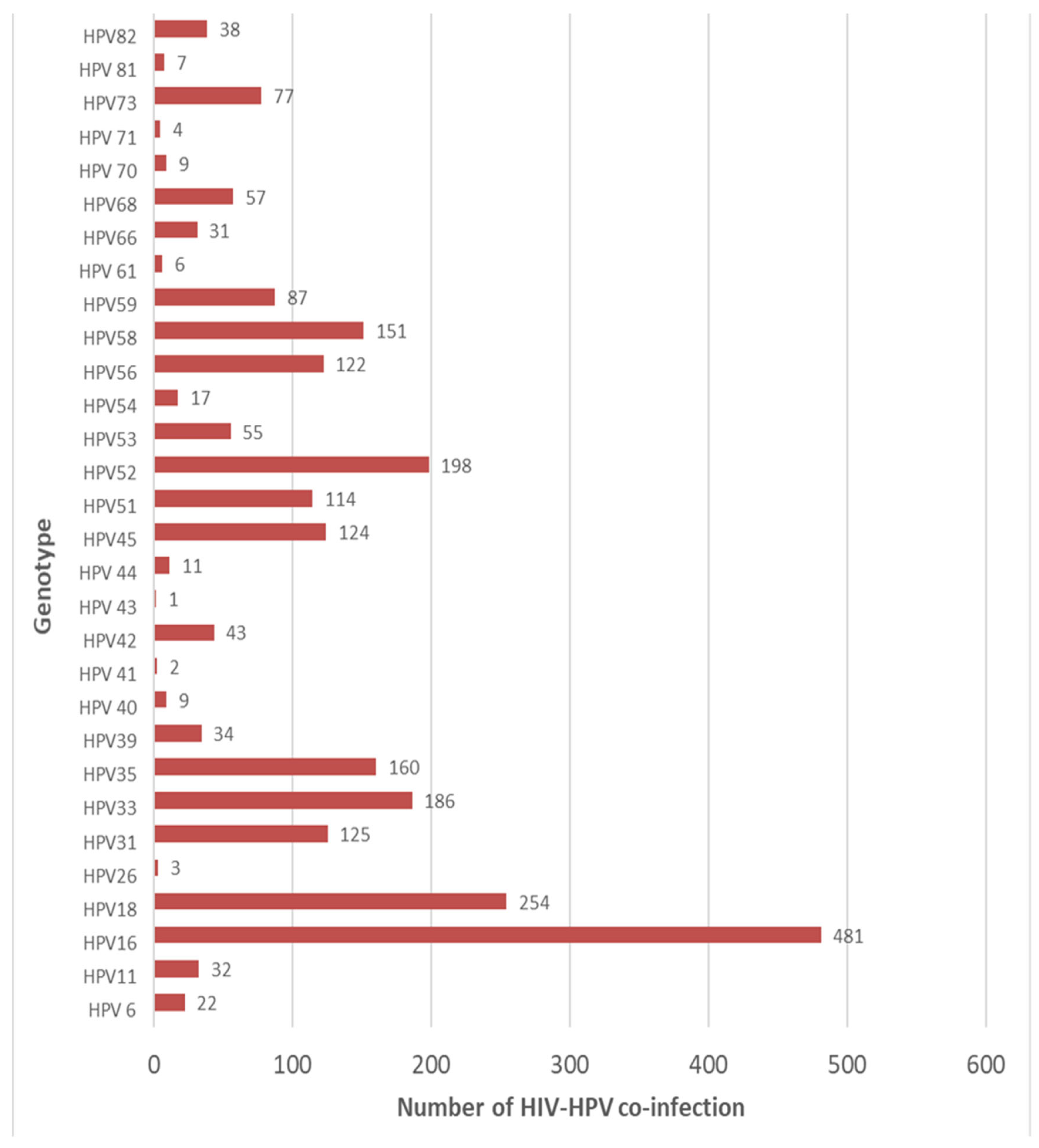
3.7. HPV Genotype Disparities Among HIV-Positive Women
3.8. Factors Associated with HPV Infection
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | Acquired Immunodeficiency Syndrome |
| CI | Confidence Interval |
| DNA | Deoxyribonucleic Acid |
| HIV | Human Immunodeficiency Virus |
| HPV | Human Papillomavirus |
| HR-HPV | High-Risk Human Papillomavirus |
| I2 | I-Squared Statistic Heterogeneity |
| LR-HPV | Low-Risk Human Papillomavirus |
| MA | Meta-Analysis |
| PCR | Polymerase Chain Reaction |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | Prospective Register of Systematic Reviews |
| ROB | Risk of Bias |
| SR | Systematic Review |
| SRMA | Systematic Review and Meta-Analysis |
| WLHIV | Women Living with HIV |
References
- Bogale, A.L.; Belay, N.B.; Medhin, G.; Ali, J.H. Molecular epidemiology of human papillomavirus among HIV infected women in developing countries: Systematic review and meta-analysis. Virol. J. 2020, 17, 179. [Google Scholar] [CrossRef]
- McBride, A.A. Human papillomaviruses: Diversity, infection and host interactions. Nat. Rev. Microbiol. 2022, 20, 95–108. [Google Scholar] [CrossRef]
- Farahmand, M.; Moghoofei, M.; Dorost, A.; Abbasi, S.; Monavari, S.H.; Kiani, S.J.; Tavakoli, A. Prevalence and genotype distribution of genital human papillomavirus in-fection in female sex workers in the world: A systematic review and meta-analysis. BMC Public Health 2020, 20, 1455. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Albero, G.; Rowley, J.; Alemany, L.; Arbyn, M.; Giuliano, A.R.; Markowitz, L.E.; Broutet, N.; Taylor, M. Global and regional estimates of genital human papillomavirus prevalence among men: A systematic review and meta-analysis. Lancet Glob. Health 2023, 11, e1345–e1362. [Google Scholar] [CrossRef] [PubMed]
- Molina-Pineda, A.; López-Cardona, M.G.; Limón-Toledo, L.P.; Cantón-Romero, J.C.; Martínez-Silva, M.G.; Ramos-Sánchez, H.V.; Flores-Miramontes, M.G.; de la Mata-González, P.; Jave-Suárez, L.F.; Aguilar-Lemarroy, A. High frequency of HPV genotypes 59, 66, 52, 51, 39 and 56 in women from Western Mexico. BMC Infect Dis. 2020, 20, 889. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, M.; Loux, T.; Shacham, E.; Tiro, J.A.; Arnold, L.D. Barriers to human papillomavirus (HPV) vaccination among young adults, aged 18–35. Prev. Med. Rep. 2022, 29, 101942. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and burden of human papillomavirus and related diseases, molecu-lar pathogenesis, and vaccine evaluation. Front. Public Health 2021, 8, 552028. [Google Scholar] [CrossRef]
- Mcharo, R.; Lennemann, T.; France, J.; Torres, L.; Gari, M.; Mbuya, W.; Mwalongo, W.; Mahenge, A.; Bauer, A.; Mnkai, J.; et al. HPV type distribution in HIV positive and negative women with or without cervical dysplasia or cancer in East Africa. Front. Oncol. 2021, 11, 763717. [Google Scholar] [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1024–1034. [Google Scholar] [CrossRef]
- Cadeddu, C. Vaccine hesitancy in Europe: The long and winding road. Eur. J. Public Health 2023, 33, ckad160.011. [Google Scholar] [CrossRef]
- Kakotkin, V.V.; Semina, E.V.; Zadorkina, T.G.; Agapov, M.A. Prevention strategies and early diagnosis of cervical cancer: Current state and prospects. Diagnostics 2023, 13, 610. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Jeve, Y.B.; Sherman, S.M.; Moss, E.L. Knowledge of human papillomavirus and the human papillomavirus vaccine in Eu-ropean adolescents: A systematic review. Sex. Transm. Infect. 2016, 92, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.A.; Logie, C.H.; Lacombe-Duncan, A.; Baiden, P.; Tepjan, S.; Rubincam, C.; Doukas, N.; Asey, F. “Parents’ uptake of human papilloma-virus vaccines for their children: A systematic review and meta-analysis of observational studies. BMJ Open 2018, 8, e019206. [Google Scholar] [CrossRef] [PubMed]
- Meites, E.; Gee, J.; Unger, E.; Markowitz, L.E. Human papillomavirus. In Epidemiology and Prevention of Vaccine-Preventable Diseases, 13th ed.; Hamborsky, J., Ed.; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021; pp. 175–186. Available online: https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-11-human-papillomavirus.html (accessed on 22 June 2025).
- Bortoletto, T.G.; Silva, T.V.; Borovac-Pinheiro, A.; Pereira, C.M.; Silva, A.D.; França, M.S.; Zeferino, L. Quality assessment tool for observational cohort and cross-sectional studies—National Heart, Lung and Blood Institute. PLoS ONE 2021, 16, e0244967. [Google Scholar]
- Akakpo, P.K.; Ken-Amoah, S.; Enyan, N.I.E.; Agyare, E.; Salia, E.; Baidoo, I.; Derkyi-Kwarteng, L.; Asare, M.; Adjei, G.; Addo, S.A.; et al. High-risk human papillomavirus genotype distribution among women living with HIV; implication for cervical cancer prevention in a resource limited setting. Infect. Agents Cancer 2023, 18, 33. [Google Scholar] [CrossRef]
- Bogale, A.L.; Teklehaymanot, T.; Ali, J.H.; Kassie, G.M.; Medhin, G.; Baye, A.Y.; Shiferaw, A.Y. Comparison of self-collected versus clinician collected cervicovaginal specimens for detection of high risk human papillomavirus among HIV infected women in Ethiopia. BMC Women’s Health 2022, 22, 360. [Google Scholar] [CrossRef]
- Megersa, T.; Dango, S.; Kumsa, K.; Lemma, K.; Lencha, B. Prevalence of high-risk human papillomavirus infections and associated factors among women living with HIV in Shashemene town public health facilities, Southern Ethiopia. BMC Women’s Health 2023, 23, 125. [Google Scholar] [CrossRef]
- Guthrie, B.L.; Rositch, A.F.; Cooper, J.A.; Farquhar, C.; Bosire, R.; Choi, R.; Kiarie, J.; Smith, J.S. Human papillomavirus and abnormal cervical lesions among HIV-infected women in HIV-discordant couples from Kenya. Sex. Transm. Infect. 2020, 96, 457–463. [Google Scholar] [CrossRef]
- Kangethe, J.M.; Gichuhi, S.; Odari, E.; Pintye, J.; Mutai, K.; Abdullahi, L.; Maiyo, A.; Mureithi, M.W. Confronting the human papillomavirus–HIV intersection: Cervical cytology implications for Kenyan women living with HIV. S. Afr. J. HIV Med. 2023, 24, 12. [Google Scholar] [CrossRef]
- Omire, A.; Budambula, N.L.M.; Kirumbi, L.; Langat, H.; Kerosi, D.; Ochieng, W.; Lwembe, R.; Quarleri, J.F. Cervical Dysplasia, Infection, and Phylogeny of Human Papillomavirus in HIV-Infected and HIV-Uninfected Women at a Reproductive Health Clinic in Nairobi, Kenya. BioMed Res. Int. 2020, 2020, 4945608. [Google Scholar] [CrossRef]
- Sweet, K.; Bosire, C.; Sanusi, B.; Sherrod, C.J.; Kwatampora, J.; Waweru, W.; Mugo, N.; Kimani, J.; Ting, J.; Clark, J.; et al. Prevalence, incidence, and distribution of human papillomavirus types in female sex workers in Kenya. Int. J. STD AIDS 2020, 31, 109–118. [Google Scholar] [CrossRef]
- Kinotia, N.J.; Muturib, M.; Kamauc, L.; Lwembed, R. Human Papillomavirus types prevalence and their association with cervical dysplasia among HIV and non-HIV infected women attending reproductive health clinics in Eastern Kenya. Afr. Health Sci. 2022, 22, 106–114. [Google Scholar] [CrossRef]
- Johnson, L.G.; Saidu, R.; Mbulawa, Z.; Williamson, A.; Boa, R.; Tergas, A.; Moodley, J.; Persing, D.; Campbell, S.; Tsai, W.; et al. Selecting human papillomavirus genotypes to optimize the performance of screening tests among South African women. Cancer Med. 2020, 9, 6813–6824. [Google Scholar] [CrossRef] [PubMed]
- Taku, O.; Onywera, H.; Mbulawa, Z.Z.A.; Businge, C.B.; Meiring, T.L.; Williamson, A.-L.; Shakir, S.M. Molecular Identification of Cervical Microbes in HIV-Negative and HIV-Positive Women in an African Setting Using a Customized Bacterial Vaginosis Microbial DNA Quantitative PCR (qPCR) Array. Microbiol. Spectr. 2022, 10, e0222921. [Google Scholar] [CrossRef] [PubMed]
- Lemba, P.C.T.; Boumba, L.M.A.; Pere, H.; Nganga, P.C.; Veyer, D.; Puech, J.; Bouassa, R.S.M.; Malanda-Kiminou, P.; Moukassa, D.; Belec, L. Human papillomavirus genotype distribution by cytological status and associat-ed risk factors in the general population of Congolese women living in urban and rural areas: Implications for cervical cancer prevention. Infect. Dis. Now 2023, 53, 104762. [Google Scholar] [CrossRef] [PubMed]
- Nakigozi, H.; Ndejjo, R.; Bazeyo, W.; Nabaggala, A.; Achola, C.; Iga, M.; Kalyesubula, S.; Kanamwangi, B.; Mutungi, G.; Batte, C.; et al. Prevalence of genital high-risk human papillomavirus infections and associated factors among women living with human immunodeficiency virus in Uganda. BMC Cancer 2024, 24, 243. [Google Scholar] [CrossRef]
- Nakisige, C.; Adams, S.V.; Namirembe, C.; Okoche, L.; Ferrenberg, J.; Towlerton, A.; Larsen, A.; Orem, J.; Casper, C.; Frenkel, L.; et al. Mul-tiple High Risk and Non-Vaccine HPV Types in Ugandan Women Living with HIV on Long-Term Antiretroviral Therapy. Available online: http://dx.doi.org/10.2139/ssrn.3785994 (accessed on 2 March 2024).
- Rais, M.; Ouyahia, A.; Mohammedi, D.; Sadouki, N.; Laouamri, S.; Abdoun, M.; Gasmi, A.; Lacheheb, A. First study of genital HPV infection among women living with HIV recruited from May to September 2018 in Eastern Algeria. Int. J. STD AIDS 2023, 34, 890–896. [Google Scholar] [CrossRef]
- Faye, B.; Gueye, S.A.A.M.; Tine, J.A.D.; Sarr, H.; Dièye, A. Molecular Genotyping of Human Papillomaviruses (HPV) in HIV+ and HIV− Women in Senegal. Am. J. Mol. Biol. 2022, 12, 54–66. [Google Scholar] [CrossRef]
- Kuguyo, O.; Mandishora, R.S.D.; Thomford, N.E.; Makunike-Mutasa, R.; Nhachi, C.F.B.; Matimba, A.; Dandara, C.; Andrei, G. High-risk HPV genotypes in Zimbabwean women with cervical cancer: Comparative analyses between HIV-negative and HIV-positive women. PLoS ONE 2021, 16, e0257324. [Google Scholar] [CrossRef]
- Ali, M.J.; Godwin, I.E.; Achenbach, C.; Sagay, A.S.; Daru, P.H.; Ocheke, A.N.; Afolaranmi, T.; Hou, L.; Murphy, R.L.; Musa, J. Prevalence and distribution of high-risk human papillomavirus genotypes in HIV positive and HIV negative women with Abnormal cervical cytology. Trop. J. Obstet. Gynaecol. 2021, 38, 144–153. [Google Scholar]
- Maueia, C.; Murahwa, A.; Manjate, A.; Andersson, S.; Sacarlal, J.; Kenga, D.; Mussá, T.; Williamson, A.-L. Identification of the Human Papillomavirus Genotypes, According to the Human Immunodeficiency Virus Status in a Cohort of Women from Maputo, Mozambique. Viruses 2021, 14, 24. [Google Scholar] [CrossRef]
- Sineque, A.; Catalao, C.; Ceffa, S.; Fonseca, A.M.; Parruque, F.; Guidotti, G.; Massango, C.; Carrilho, C.; Bicho, C.; Rangeiro, R.; et al. Screening approaches for cervical cancer in Mozambique in HIV positive and negative women. Eur. J. Cancer Prev. 2023, 32, 431–437. [Google Scholar] [CrossRef]
- Jary, A.; Teguete, I.; Sidibé, Y.; Kodio, A.; Dolo, O.; Burrel, S.; Boutolleau, D.; Beauvais-Remigereau, L.; Sayon, S.; Kampo, M.; et al. Prevalence of cervical HPV infection, sexually transmitted infections and associated antimicrobial resistance in women attending cervical cancer screening in Mali. Int. J. Infect. Dis. 2021, 108, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Sosso, S.M.; Tchouaket, M.C.T.; Fokam, J.; Simo, R.K.; Torimiro, J.; Tiga, A.; Lobe, E.E.; Ambada, G.; Nange, A.; Semengue, E.N.J.; et al. Human immunodeficiency virus is a driven factor of human papilloma virus among women: Evidence from a cross-sectional analysis in Yaounde, Cameroon. Virol. J. 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Adedimeji, A.; Ajeh, R.; Dzudie, A.; Kendowo, E.; Fuhngwa, N.; Nsame, D.; Simo-Wambo, A.G.; Orock, E.; Hebert, T.M.; Pierz, A.J.; et al. Cervical human papillomavirus DNA detection in women living with HIV and HIV-uninfected women living in Limbe, Cameroon. J. Clin. Virol. 2020, 128, 104445. [Google Scholar] [CrossRef]
- Simo, R.T.; Kiafon, F.; Nangue, C.; Goura, A.P.; Ebune, J.L.; Usani, M.C.; Kamdje, A.H.N.; Etet, P.F.S.; Telefo, P.B. Influence of HIV infection on the distribution of high-risk HPV types among women with cervical precancerous lesions in Yaounde, Cameroon. Int. J. Infect. Dis. 2021, 110, 426–432. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Menon, S.; Wusiman, A.; Boily, M.C.; Kariisa, M.; Mabeya, H.; Luchters, S.; Forland, F.; Rossi, R.; Callens, S.; Broeck, D.V.; et al. Epidemiology of HPV genotypes among HIV positive women in Kenya: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0163965. [Google Scholar] [CrossRef]
- Clifford, G.M.; Tully, S.; Franceschi, S. Carcinogenicity of Human Papillomavirus (HPV) types in HIV-positive women: A meta-analysis from HPV infection to cervical cancer. Clin. Infect. Dis. 2017, 64, 1228–1235. [Google Scholar] [CrossRef]
- Adeyanju, G.C.; Essoh, T.-A.; Sidibe, A.R.; Kyesi, F.; Aina, M. Human Papillomavirus Vaccination Acceleration and Introduction in Sub-Saharan Africa: A Multi-Country Cohort Analysis. Vaccines 2024, 12, 489. [Google Scholar] [CrossRef]
- Ajenifuja, O.K.; Ikeri, N.Z.; Adeteye, O.V.; Banjo, A.A. Comparison between self sampling and provider collected samples for Human Papillomavirus (HPV) Deoxyribonucleic acid (DNA) testing in a Nigerian facility. Pan Afr. Med. J. 2018, 30, 110. [Google Scholar] [CrossRef]


| Author & Year | Study Location | Study Design | Study Setting | Age of Study Subjects | Sample Size | Total Event | Hr HPV | Lr HPV | Quality Assessment % |
|---|---|---|---|---|---|---|---|---|---|
| Adedimeji et al., 2020 [37] | Cameroon | Cross-sectional | HIV treatment center | Median = 42 Range = 25–59 | 560 | 260 | - | - | 84 |
| Akakpo et al., 2023 [16] | Ghana | Cross-sectional | Hospital | Mean = 47.2 std = 10 | 330 | 156 | - | - | 74.5 |
| Bogale et al., 2022 [17] | Ethiopia | Cross-sectional | Hospital | - | 497 | 146 | - | - | 85 |
| Guthrie et al., 2020 [19] | Kenya | Cross-sectional | - | - | 283 | 175 | - | - | 69.5 |
| Johnson et al., 2020 [24] | S. Africa | Cross-sectional | University | Range = 30–65 | 535 | 258 | - | - | 77.5 |
| Kangethe et al., 2023 [20] | Kenya | Cross-sectional | Hospital | Mean = 42.8 std = 8.7 | 647 | 224 | - | - | 88.5 |
| Paola et al., 2023 [26] | Congo | Cross-sectional | Hospital | Mean = 53 Std = 18.6 | 53 | 42 | - | 4 | 65 |
| Sweet et al., 2020 [22] | Kenya | Cross-sectional | Clinic | Median = 32 | 84 | 27 | 23 | - | 68.5 |
| Nakigozi et al., 2024 [27] | Uganda | Cross-sectional | Clinic | Range = 25–49 | 5856 | 2006 | - | - | 95 |
| Richard, Simo et al., 2021 [38] | Cameroon | Cross-sectional | Health center | Range = 20–84 | 102 | 53 | - | - | 66.5 |
| Maueia et al., 2021 [33] | Mozambique | Cross-sectional | - | Range = 14–45 | 56 | 44 | - | - | 64.5 |
| Kinotia et al., 2022 [23] | Kenya | Cross-sectional | Hospital | Mean = 34.3 std = 10.4 | 317 | 73 | 14 | 59 | 79 |
| Sosso et al., 2020 [36] | Cameroon | Cross-sectional | Hospital | Mean = 37 std = 3 | 184 | 80 | - | - | 68 |
| Omire, Agnes et al., 2020 [21] | Kenya | Cross-sectional | Hospital | Mean = 40.7 std = 6.6 | 29 | 14 | 13 | 1 | 56.5 |
| Rais et al., 2023 [29] | Algeria | Cross-sectional | - | - | 100 | 32 | - | - | 70 |
| Faye et al., 2022 [30] | Senegal | Cross-sectional | Hospital | ≥20 | 133 | 105 | 96 | 76 | 71 |
| Kuguyo et al., 2021 [31] | Zimbabwe | Cross-sectional | Hospital | Median = 46 IQR = 41–54 | 116 | - | 56 | 12 | 53 |
| Jamila, Maryam et al., 2021 [32] | Nigeria | Cross-sectional | Hospital and clinic | Median = 49.5 IQR = 41.4–55.0 | 75 | - | 43 | - | 66 |
| Sineque et al., 2023 [34] | Mozambique | Cross-sectional | Hospital | Median = 43 IQR = 38–47 | 577 | - | 212 | - | 88 |
| Megersa, Tariku et al., 2023 [18] | Ethiopia | Cross-sectional | Hospital | Mean = 34 std = 6 | 406 | - | 143 | - | 75.5 |
| Aude et al., 2021 [35] | Mali | Cross-sectional | Clinic | Median = 40 IQR = 34–44 | 44 | - | 34 | 25 | 65 |
| Nakisige, Carol et al., 2023 [28] | Uganda | Cohort | Clinic | Median = 34 IQR = 28–40 | 188 | 145 | 126 | 66 | 74 |
| Taku et al., 2022 [25] | South Africa | Cross-sectional | Clinic | Median = 46 IQR 38–55 | 155 | - | 63 | - | 73 |
| Study | Sample Size | Prevalence (%) | 95% CI | Weight (%) Random |
|---|---|---|---|---|
| Adedimeji et al., 2020 [37] | 560 | 46.429 | 42.23 to 50.65 | 6.28 |
| Agnes et al., 2020 [21] | 29 | 48.276 | 29.44 to 67.46 | 4.52 |
| Akakpo et al., 2023 [16] | 330 | 47.273 | 41.78 to 52.81 | 6.19 |
| Bogale et al., 2022 [17] | 497 | 29.376 | 25.40 to 33.59 | 6.27 |
| Carol et al., 2021 [28] | 188 | 77.128 | 70.45 to 82.92 | 6.02 |
| Faye et al., 2022 [30] | 133 | 78.947 | 71.03 to 85.53 | 5.87 |
| Guthrie et al., 2020 [19] | 283 | 61.837 | 55.90 to 67.52 | 6.15 |
| Johnson et al., 2020 [24] | 535 | 48.224 | 43.91 to 52.55 | 6.28 |
| Kangethe et al., 2023 [20] | 647 | 34.621 | 30.95 to 38.42 | 6.30 |
| Kinotia et al., 2022 [23] | 317 | 23.028 | 18.50 to 28.06 | 6.18 |
| Maueia et al., 2021 [33] | 56 | 78.571 | 65.56 to 88.40 | 5.26 |
| Nakigozi et al., 2024 [27] | 5856 | 34.255 | 33.04 to 35.48 | 6.41 |
| Paola et al., 2023 [26] | 53 | 79.245 | 65.89 to 89.15 | 5.21 |
| Rais et al., 2023 [29] | 100 | 32.000 | 23.02 to 42.07 | 5.71 |
| Richard et al., 2021 [38] | 102 | 51.961 | 41.84 to 61.96 | 5.72 |
| Sosso et al., 2020 [36] | 184 | 43.478 | 36.20 to 50.96 | 6.02 |
| Sweet et al., 2020 [22] | 84 | 32.143 | 22.36 to 43.22 | 5.60 |
| Total (random effects) | 9954 | 49.405 | 42.43 to 56.38 | 100.00 |
| Prediction interval | 49.4 | 0.23 to 0.77 | ||
| Test for heterogeneity | ||||
| Q | 520.9266 | |||
| DF | 16 | |||
| Significance level | p < 0.0001 | |||
| I2 | 96.93% | |||
| 95% CI for I2 | 96.03 to 97.62 |
| Study | Sample Size | Proportion (%) | 95% CI | Weight (%) | |
|---|---|---|---|---|---|
| Fixed | Random | ||||
| Agnes et al., 2020 [21] | 29 | 44.82 | 26.44 to 64.30 | 1.41 | 8.32 |
| Aude et al., 2021 [35] | 44 | 77.27 | 62.15 to 88.52 | 2.11 | 8.67 |
| Carol et al., 2021 [28] | 188 | 67.02 | 59.80 to 73.69 | 8.85 | 9.27 |
| Faye et al., 2022 [30] | 133 | 72.18 | 63.74 to 79.59 | 6.28 | 9.19 |
| Kinotia et al., 2022 [23] | 317 | 4.41 | 2.43 to 7.29 | 14.89 | 9.36 |
| Kuguyo, 2021 [31] | 116 | 48.27 | 38.90 to 57.74 | 5.48 | 9.15 |
| Maryam et al., 2021 [32] | 75 | 57.33 | 45.37 to 68.69 | 3.56 | 8.98 |
| Megersa et al., 2023 [18] | 406 | 35.22 | 30.57 to 40.08 | 19.06 | 9.38 |
| Sineque et al., 2023 [34] | 577 | 36.74 | 32.79 to 40.82 | 27.07 | 9.41 |
| Sweet et al., 2020 [22] | 84 | 27.38 | 18.21 to 38.20 | 3.98 | 9.03 |
| Taku et al., 2020 [25] | 155 | 40.64 | 32.83 to 48.81 | 7.31 | 9.23 |
| Total (random effects) | 2124 | 45.26 | 31.02 to 59.91 | 100.00 | 100.0 |
| Test for heterogeneity | |||||
| Q | 439.1812 | ||||
| DF | 10 | ||||
| Significance level | p < 0.0001 | ||||
| I2 (inconsistency) | 97.72% | ||||
| 95% CI for I2 | 96.95 to 98.30 | ||||
| Study | Sample Size | Prevalence (%) | 95% CI | Weight (%) | |
|---|---|---|---|---|---|
| Fixed | Random | ||||
| Agnes et al., 2020 [21] | 29 | 3.44 | 0.08 to 17.76 | 3.38 | 13.00 |
| Aude et al., 2021 [35] | 44 | 56.81 | 41.03 to 71.65 | 5.07 | 13.69 |
| Carol et al., 2021 [28] | 188 | 35.10 | 28.30 to 42.38 | 21.31 | 14.90 |
| Faye et al., 2022 [30] | 133 | 57.14 | 48.27 to 65.68 | 15.11 | 14.74 |
| Kinotia et al., 2022 [23] | 317 | 18.61 | 14.48 to 23.34 | 35.85 | 15.07 |
| Kuguyo, 2021 [31] | 116 | 10.34 | 5.46 to 17.37 | 13.19 | 14.65 |
| Paola et al., 2023 [26] | 53 | 7.54 | 2.09 to 18.21 | 6.09 | 13.94 |
| Total (fixed effects) | 880 | 26.21 | 23.35 to 29.24 | 100.0 | 100.00 |
| Total (random effects) | 880 | 24.98 | 12.27 to 40.41 | 100.0 | 100.00 |
| Test for heterogeneity | |||||
| Q | 134.3977 | ||||
| DF | 6 | ||||
| Significance level | p < 0.0001 | ||||
| I2 (inconsistency) | 95.54% | ||||
| 95% CI for I2 | 92.89 to 97.20 | ||||
| Main Results for Model, Random Effects (MM), Z-Distribution, Logit Event Rate | ||||||
|---|---|---|---|---|---|---|
| Covariate | Coefficient | Standard Error | 95% Lower | 95% Upper | Z-Value | p-Value |
| Publication year | −0.0991 | 0.1034 | −0.3017 | 0.1035 | −0.96 | 0.3378 |
| Comparison of Model 1 with the null model | ||||||
| Q = 0.92, df = 1, p = 0.3378 | ||||||
| Goodness of fit | ||||||
| Tau2 = 0.2942, Tau = 0.5424, I2 = 94.96%, Q = 297.72, df = 15, p = 0.0000 | ||||||
| Total between-study variance (intercept only) | ||||||
| Tau2 = 0.2983, Tau = 0.5462, I2 = 96.36%, Q = 439.85, df = 16, p = 0.0000 | ||||||
| The proportion of total between-study variance explained by Model 1 | ||||||
| R2 analog = 0.02 | ||||||
| Egger’s Test | All Types of HPV | High-Risk HPV | Low-Risk HPV |
|---|---|---|---|
| Intercept | 5.05 | 6.49 | −0.45 |
| 95% CI | 1.28 to 8.82 | −4.53 to 17.52 | −14.42 to 13.51 |
| Significance level | p = 0.012 | p = 0.215 | p = 0.936 |
| Begg’s test | |||
| Kendall’s Tau | 0.191 | 0.163 | 0.142 |
| Significance level | p = 0.284 | p = 0.483 | p = 0.652 |
| Author & Year | Study Setting | Diagnosis Method | Associated Risk Factor |
|---|---|---|---|
| Adedimeji et al., 2020 [37] | HIV Rx center | Gene Xpert | A higher CD4 count was associated with lower HPV prevalence. HPV testing of self-collected specimens appeared less specific than HPV testing of provider-collected specimens. The prevalence of HPV and HPV16 decreased with increasing age. Self-collected cervicovaginal specimens were more likely to test high-risk HPV positive than the provider-collected cervical specimens. |
| Akakpo et al., 2023 [16] | Hospital | AmpFire HPV detection system or isothermal PCR assay | Women with HIV viral load ≥ 1000 copies/mL. Women who had 1 or more pregnancies are less likely to have HPV 16 and/or 18 genotypes compared to those who had no pregnancy. |
| Bogale et al., 2022 [17] | Hospital | Abbott real-time PCR | Oncogenic HPV was higher in self-collected samples than the clinician-collected specimens. |
| Kangethe et al., 2023 [20] | Hospital | Gene Xpert® | WLHIV aged < 25 years had the highest prevalence of high-risk HPV infection. Abnormal cervical cytology and having multiple HR-HPV infections, regardless of ART duration, CD4 count and behavioral factors. |
| Nakigozi et al., 2024 [27] | ART clinic | HPV DNA gen expert assay and HPV RNA Hologic assay | Compared to women aged 25–35 years, those aged 36–49 had a lower prevalence of all high-risk HPVs. |
| Richard, Simo et al., 2021 [38] | Health Centre, | HPV DNA genotyping assays and type- pecific PCR | Smoking was associated with a higher prevalence of HPV. |
| Kinotia et al., 2022 [23] | Referral Hospital | HPV DNA PCR, HPV DNA sequencing | Cervical dysplasia was associated with more mixed low-risk/high-risk HPV genotypes among HIV-infected than HIV-uninfected women. |
| Sosso et al., 2020 [36] | Hospital | Real-time PCR | The lower the CD4 count, the higher the rate of HPV positivity. |
| Omire Agnes et al., 2020 [21] | Referral hospital | Roche Linear Array test | Low CD4 T cell count < 200/mm3. |
| Megersa, Tariku et al., 2023 [18] | Hospital | Cobas 4800 HPV Test | End line CD4 count < 200 cells/mm3. End-line HIV viral load ≥ 50 copies/mL. More than one lifetime sexual partner was significantly associated with high-risk HPV infections. |
| Nakisige, Carol et al., 2023 [28] | - | Roche Linear Array test | A lower CD4/8 ratio was significantly associated with measures of high-risk HPV. |
| Taku et al., 2022 [25] | Clinic | Taxon-directed 16S rRNA gene quantitative PCR (qPCR) assay | HIV-positive women had significantly higher high-risk HPV viral load. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amare, Y.; Gelgalo, D.; Pozsgai, É.; Kiss, I. Systematic Review and Meta-Analysis of Human Papillomavirus Prevalence and Genotypic Disparities Among Human Immunodeficiency Virus-Positive Women in Africa. J. Clin. Med. 2025, 14, 5924. https://doi.org/10.3390/jcm14175924
Amare Y, Gelgalo D, Pozsgai É, Kiss I. Systematic Review and Meta-Analysis of Human Papillomavirus Prevalence and Genotypic Disparities Among Human Immunodeficiency Virus-Positive Women in Africa. Journal of Clinical Medicine. 2025; 14(17):5924. https://doi.org/10.3390/jcm14175924
Chicago/Turabian StyleAmare, Yirga, Dahabo Gelgalo, Éva Pozsgai, and István Kiss. 2025. "Systematic Review and Meta-Analysis of Human Papillomavirus Prevalence and Genotypic Disparities Among Human Immunodeficiency Virus-Positive Women in Africa" Journal of Clinical Medicine 14, no. 17: 5924. https://doi.org/10.3390/jcm14175924
APA StyleAmare, Y., Gelgalo, D., Pozsgai, É., & Kiss, I. (2025). Systematic Review and Meta-Analysis of Human Papillomavirus Prevalence and Genotypic Disparities Among Human Immunodeficiency Virus-Positive Women in Africa. Journal of Clinical Medicine, 14(17), 5924. https://doi.org/10.3390/jcm14175924








