The Significance of Enzymatic Cholestasis in Inflammatory Bowel Disease Patients for the Diagnosis of Primary Sclerosing Cholangitis—A Retrospective Study
Abstract
1. Introduction
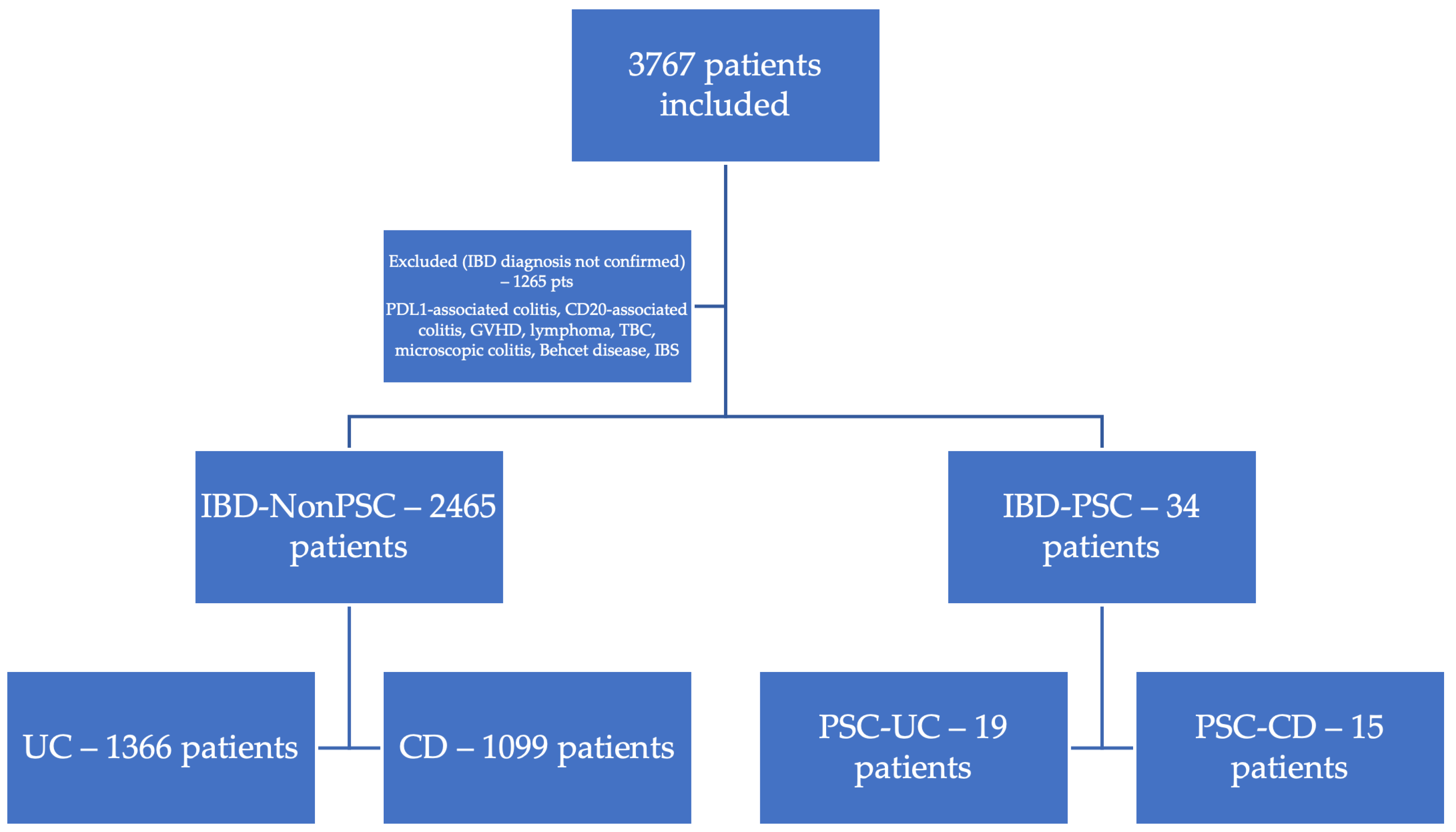
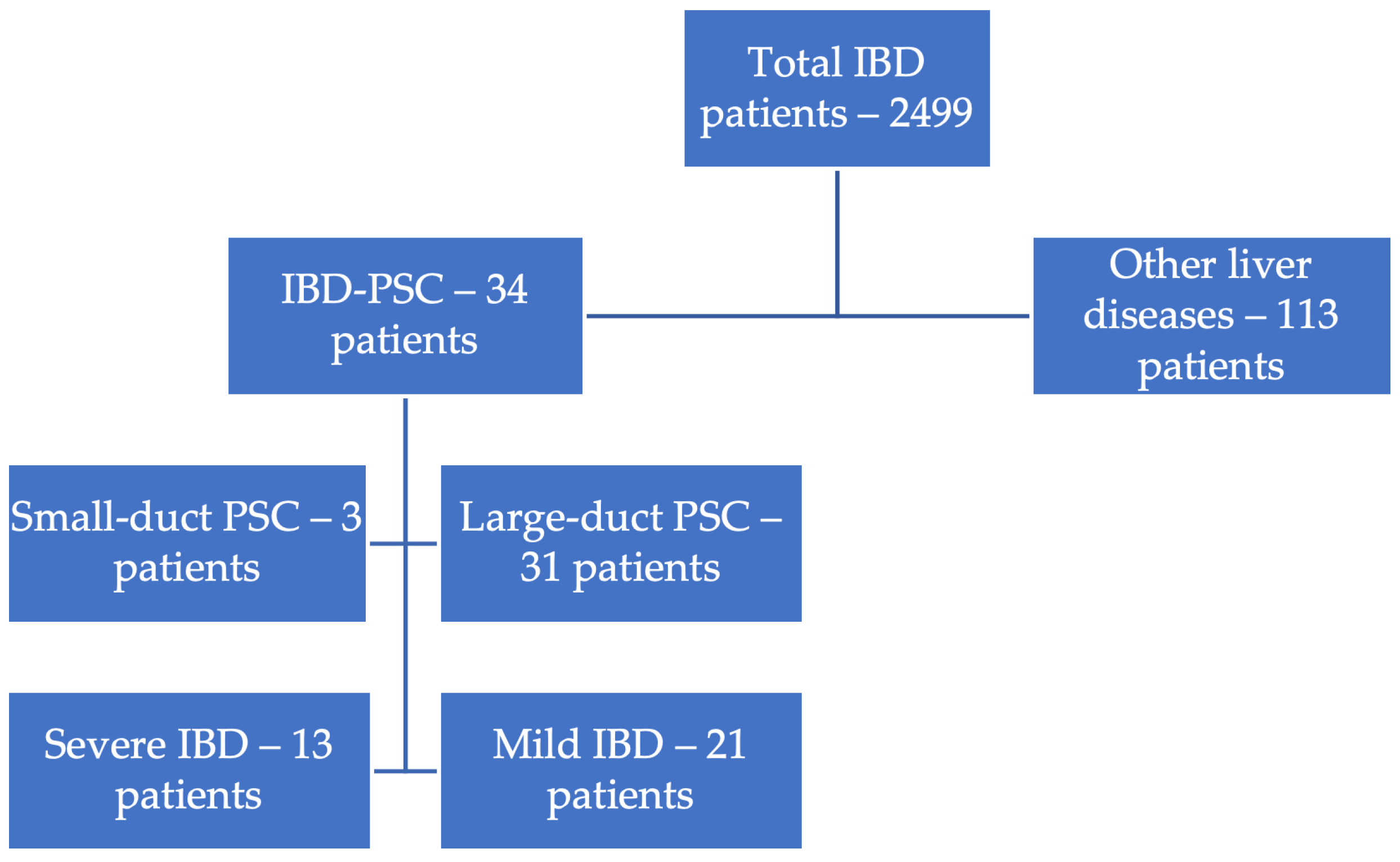
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSC | Primary Sclerosing Cholangitis |
| IBD | Inflammatory Bowel Disease |
| UC | Ulcerative Colitis |
| CD | Crohn’s Disease |
| GGT | Gamma-Glutamyl Transpeptidase |
| ALP | Alkaline Phosphatase |
| MASLD | Metabolic-Associated Steatotic Liver Disease |
| MRCP | Magnetic Resonance Cholangiopancreatography |
| ECS | Enzymatic Cholestasis Syndrome |
| ULN | Upper Limit of Normal |
| IBS | Irritable Bowel Syndrome |
| PDL 1 | Programmed Death-Ligand 1 |
| GVHD | Graft-Versus-Host Disease |
| PBC | Primary Biliary Cholangitis |
| CCA | Cholangiocarcinoma |
| CRC | Colorectal Cancer |
| 5-ASA | 5-Aminosalicylic Acid |
| AIH | Autoimmune Hepatitis |
| SD | Standard Deviation |
| EASL | European Association for the Study of the Liver |
| U/L | Units per Liter |
References
- Mertz, A.; Nguyen, N.A.; Katsanos, K.H.; Kwok, R.M. Primary sclerosing cholangitis and inflammatory bowel disease comorbidity: An update of the evidence. Ann. Gastroenterol. 2019, 32, 124–133. [Google Scholar] [CrossRef]
- Vernero, M.; Saibeni, S.; Scalvini, D.; Cicalini, C.; Chiarello, L.; Nardi, S.; Ribaldone, D.G.; Bezzio, C. Prevalence and Clinical Impact of Immune-Mediated Inflammatory Diseases in Patients with Inflammatory Bowel Disease: Results from a Large Retrospective Observational Study. J. Clin. Med. 2024, 13, 1019. [Google Scholar] [CrossRef]
- Franceschet, I.; Cazzagon, N.; Del Ross, T.; D’Incà, R.; Buja, A.; Floreani, A. Primary sclerosing cholangitis associated with inflammatory bowel disease: An observational study in a Southern Europe population focusing on new therapeutic options. Eur. J. Gastroenterol. Hepatol. 2016, 28, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, T.; Vaziri, H.; Wu, G.Y. Primary Sclerosing Cholangitis and Inflammatory Bowel Disease: A Review. J. Clin. Transl. Hepatol. 2022, 10, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Nayagam, J.S.; Pereira, S.P.; Devlin, J.; Harrison, P.M.; Joshi, D. Controversies in the management of primary sclerosing cholangitis. World J. Hepatol. 2016, 8, 265–272. [Google Scholar] [CrossRef]
- Vlăduţ, C.; Ciocîrlan, M.; Bilous, D.; Șandru, V.; Stan-Ilie, M.; Panic, N.; Becheanu, G.; Jinga, M.; Costache, R.S.; Costache, D.O.; et al. An Overview on Primary Sclerosing Cholangitis. J. Clin. Med. 2020, 9, 754. [Google Scholar] [CrossRef]
- Tsaitas, C.; Semertzidou, A.; Sinakos, E. Update on inflammatory bowel disease in patients with primary sclerosing cholangitis. World J. Hepatol. 2014, 6, 178–187. [Google Scholar] [CrossRef]
- Weismüller, T.J.; Trivedi, P.J.; Bergquist, A.; Imam, M.; Lenzen, H.; Ponsioen, C.Y.; Holm, K.; Gotthardt, D.; Färkkilä, M.A.; Marschall, H.-U.; et al. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate with Course of Primary Sclerosing Cholangitis. Gastroenterology 2017, 152, 1975–1984.e8. [Google Scholar] [CrossRef]
- Guerra, I.; Bujanda, L.; Castro, J.; Merino, O.; Tosca, J.; Camps, B.; Gutiérrez, A.; Gordillo Ábalos, J.; de Castro, L.; Iborra, M.; et al. Clinical Characteristics, Associated Malignancies and Management of Primary Sclerosing Cholangitis in Inflammatory Bowel Disease Patients: A Multicentre Retrospective Cohort Study. J. Crohns Colitis 2019, 13, 1492–1500. [Google Scholar] [CrossRef]
- Barberio, B.; Massimi, D.; Cazzagon, N.; Zingone, F.; Ford, A.C.; Savarino, E.V. Prevalence of Primary Sclerosing Cholangitis in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Gastroenterology 2021, 161, 1865–1877. [Google Scholar] [CrossRef]
- Moncrief, K.J.; Savu, A.; Ma, M.M.; Bain, V.G.; Wong, W.W.; Tandon, P. The natural history of inflammatory bowel disease and primary sclerosing cholangitis after liver transplantation—A single-centre experience. Can. J. Gastroenterol. 2010, 24, 40–46. [Google Scholar] [CrossRef]
- Liu, K.; Wang, R.; Kariyawasam, V.; Wells, M.; Strasser, S.I.; McCaughan, G.; Corte, C.; Leong, R.W. Epidemiology and outcomes of primary sclerosing cholangitis with and without inflammatory bowel disease in an Australian cohort. Liver Int. 2017, 37, 442–448. [Google Scholar] [CrossRef]
- Peviani, M.; Cazzagon, N.; Gambato, M.; Bertin, L.; Zingone, F.; Savarino, E.V.; Barberio, B. Primary sclerosing cholangitis and inflammatory bowel disease: A complicated yet unique relationship. Minerva Gastroenterol. 2024, 71, 33–47. [Google Scholar] [CrossRef]
- Kuo, A.; Gomel, R.; Safer, R.; Lindor, K.D.; Everson, G.T.; Bowlus, C.L. Characteristics and Outcomes Reported by Patients with Primary Sclerosing Cholangitis Through an Online Registry. Clin. Gastroenterol. Hepatol. 2019, 17, 1372–1378. [Google Scholar] [CrossRef]
- Khrom, M.; Long, M.; Dube, S.; Robbins, L.; Botwin, G.J.; Yang, S.; Mengesha, E.; Li, D.; Naito, T.; Bonthala, N.N.; et al. Comprehensive Association Analyses of Extraintestinal Manifestations in Inflammatory Bowel Disease. Gastroenterology 2024, 167, 315–332. [Google Scholar] [CrossRef]
- Crothers, H.; Ferguson, J.; Quraishi, M.N.; Cooney, R.; Iqbal, T.H.; Trivedi, P.J. Past, current, and future trends in the prevalence of primary sclerosing cholangitis and inflammatory bowel disease across England (2015–2027): A nationwide, population-based study. The Lancet regional health. Europe 2024, 44, 101002. [Google Scholar] [CrossRef]
- Losurdo, G.; Brescia, I.V.; Lillo, C.; Mezzapesa, M.; Barone, M.; Principi, M.; Ierardi, E.; Di Leo, A.; Rendina, M. Liver involvement in inflammatory bowel disease: What should the clinician know? World J. Hepatol. 2021, 13, 1534–1551. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Palmela, C.; Peerani, F.; Castaneda, D.; Torres, J.; Itzkowitz, S.H. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis: A Review of the Phenotype and Associated Specific Features. Gut Liver 2018, 12, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Rocha, H.C.; Vilela, E.G. Clinical aspects and prognosis of patients with inflammatory bowel disease associated with autoimmune liver diseases. Gastroenterol. Hepatol. 2022, 45, 83–90. [Google Scholar] [CrossRef]
- Girardin, M.; Hadengue, A.; Frossard, J.-L. Swiss IBD Cohort Study Group: High prevalence of cholestasis, with increased conjugated bile acids in inflammatory bowel diseases patients. World J. Clin. Cases 2018, 6, 44–53. [Google Scholar] [CrossRef]
- Bowlus, C.L.; Arrivé, L.; Bergquist, A.; Deneau, M.; Forman, L.; Ilyas, S.I.; Lunsford, K.E.; Martinez, M.; Sapisochin, G.; Shroff, R.; et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2023, 77, 659–702. [Google Scholar] [CrossRef] [PubMed]
- EASL. Clinical Practice Guidelines on sclerosing cholangitis. J. Hepatol. 2022, 77, 761–806. [Google Scholar] [CrossRef] [PubMed]
- Núñez, F.P.; Castro, F.; Mezzano, G.; Quera, R.; Diaz, D.; Castro, L. Hepatobiliary manifestations in inflammatory bowel disease: A practical approach. World J. Hepatol. 2022, 14, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Isayama, H.; Tazuma, S.; Kokudo, N.; Tanaka, A.; Tsuyuguchi, T.; Nakazawa, T.; Notohara, K.; Mizuno, S.; Akamatsu, N.; Serikawa, M.; et al. PSC guideline committee Members: Ministry of Health the Intractable Hepatobiliary Disease Study Group, L. and W. (Japan) R.P. Clinical guidelines for primary sclerosing cholangitis 2017. J. Gastroenterol. 2018, 53, 1006–1034. [Google Scholar] [CrossRef]
- Leung, K.K.; Li, W.; Hansen, B.; Gulamhusein, A.; Lapointe-Shaw, L.; Shaheen, A.A.; Ricciuto, A.; Benchimol, E.I.; Flemming, J.A.; Hirschfield, G.M. Primary sclerosing cholangitis–inflammatory bowel disease: Epidemiology, mortality, and impact of diagnostic sequence. JHEP Rep. 2025, 7, 101272. [Google Scholar] [CrossRef]
- Manns, M.P.; Bergquist, A.; Karlsen, T.H.; Levy, C.; Muir, A.J.; Ponsioen, C.; Trauner, M.; Wong, G.; Younossi, Z.M. Primary sclerosing cholangitis. Nat. Rev. Dis. Primers 2025, 11, 17. [Google Scholar] [CrossRef]
- Belle, A.; Laurent, V.; Pouillon, L.; Baumann, C.; Orry, X.; Lopez, A.; Rousseau, H.; Bronowicki, J.-P.; Peyrin-Biroulet, L. Systematic screening for primary sclerosing cholangitis with magnetic resonance cholangiography in inflammatory bowel disease. Dig. Liver Dis. 2018, 50, 1012–1018. [Google Scholar] [CrossRef]
- Nakazawa, T.; Naitoh, I.; Hayashi, K.; Sano, H.; Miyabe, K.; Shimizu, S.; Joh, T. Inflammatory bowel disease of primary sclerosing cholangitis: A distinct entity? World J. Gastroenterol. 2014, 20, 3245–3254. [Google Scholar] [CrossRef]
- Gaspar, R.; Branco, C.C.; Macedo, G. Liver manifestations and complications in inflammatory bowel disease: A review. World J. Hepatol. 2021, 13, 1956–1967. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, R.; Li, H.; Shuai, Z. Clinical management of autoimmune liver diseases: Juncture, opportunities, and challenges ahead. Immunol. Res. 2025, 73, 67. [Google Scholar] [CrossRef]
- Marya, N.B.; Tabibian, J.H. Role of endoscopy in the management of primary sclerosing cholangitis. World J. Gastrointest. Endosc. 2019, 11, 84–94. [Google Scholar] [CrossRef]
- Berhane, B.; van Rheenen, P.F.; Verkade, H.J. Gamma-glutamyl transferase and disease course in pediatric-onset primary sclerosing cholangitis: A single-center cohort study. Health Sci. Rep. 2023, 6, e1086. [Google Scholar] [CrossRef]
- Rabiee, A.; Silveira, M.G. Primary sclerosing cholangitis. Transl. Gastroenterol. Hepatol. 2021, 6, 29. [Google Scholar] [CrossRef]
- Chapman, M.H.; Thorburn, D.; Hirschfield, G.M.; Webster, G.G.J.; Rushbrook, S.M.; Alexander, G.; Collier, J.; Dyson, J.K.; Jones, D.E.; Patanwala, I.; et al. British Society of Gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut 2019, 68, 1356–1378. [Google Scholar] [CrossRef]
| Value | Inflammatory Bowel Disease Patients | ||
|---|---|---|---|
| IBD-PSC | IBD Non-PSC | p Value | |
| Mean age, years (95%CI) | 37.35 (32.4–42.2) | 42.87 (42.2–43.5) | 0.059 |
| Female sex % | 52.94% | 46.43% | 0.450 |
| Severe IBD % | 35.25% | 34.73% | 0.710 |
| Cholangiocarcinoma % | 11.76% | 0.24% | <0.001 |
| Colonic surgery % | 11.76% | 14.59% | 0.643 |
| Mean ALP U/L | 308.82 (232.4–385.1) | 79.21 (77.5–80.8) | <0.001 |
| Mean GGT U/L (95%CI) | 243 (134.9–351.1) | 30.44 (28.5–32.3) | <0.001 |
| Disease-related death % | 8.82% | 0.65% | <0.001 |
| Ulcerative Colitis Patients (n = 1386) | |||
|---|---|---|---|
| PSC-UC (n = 19) | UC Non-PSC (n = 1367) | p Value | |
| Mean age, years (95%CI) | 43 (36.1–49.8) | 48.3 (47.4–49.2) | <0.001 |
| Female sex % | 45.72% | 47.37% | 0.880 |
| Montreal E1 % | 26.12% | 5.26% | 0.039 |
| Montreal E2 % | 51.94% | 15.79% | 0.002 |
| Montreal E3 % | 21.87% | 78.95% | <0.001 |
| Severe disease % | 23.34% | 10.53% | 0.189 |
| Biological treatment % | 22.75% | 15.79% | 0.472 |
| Azathioprine treatment % | 26.12% | 31.58% | 0.591 |
| 5-ASA treatment % | 95.61% | 100% | 0.350 |
| Liver disease % | - | 4.17% | - |
| CCA % | 10.5% | 0.43% | <0.001 |
| CRC % | 15.79% | 0.88% | <0.001 |
| Colonic surgery | 5.26% | 5.56% | 0.955 |
| Mean GGT, U/L (SD) * | 217.32 (190.7) | 31.76 (54) | <0.001 |
| Mean ALP, U/L (SD) ** | 278.37 (196.8) | 78.12 (41.71) | <0.001 |
| Crohn’s Disease Patients (n = 1116) | |||
|---|---|---|---|
| PSC-CD (n = 15) | CD Non-PSC (n = 1101) | p Value | |
| Mean age, years (95%CI) | 30.2 (24.3–36.1) | 36.08 (35.1–36.9) | 0.043 |
| Female sex | 60% | 47.32% | 0.329 |
| Montreal Classification | |||
| Extension | |||
| L1 | 13.3% | 21.8% | 0.429 |
| L2 | 46.67% | 43.87% | 0.828 |
| L3 | 40% | 33.79% | 0.614 |
| L4 | 0.27% | 0.27% | 0.840 |
| Behavior | |||
| B1 | 60% | 59.95% | 0.997 |
| B2 | 33.33% | 31.34% | 0.868 |
| B3 | 20% | 12.99% | 0.424 |
| Perianal disease | 13.33% | 16.35% | 0.754 |
| Severe disease | 73.33% | 49.95% | 0.671 |
| Biological treatment | 60% | 54.5% | 0.072 |
| Azathioprine treatment | 66.67% | 48.96% | 0.173 |
| 5-ASA treatment | 73.33% | 59.4% | 0.275 |
| Liver disease | - | 5.02% | - |
| CCA | 2 | 1 | - |
| CRC | 0 | 1.25% | - |
| Enterocolonic surgery | 20% | 25.45% | 0.610 |
| Mean GGT, U/L (95%CI) * | 275.53 (42.4–508.6) | 28.87 (26.9–30.7) | <0.001 |
| Mean ALP, U/L (95%CI) ** | 347.4 (211.6–483.1) | 80.51 (78.4–82.5) | <0.001 |
| PSC-UC (n = 19) | PSC-CD (n = 15) | p Value | |
|---|---|---|---|
| Mean age | 43 | 30.2 | 0.005 |
| CCA | 10.5% | 13.3% | 0.801 |
| Biological treatment | 15.7% | 60% | 0.007 |
| Death | 0 | 20% | 0.041 |
| Severe IBD | 10.5% | 73.3% | <0.001 |
| Mean GGT, U/L (95%CI) * | 217.3 (125.4–309.2) | 275.5 (42.4–508.6) | 0.594 |
| Mean ALP, U/L (95%CI) ** | 278.37 (183.4–373.2) | 347.4 (211.6–483.1) | 0.369 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandea, M.; Oancea, D.M.; Ghioca, M.C.; Iacob, S.M.; Iacob, R.A.; Lupescu, I.G.; Gheorghe, L.S. The Significance of Enzymatic Cholestasis in Inflammatory Bowel Disease Patients for the Diagnosis of Primary Sclerosing Cholangitis—A Retrospective Study. J. Clin. Med. 2025, 14, 5915. https://doi.org/10.3390/jcm14165915
Mandea M, Oancea DM, Ghioca MC, Iacob SM, Iacob RA, Lupescu IG, Gheorghe LS. The Significance of Enzymatic Cholestasis in Inflammatory Bowel Disease Patients for the Diagnosis of Primary Sclerosing Cholangitis—A Retrospective Study. Journal of Clinical Medicine. 2025; 14(16):5915. https://doi.org/10.3390/jcm14165915
Chicago/Turabian StyleMandea, Matei, Dragos M. Oancea, Mihaela C. Ghioca, Speranta M. Iacob, Razvan A. Iacob, Ioana. G. Lupescu, and Liliana S. Gheorghe. 2025. "The Significance of Enzymatic Cholestasis in Inflammatory Bowel Disease Patients for the Diagnosis of Primary Sclerosing Cholangitis—A Retrospective Study" Journal of Clinical Medicine 14, no. 16: 5915. https://doi.org/10.3390/jcm14165915
APA StyleMandea, M., Oancea, D. M., Ghioca, M. C., Iacob, S. M., Iacob, R. A., Lupescu, I. G., & Gheorghe, L. S. (2025). The Significance of Enzymatic Cholestasis in Inflammatory Bowel Disease Patients for the Diagnosis of Primary Sclerosing Cholangitis—A Retrospective Study. Journal of Clinical Medicine, 14(16), 5915. https://doi.org/10.3390/jcm14165915








