Pulmonary Infiltrates in a Non-Cystic Fibrosis Bronchiectasis Patient: A Case Report
Abstract
1. Introduction
2. Case Presentation
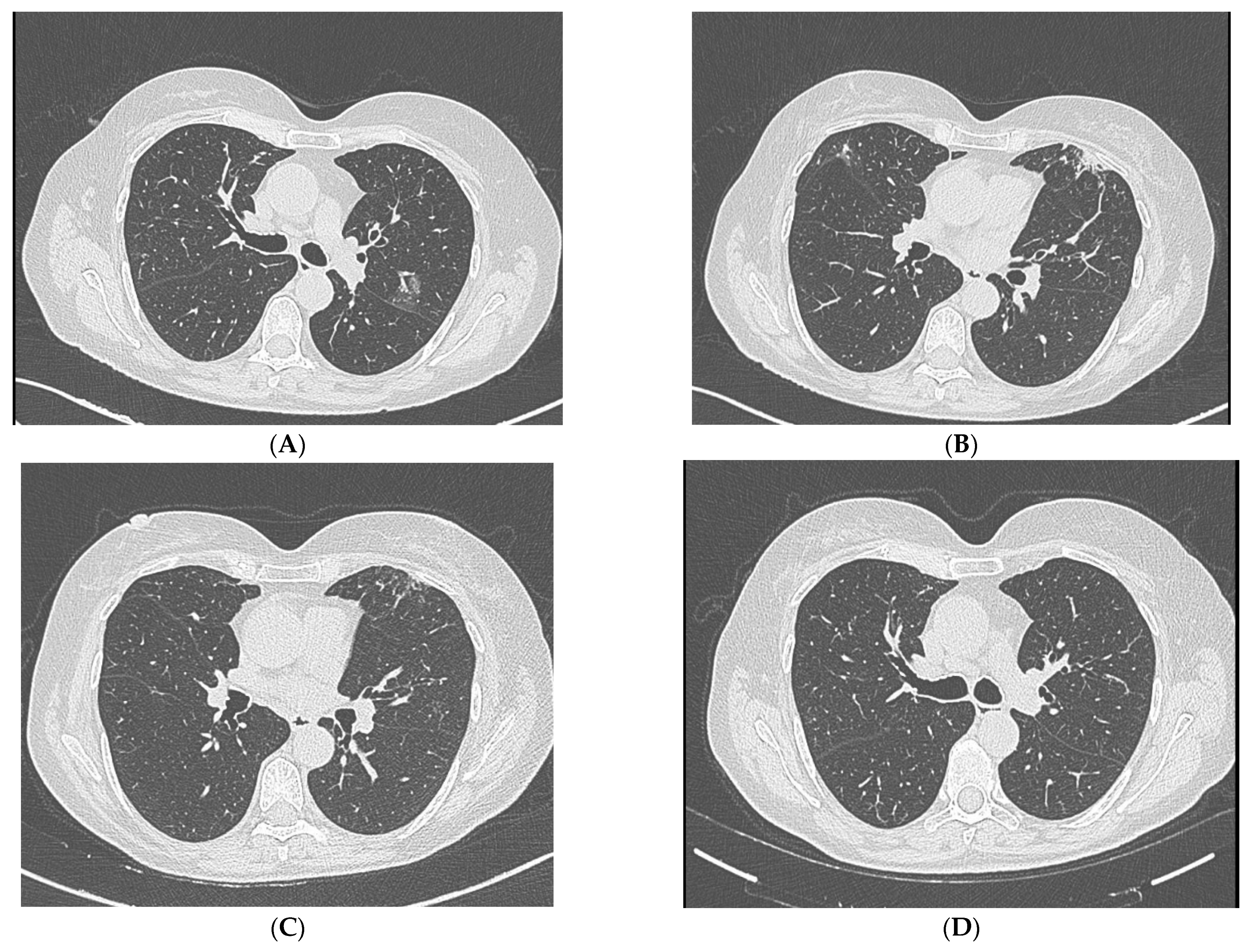
3. Physical Examination Findings
4. Diagnostic Studies
5. Clinical Course
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilgado, F.; Cano, J.; Gené, J.; Guarro, J. Molecular Phylogeny of the Pseudallescheria Boydii Species Complex: Proposal of Two New Species. J. Clin. Microbiol. 2005, 43, 4930–4942. [Google Scholar] [CrossRef] [PubMed]
- Gilgado, F.; Cano, J.; Gené, J.; Sutton, D.A.; Guarro, J. Molecular and Phenotypic Data Supporting Distinct Species Statuses for Scedosporium apiospermum and Pseudallescheria boydii and the Proposed New Species Scedosporium dehoogii. J. Clin. Microbiol. 2008, 46, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Cortez, K.J.; Roilides, E.; Quiroz-Telles, F.; Meletiadis, J.; Antachopoulos, C.; Knudsen, T.; Buchanan, W.; Milanovich, J.; Sutton, D.A.; Fothergill, A.; et al. Infections Caused by Scedosporium spp. Clin. Microbiol. Rev. 2008, 21, 157–197. [Google Scholar] [CrossRef]
- Kowacs, P.A.; Soares Silvado, C.E.; Monteiro de Almeida, S.; Ramos, M.; Abrão, K.; Madaloso, L.E.; Pinheiro, R.L.; Werneck, L.C. Infection of the CNS by Scedosporium apiospermum after near Drowning. Report of a Fatal Case and Analysis of Its Confounding Factors. J. Clin. Pathol. 2004, 57, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Cobo, F.; Lara-Oya, A.; Rodríguez-Granger, J.; Sampedro, A.; Aliaga-Martínez, L.; Navarro-Marí, J.M. Infections Caused by Scedosporium/Lomentospora Species: Clinical and Microbiological Findings in 21 Cases. Med. Mycol. 2018, 56, 917–925. [Google Scholar] [CrossRef]
- Liu, W.; Feng, R.-Z.; Jiang, H.-L. Scedosporium Spp. Lung Infection in Immunocompetent Patients: A Systematic Review and MOOSE-Compliant Meta-Analysis. Medicine 2019, 98, e17535. [Google Scholar] [CrossRef]
- Mursch, K.; Trnovec, S.; Ratz, H.; Hammer, D.; Horré, R.; Klinghammer, A.; de Hoog, S.; Behnke-Mursch, J. Successful Treatment of Multiple Pseudallescheria boydii Brain Abscesses and Ventriculitis/Ependymitis in a 2-Year-Old Child after a near-Drowning Episode. Child’s Nerv. Syst. 2006, 22, 189–192. [Google Scholar] [CrossRef]
- Messori, A.; Lanza, C.; de Nicola, M.; Menichelli, F.; Capriotti, T.; Morabito, L.; Salvolini, U. Mycotic Aneurysms as Lethal Complication of Brain Pseudallescheriasis in a Near-Drowned Child: A CT Demonstration. AJNR Am. J. Neuroradiol. 2002, 23, 1697–1699. [Google Scholar]
- Meletiadis, J.; Meis, J.F.G.M.; Mouton, J.W.; Rodriquez-Tudela, J.L.; Donnelly, J.P.; Verweij, P.E. In Vitro Activities of New and Conventional Antifungal Agents against Clinical Scedosporium Isolates. Antimicrob. Agents Chemother. 2002, 46, 62–68. [Google Scholar] [CrossRef]
- Lackner, M.; de Hoog, G.S.; Verweij, P.E.; Najafzadeh, M.J.; Curfs-Breuker, I.; Klaassen, C.H.; Meis, J.F. Species-Specific Antifungal Susceptibility Patterns of Scedosporium and Pseudallescheria Species. Antimicrob. Agents Chemother. 2012, 56, 2635–2642. [Google Scholar] [CrossRef]
- Ito, Y.; Miwa, S.; Watanabe, A.; Shirai, M. Clinical Characterization of Immunocompetent Patients with Scedosporium Detected in Respiratory Samples: A Case Series. Respir. Med. Case Rep. 2025, 57, 102256. [Google Scholar] [CrossRef] [PubMed]
- Serda Kantarcioglu, A.; Sybren de Hoog, G.; Guarro, J. Clinical Characteristics and Epidemiology of Pulmonary Pseudallescheriasis. Rev. Iberoam. Micol. 2012, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hysi, I.; Le Pimpec-Barthes, F.; Alifano, M.; Venissac, N.; Mouroux, J.; Regnard, J.-F.; Riquet, M.; Porte, H. Lymph Node Involvement and Metastatic Lymph Node Ratio Influence the Survival of Malignant Pleural Mesothelioma: A French Multicenter Retrospective Study. Oncol. Rep. 2014, 31, 415–421. [Google Scholar] [CrossRef]
- Han, J.; Liang, L.; Li, Q.; Deng, R.; Liu, C.; Wu, X.; Zhang, Y.; Zhang, R.; Dai, H. Diagnosis of Pulmonary Scedosporium apiospermum Infection from Bronchoalveolar Lavage Fluid by Metagenomic Next-Generation Sequencing in an Immunocompetent Female Patient with Normal Lung Structure: A Case Report and Literature Review. BMC Infect. Dis. 2024, 24, 308. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.-A.; Halliday, C.L.; Hoenigl, M.; Cornely, O.A.; Meyer, W. Scedosporium and Lomentospora Infections: Contemporary Microbiological Tools for the Diagnosis of Invasive Disease. J. Fungi 2021, 7, 23. [Google Scholar] [CrossRef]
- Reinhold, I.; Quiblier, C.; Blaser, F.; Bögeholz, J.; Imkamp, F.; Schuurmans, M.M.; Soyka, M.B.; Zbinden, R.; Mueller, N.J. Detection of Scedosporium spp.: Colonizer or Pathogen? A Retrospective Analysis of Clinical Significance and Management in a Large Tertiary Center. Med. Mycol. 2024, 62, myae002. [Google Scholar] [CrossRef]
- Mir, W.A.Y.; Shrestha, D.B.; Khan Suheb, M.Z.; Reddy, S.; Gaire, S. Scedosporium Apiospermum Pneumonia in an Immunocompetent Host. Cureus 2021, 13, e16891. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Yang, X.; Liu, Y.; Du, J.; Bossios, A.; Zhang, X.; Su, G.; Wu, L.; Zhang, Z.; et al. Pulmonary Microbiology and Microbiota in Adults with Non-Cystic Fibrosis Bronchiectasis: A Systematic Review and Meta-Analysis. Respir. Res. 2025, 26, 77. [Google Scholar] [CrossRef]
- Máiz, L.; Nieto, R.; Cantón, R.; de la Pedrosa, E.; Martinez-García, M.Á. Fungi in Bronchiectasis: A Concise Review. Int. J. Mol. Sci. 2018, 19, 142. [Google Scholar] [CrossRef]
- Steinbach, W.J.; Juvvadi, P.R.; Fortwendel, J.R.; Rogg, L.E. Newer Combination Antifungal Therapies for Invasive Aspergillosis. Med. Mycol. 2011, 49 (Suppl. S1), S77–S81. [Google Scholar] [CrossRef]
- Troke, P.; Aguirrebengoa, K.; Arteaga, C.; Ellis, D.; Heath, C.H.; Lutsar, I.; Rovira, M.; Nguyen, Q.; Slavin, M.; Chen, S.C.A. Treatment of Scedosporiosis with Voriconazole: Clinical Experience with 107 Patients. Antimicrob. Agents Chemother. 2008, 52, 1743–1750. [Google Scholar] [CrossRef]
- Henao-Martínez, A.F.; Castillo-Mancilla, J.R.; Barron, M.A.; Nichol, A.C. Combination Antifungal Therapy in the Treatment of Scedosporium apiospermum Central Nervous System Infections. Case Rep. Infect. Dis. 2013, 2013, 589490. [Google Scholar] [CrossRef]
- McCarthy, M.W.; Katragkou, A.; Iosifidis, E.; Roilides, E.; Walsh, T.J. Recent Advances in the Treatment of Scedosporiosis and Fusariosis. J. Fungi 2018, 4, 73. [Google Scholar] [CrossRef]
- Vuong, N.N.; Hammond, D.; Kontoyiannis, D.P. Clinical Uses of Inhaled Antifungals for Invasive Pulmonary Fungal Disease: Promises and Challenges. J. Fungi 2023, 9, 464. [Google Scholar] [CrossRef]
- Safdar, A.; Shelburne, S.A.; Evans, S.E.; Dickey, B.F. Inhaled Therapeutics for Prevention and Treatment of Pneumonia. Expert Opin. Drug Saf. 2009, 8, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Solé, A. Invasive Fungal Infections in Lung Transplantation: Role of Aerosolised Amphotericin B. Int. J. Antimicrob. Agents 2008, 32 (Suppl. S2), S161–S165. [Google Scholar] [CrossRef] [PubMed]
- Brunet, K.; Martellosio, J.-P.; Tewes, F.; Marchand, S.; Rammaert, B. Inhaled Antifungal Agents for Treatment and Prophylaxis of Bronchopulmonary Invasive Mold Infections. Pharmaceutics 2022, 14, 641. [Google Scholar] [CrossRef] [PubMed]
- Tolman, J.A.; Wiederhold, N.P.; McConville, J.T.; Najvar, L.K.; Bocanegra, R.; Peters, J.I.; Coalson, J.J.; Graybill, J.R.; Patterson, T.F.; Williams, R.O. 3rd Inhaled Voriconazole for Prevention of Invasive Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2009, 53, 2613–2615. [Google Scholar] [CrossRef]
- Vikelouda, K.; Simitsopoulou, M.; Skoura, L.; Antachopoulos, C.; Roilides, E. Activity of Amphotericin B Formulations and Voriconazole, Alone or in Combination, against Biofilms of Scedosporium and Fusarium spp. Antimicrob. Agents Chemother. 2021, 65, e0063821. [Google Scholar] [CrossRef]
- Hassan, T.; Nicholson, S.; Fahy, R. Pneumothorax and Empyema Complicating Scedosporium apiospermum Mycetoma: Not Just a Problem in the Immunocompromised Patients. Ir. J. Med. Sci. 2011, 180, 931–932. [Google Scholar] [CrossRef]
- Cruz, R.; Barros, M.; Reyes, M. Pulmonary non invasive infection by Scedosporium apiospermum. Rev. Chil. Infectol. 2015, 32, 472–475. [Google Scholar] [CrossRef]
- Durand, C.M.; Durand, D.J.; Lee, R.; Ray, S.C.; Neofytos, D. A 61 Year-Old Female with a Prior History of Tuberculosis Presenting with Hemoptysis. Clin. Infect. Dis. 2011, 52, 910, 957–959. [Google Scholar] [CrossRef]
- Jimeno, V.M.; Muñoz, E.C. Diagnosis of Atypical Mycobacterial and Fungal Coinfection. Int. J. Mycobacteriology 2020, 9, 435–437. [Google Scholar] [CrossRef]
| Risk Group | Underlying Conditions/Risk Factors | Typical Infection Site(s) |
|---|---|---|
| Immunocompromised patients | Hematologic malignancies, solid organ/stem cell transplantation, prolonged neutropenia, corticosteroid therapy | Lungs, CNS, disseminated |
| Immunocompetent with structural lung disease | Non-CF bronchiectasis, prior pulmonary tuberculosis, COPD, cystic lung disease | Lungs |
| Post-trauma or surgery | Penetrating injuries, orthopedic implants, post-surgical wounds | Skin, soft tissue, bone |
| Near-drowning victims | Aspiration of contaminated water | Lungs, CNS |
| Patients with chronic sinusitis or otitis | Anatomical alterations, recurrent bacterial infections | Paranasal sinuses, ear |
| Environmental exposure without apparent host factors | Soil or sewage contact in endemic areas | Variable |
| Reference (Year) | Age/Sex | Imaging Findings | Symptoms | Diagnostic Method | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Liu et al. (2020) [6] | 44/F | Cavitary lesion and consolidation in the left upper lobe | Hemoptysis, cough, weight loss, anorexia | BAL culture | Voriconazole 200 mg BID × 8 wk → lobectomy → VRC × 6 mo | Complete resolution |
| Hassan et al. (2010) [30] | 26/M | Pneumothorax and cavitary mycetoma | Cough, expectoration, fever, spontaneous pneumothorax, fungal empyema | BAL culture | Voriconazole and surgical resection | Recovery |
| Mir et al. (2021) [17] | 83/F | Persistent infiltrates and tree in bud | shortness of breath, cough with blood-tinged sputum, and fatigue for the past several months | Sputum culture | Voriconazole 6 mg/kg intravenously twice a day for the first 24 h, followed by 4 mg/kg twice-daily dosing. At the time of discharge to home, the patient was kept on oral voriconazole 200 mg twice daily for 6 months | Clinical improvement, radiologic resolution |
| Cruz et al. (2015) [31] | 67/F | Cavitary lesion with adjacent bronchiectasis | Persistent cough with bronchorrhea, hemoptisis, fever and general condition impairment | BAL culture | Itraconazole (failed) → Voriconazole × 16 wk | Favorable response |
| Jimeno et al. (2020) [33] | 74/F | RUL cavitary lesion | Asymptomatic | BAL culture | Voriconazole × 3 wk | Improvement, later deterioration due to other causes |
| Durant et al. (2011) [32] | 61/F | Mycetoma in right upper lobe; | Hemoptysis | Sputum and BAL Culture | Voriconazole × 3 months | Hemoptysis resolved, stable imaging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertuccio, F.R.; Baio, N.; Montini, S.; Ferroni, V.; Chino, V.; Pisanu, L.; Russo, M.; Giana, I.; Gallo, E.; Arlando, L.; et al. Pulmonary Infiltrates in a Non-Cystic Fibrosis Bronchiectasis Patient: A Case Report. J. Clin. Med. 2025, 14, 5914. https://doi.org/10.3390/jcm14165914
Bertuccio FR, Baio N, Montini S, Ferroni V, Chino V, Pisanu L, Russo M, Giana I, Gallo E, Arlando L, et al. Pulmonary Infiltrates in a Non-Cystic Fibrosis Bronchiectasis Patient: A Case Report. Journal of Clinical Medicine. 2025; 14(16):5914. https://doi.org/10.3390/jcm14165914
Chicago/Turabian StyleBertuccio, Francesco Rocco, Nicola Baio, Simone Montini, Valentina Ferroni, Vittorio Chino, Lucrezia Pisanu, Marianna Russo, Ilaria Giana, Elisabetta Gallo, Lorenzo Arlando, and et al. 2025. "Pulmonary Infiltrates in a Non-Cystic Fibrosis Bronchiectasis Patient: A Case Report" Journal of Clinical Medicine 14, no. 16: 5914. https://doi.org/10.3390/jcm14165914
APA StyleBertuccio, F. R., Baio, N., Montini, S., Ferroni, V., Chino, V., Pisanu, L., Russo, M., Giana, I., Gallo, E., Arlando, L., Mucaj, K., Tafa, M., Arminio, M., De Stefano, E., Cascina, A., Corsico, A. G., Stella, G. M., & Conio, V. (2025). Pulmonary Infiltrates in a Non-Cystic Fibrosis Bronchiectasis Patient: A Case Report. Journal of Clinical Medicine, 14(16), 5914. https://doi.org/10.3390/jcm14165914






