Optimizing Ovarian Stimulation for IVF in PCOS Patients: A Novel Day 1 GnRH Antagonist Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, Ethics, Sample Size Determination, and Informed Consent
2.2. Exclusion Criteria and Outcome Measures
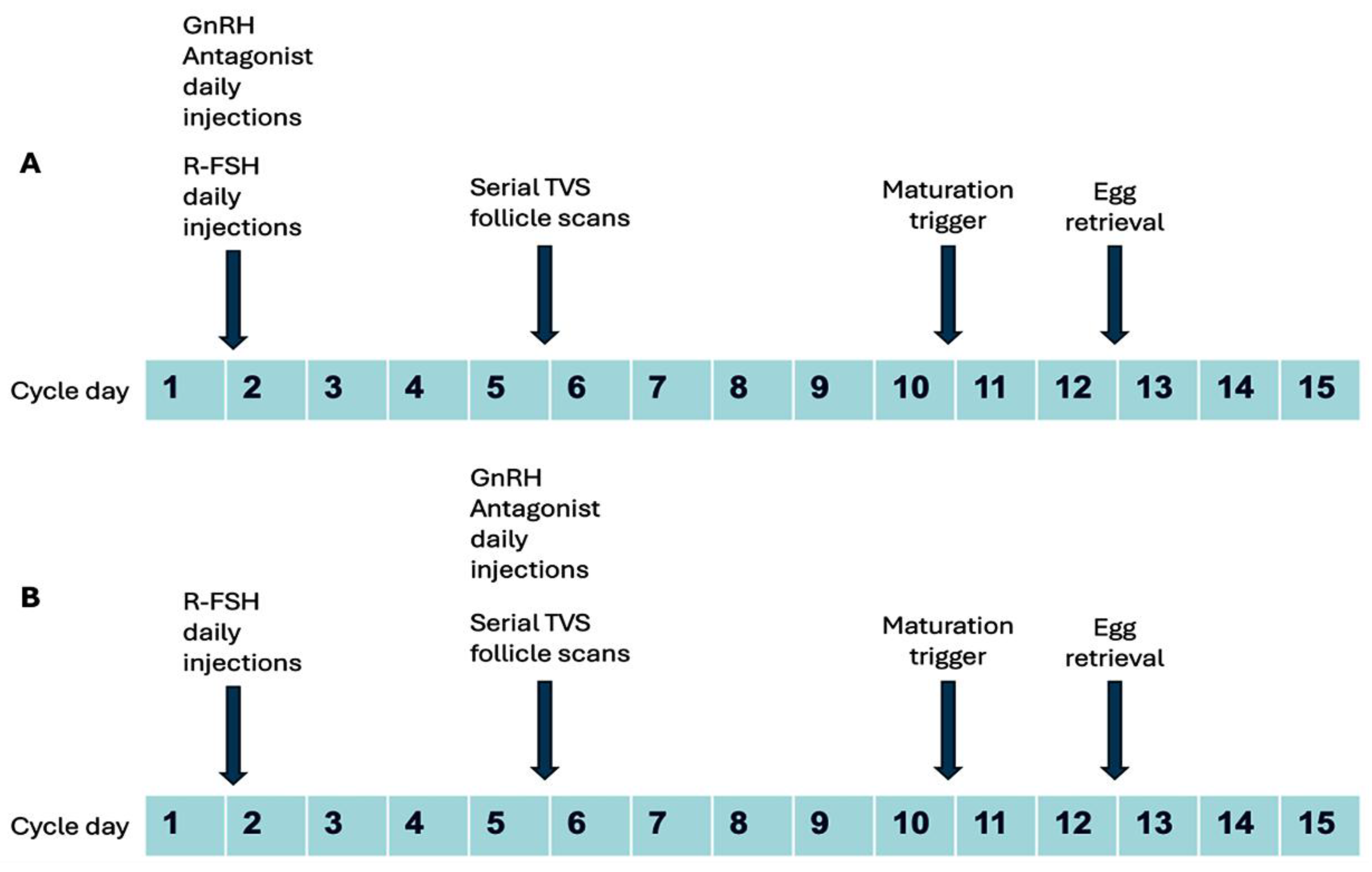
2.3. Clinical Protocol
2.4. Laboratory Protocol
2.5. Embryo Transfer and Cryopreservation
2.6. Luteal Support and Documentation of Pregnancy
2.7. Retrospective Cohort Analysis: Embryo Grading
2.8. Statistical Analysis
2.9. Final Data Presentation
3. Results
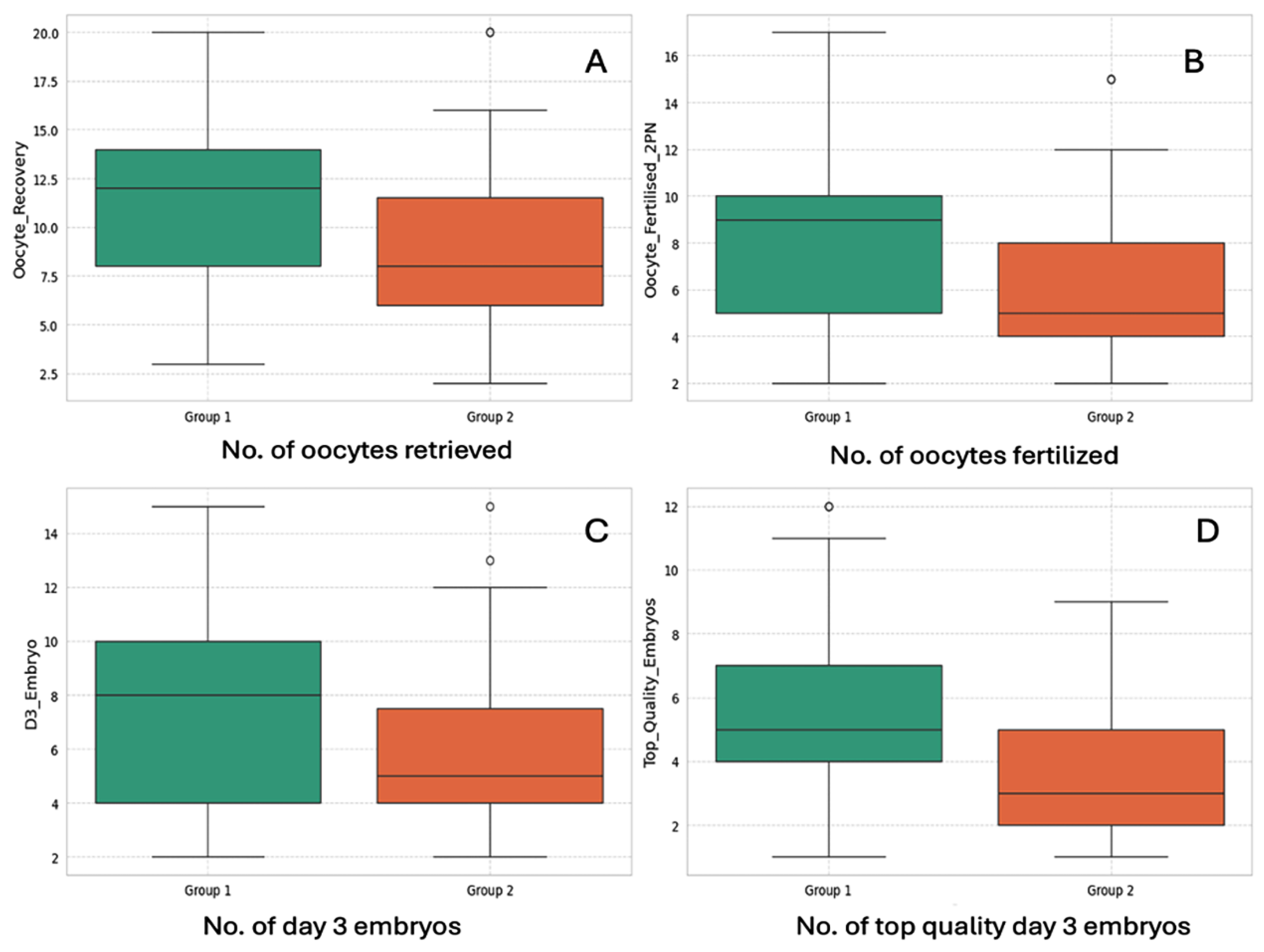
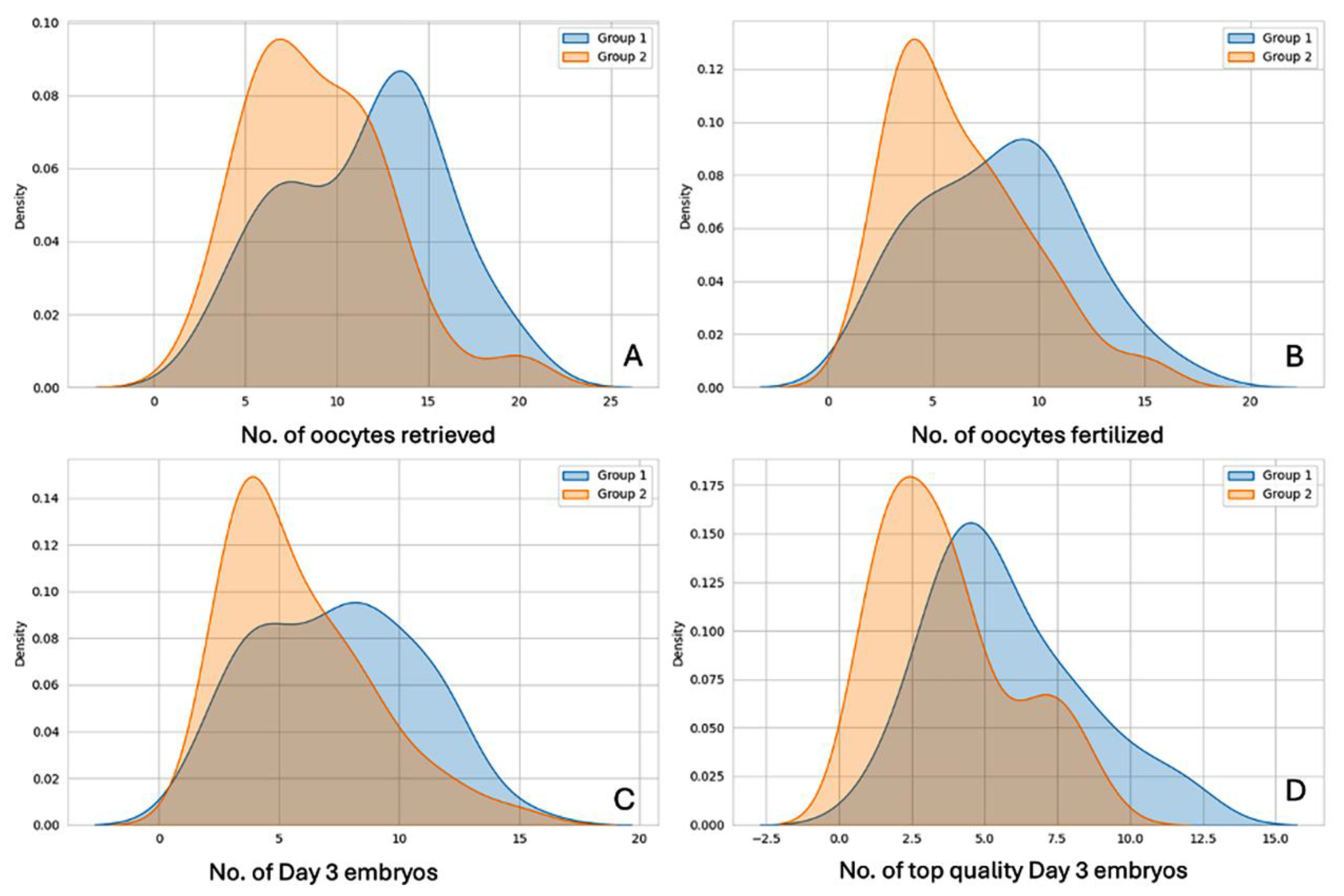
3.1. Laboratory Outcome Measures
3.2. Clinical Outcome Parameters
4. Discussion
4.1. Number of COCs Retrieved and Oocyte Fertilization Rate
4.2. Proportion of Top-Quality Embryos Achieved
4.3. Cumulative Clinical Pregnancy Rate
5. Conclusions
6. Limitations and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GnRH | gonadotropin-releasing hormone |
| PCOS | polycystic ovary syndrome |
| IVF | in vitro fertilization |
| FSH | follicle-stimulating hormone |
| LH | luteinizing hormone |
| PCOM | polycystic ovary morphology |
| AEPCOS | Androgen Excess and PCOS Society |
| AMH | anti-Mullerian hormone |
| HPO | hypothalamic–pituitary–ovarian |
| OS | ovarian stimulation |
| OHSS | ovarian hyperstimulation syndrome |
| FET | frozen embryo transfer |
| 2PN | 2 pronuclear |
| ASRM | American Society for Reproductive Medicine |
| GDIFR | Institute for Fertility Research |
| IRM | Institute of Reproductive Medicine |
| ICSI | intra-cytoplasmic sperm injection |
| CPR | clinical pregnancy rate |
| COC | cumulus–oocyte complex |
| IU | international unit |
| E2 | estradiol |
| TVS | transvaginal sonography |
| r-hCG | recombinant human chorionic gonadotropin |
| HPI | hours post-insemination |
| ET | embryo transfer |
| HRT | hormone replacement therapy |
| MR | miscarriage rate |
| OPR | ongoing pregnancy rate |
| BMI | body mass index |
| TSH | thyroid-stimulating hormone |
References
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.; Norman, R.J.; Teede, H. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef] [PubMed]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: The complete task force report. Fertil. Steril. 2009, 91, 456–488. [Google Scholar] [CrossRef]
- Carmina, E.; Lobo, R.A. Polycystic ovary syndrome (PCOS): Arguably the most common endocrinopathy is associated with significant morbidity in women. J. Clin. Endocrinol. Metab. 1999, 84, 1897–1899. [Google Scholar] [CrossRef]
- Peigné, M.; Dewailly, D. Long term complications of polycystic ovary syndrome (PCOS). In Annales D’endocrinologie; Elsevier Masson: Paris, France, 2014; Volume 75, pp. 194–199. [Google Scholar]
- Daniilidis, A.; Dinas, K. Long term health consequences of polycystic ovarian syndrome: A review analysis. Hippokratia 2009, 13, 90. [Google Scholar]
- Azziz, R. Polycystic ovary syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef]
- Pierre, A.; Peigné, M.; Grynberg, M.; Arouche, N.; Taieb, J.; Hesters, L.; Gonzalès, J.; Picard, J.-Y.; Dewailly, D.; Fanchin, R.; et al. Loss of LH-induced down-regulation of anti-Müllerian hormone receptor expression may contribute to anovulation in women with polycystic ovary syndrome. Hum. Reprod. 2013, 28, 762–769. [Google Scholar] [CrossRef]
- Carlsen, S.; Vanky, E.; Fleming, R. Anti-Müllerian hormone concentrations in androgen-suppressed women with polycystic ovary syndrome. Hum. Reprod. 2009, 24, 1732–1738. [Google Scholar] [CrossRef][Green Version]
- Tal, R.; Seifer, D.B.; Khanimov, M.; Malter, H.E.; Grazi, R.V.; Leader, B. Characterization of women with elevated antimüllerian hormone levels (AMH): Correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am. J. Obstet. Gynecol. 2014, 211, 59-e1. [Google Scholar] [CrossRef]
- Nestler, J.E.; Powers, L.P.; Matt, D.W.; Steingold, K.A.; Plymate, S.R.; Rittmaster, R.S.; Clore, J.N.; Blackard, W.G. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1991, 72, 83–89. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Prevention of moderate and severe ovarian hyperstimulation syndrome: A guideline. Fertil. Steril. 2024, 121, 230–245. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.; Li, L.; Wang, W.; Yang, D.; Zhang, Q. Is a GnRH antagonist protocol better in PCOS patients? A meta-analysis of RCTs. PLoS ONE 2014, 9, e91796. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, S.; Zhang, C.; Chang, S. Effectiveness of GnRH antagonist in the treatment of patients with polycystic ovary syndrome undergoing IVF: A systematic review and meta analysis. Gynecol. Endocrinol. 2013, 29, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Onofriescu, A.; Bors, A.; Luca, A.; Holicov, M.; Onofriescu, M.; Vulpoi, C. GnRH antagonist IVF protocol in PCOS. Curr. Health Sci. J. 2013, 39, 20. [Google Scholar] [PubMed]
- Tehraninejad, E.S.; Nasiri, R.; Rashidi, B.; Haghollahi, F.; Ataie, M. Comparison of GnRH antagonist with long GnRH agonist protocol after OCP pretreatment in PCOs patients. Arch. Gynecol. Obstet. 2010, 282, 319–325. [Google Scholar] [CrossRef]
- Haydardedeoglu, B.; Kilicdag, E.B.; Parlakgumus, A.H.; Zeyneloglu, H.B. IVF/ICSI outcomes of the OCP plus GnRH agonist protocol versus the OCP plus GnRH antagonist fixed protocol in women with PCOS: A randomized trial. Arch. Gynecol. Obstet. 2012, 286, 763–769. [Google Scholar] [CrossRef]
- Hosseini, M.A.; Aleyasin, A.; Saeedi, H.; Mahdavi, A. Comparison of gonadotropin-releasing hormone agonists and antagonists in assisted reproduction cycles of polycystic ovarian syndrome patients. J. Obstet. Gynaecol. Res. 2010, 36, 605–610. [Google Scholar] [CrossRef]
- Trenkić, M.; Popović, J.; Kopitović, V.; Bjelica, A.; Živadinović, R.; Pop-Trajković, S. Flexible GnRH antagonist protocol vs. long GnRH agonist protocol in patients with polycystic ovary syndrome treated for IVF: Comparison of clinical outcome and embryo quality. Ginekol. Pol. 2016, 87, 265–270. [Google Scholar] [CrossRef]
- Behery, M.A.; Hasan, E.A.; Ali, E.A.; Eltabakh, A.A. Comparative study between agonist and antagonist protocols in PCOS patients undergoing ICSI: A cross-sectional study. Middle East Fertil. Soc. J. 2020, 24, 2. [Google Scholar] [CrossRef]
- Olivennes, F.; Cunha-Filho, J.S.; Fanchin, R.; Bouchard, P.; Frydman, R. The use of GnRH antagonists in ovarian stimulation. Hum. Reprod. Update 2002, 8, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, G.; Diedrich, K.; Tarlatzis, B.C.; Kolibianakis, E.M. GnRH-antagonists in ovarian stimulation for IVF in patients with poor response to gonadotrophins, polycystic ovary syndrome, and risk of ovarian hyperstimulation: A meta-analysis. Reprod. Biomed. Online 2006, 13, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Kadoura, S.; Alhalabi, M.; Nattouf, A.H. Conventional GnRH antagonist protocols versus long GnRH agonist protocol in IVF/ICSI cycles of polycystic ovary syndrome women: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 4456. [Google Scholar] [CrossRef]
- Lainas, T.G.; Sfontouris, I.A.; Zorzovilis, I.Z.; Petsas, G.K.; Lainas, G.T.; Alexopoulou, E.; Kolibianakis, E.M. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients with polycystic ovary syndrome treated for IVF: A prospective randomised controlled trial (RCT). Hum. Reprod. 2010, 25, 683–689. [Google Scholar] [CrossRef]
- Lainas, T.G.; Petsas, G.K.; Zorzovilis, I.Z.; Iliadis, G.S.; Lainas, G.T.; Cazlaris, H.E.; Kolibianakis, E.M. Initiation of GnRH antagonist on Day 1 of stimulation as compared to the long agonist protocol in PCOS patients. A randomized controlled trial: Effect on hormonal levels and follicular development. Hum. Reprod. 2007, 22, 1540–1546. [Google Scholar] [CrossRef]
- Shin, J.J.; Park, K.E.; Choi, Y.M.; Kim, H.O.; Choi, D.H.; Lee, W.S.; Cho, J.H. Early gonadotropin-releasing hormone antagonist protocol in women with polycystic ovary syndrome: A preliminary randomized trial. Clin. Exp. Reprod. Med. 2018, 45, 135. [Google Scholar] [CrossRef]
- Dastidar, S.G. A novel ovarian stimulation protocol with gonadotropin-releasing hormone (GnRH) antagonist and follicle stimulating hormone from cycle day 1 in IVF in patients with polycystic ovary syndrome (PCOS). Fertil. Steril. 2008, 90, S362. [Google Scholar] [CrossRef]
- Balaban, B.; Brison, D.; Calderon, G.; Catt, J.; Conaghan, J.; Cowan, L.; Ebner, T.; Gardner, D.; Hardarson, T.; Lundin, K.; et al. Alpha scientists in reproductive medicine and ESHRE special interest group of embryology. The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [Google Scholar]
- Di Guardo, F.; De Rijdt, S.; Racca, A.; Drakopoulos, P.; Mackens, S.; Strypstein, L.; Tournaye, H.; De Vos, M.; Blockeel, C.; Maged, A.M. Impact of GnRH antagonist pretreatment on oocyte yield after ovarian stimulation: A retrospective analysis. PLoS ONE 2024, 19, e0308666. [Google Scholar] [CrossRef]
- Palomba, S. Is fertility reduced in ovulatory women with polycystic ovary syndrome? An opinion paper. Hum. Reprod. 2021, 36, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Daolio, J.; La Sala, G.B. Oocyte competence in women with polycysticovary syndrome. Trends Endocrinol. Metab. 2017, 28, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Dovey, S.; McIntyre, K.; Jacobson, D.; Catov, J.; Wakim, A. Is a premature rise in luteinizing hormone in the absence of increased progesterone levels detrimental to pregnancy outcome in GnRH antagonist in vitro fertilization cycles. Fertil. Steril. 2011, 96, 585–589. [Google Scholar] [CrossRef]
- Wang, J.; Ding, J.; Qu, B.; Zhang, Y.; Zhou, Q. Does serum LH level influence IVF outcomes in women with PCOS undergoing GnRH-antagonist stimulation: A novel indicator. J. Clin. Med. 2022, 11, 4670. [Google Scholar] [CrossRef]
- Jiang, N.X.; Li, X.L. The disorders of endometrial receptivity in PCOS and its mechanisms. Reprod. Sci. 2022, 29, 2465–2476. [Google Scholar] [CrossRef]

| Baseline Parameter | Group 1 (Day 1 Antagonist): Mean ± SD | Group 2 (Day 5/Day 6 Antagonist): Mean ± SD | p Value | Statistical Significance |
|---|---|---|---|---|
| Age (years) | 29.13 ± 4.13 | 29.89 ± 4.55 | >0.05 | NS * |
| BMI (kg/m2) | 24.48 ± 3.04 | 25.33 ± 3.16 | >0.05 | NS |
| AMH (ng/mL) | 6.12 ± 2.31 | 5.61 ± 2.42 | >0.05 | NS |
| TSH (mIU/mL) | 2.70 ± 1.29 | 3.09 ± 1.76 | >0.05 | NS |
| Average duration of stimulation (days) | 10.6 ± 2.1 | 9.9 ± 1.9 | >0.05 | NS |
| Outcome Measure | Group 1 (Day 1 Antagonist): Mean ± SD | Group 2 (Day 5/Day 6 Antagonist): Mean ± SD | t-Value | p Value | Statistical Significance |
|---|---|---|---|---|---|
| No. of oocytes retrieved (COCs) | 11.47 ± 4.37 | 8.95 ± 3.88 | 3.05 | <0.01 | S ** |
| No. of oocytes fertilized (2PN Number) | 8.13 ± 3.71 | 6.27 ± 3.20 | 2.69 | <0.01 | S |
| No. of day 3 cleavage-stage embryos formed | 7.40 ± 3.33 | 5.82 ± 2.96 | 2.51 | p = 0.01 | S |
| No. of top-quality day 3 embryos | 5.73 ± 2.63 | 3.69 ± 2.25 | 4.15 | <0.01 | S |
| Outcome Measure | Group 1 (Day 1 Antagonist) | Group 2 (Day 5/Day 6 Antagonist): | Z-Statistic | p Value | Statistical Significance |
|---|---|---|---|---|---|
| Proportion of oocytes fertilized (%) | 0.709 (70.9%) | 0.701 (70.1%) | 0.28 | >0.05 | NS * |
| Proportion of day 3 embryos graded as top-quality embryos (%) | 0.77 (77.5%) | 0.63 (63.4%) | 3.93 | <0.01 | S ** |
| Study Groups | No. of Patients Who Had Embryo Transfer/s | No. of Embryo Transfer (ET) Events | Total No. of Embryos Transferred | No. of Pregnancies | Cumulative Clinical Pregnancy Rate (CPR) per Stimulation Cycle | Clinical Pregnancy Rate (CPR) per ET |
|---|---|---|---|---|---|---|
| Group 1 (day 1 antagonist) | 42 | 50 | 91 | 22 | 52.4% | 44% |
| Group 2 (day 5/day 6 antagonist) | 55 | 68 | 124 | 19 | 34.5% | 27.9% |
| Statistical significance | * NS p > 0.05 | NS p = 0.07 | NS p = 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh Dastidar, S.; Ghosh Dastidar, B.; Chattopadhyay, R.; Chakraborty, C. Optimizing Ovarian Stimulation for IVF in PCOS Patients: A Novel Day 1 GnRH Antagonist Protocol. J. Clin. Med. 2025, 14, 5901. https://doi.org/10.3390/jcm14165901
Ghosh Dastidar S, Ghosh Dastidar B, Chattopadhyay R, Chakraborty C. Optimizing Ovarian Stimulation for IVF in PCOS Patients: A Novel Day 1 GnRH Antagonist Protocol. Journal of Clinical Medicine. 2025; 14(16):5901. https://doi.org/10.3390/jcm14165901
Chicago/Turabian StyleGhosh Dastidar, Sudarsan, Biswanath Ghosh Dastidar, Ratna Chattopadhyay, and Chandan Chakraborty. 2025. "Optimizing Ovarian Stimulation for IVF in PCOS Patients: A Novel Day 1 GnRH Antagonist Protocol" Journal of Clinical Medicine 14, no. 16: 5901. https://doi.org/10.3390/jcm14165901
APA StyleGhosh Dastidar, S., Ghosh Dastidar, B., Chattopadhyay, R., & Chakraborty, C. (2025). Optimizing Ovarian Stimulation for IVF in PCOS Patients: A Novel Day 1 GnRH Antagonist Protocol. Journal of Clinical Medicine, 14(16), 5901. https://doi.org/10.3390/jcm14165901







