High Myopia as a Risk Factor for Severe Liver Disease in Individuals with Liver Dysfunction: Evidence from a Prospective Cohort
Abstract
1. Introduction
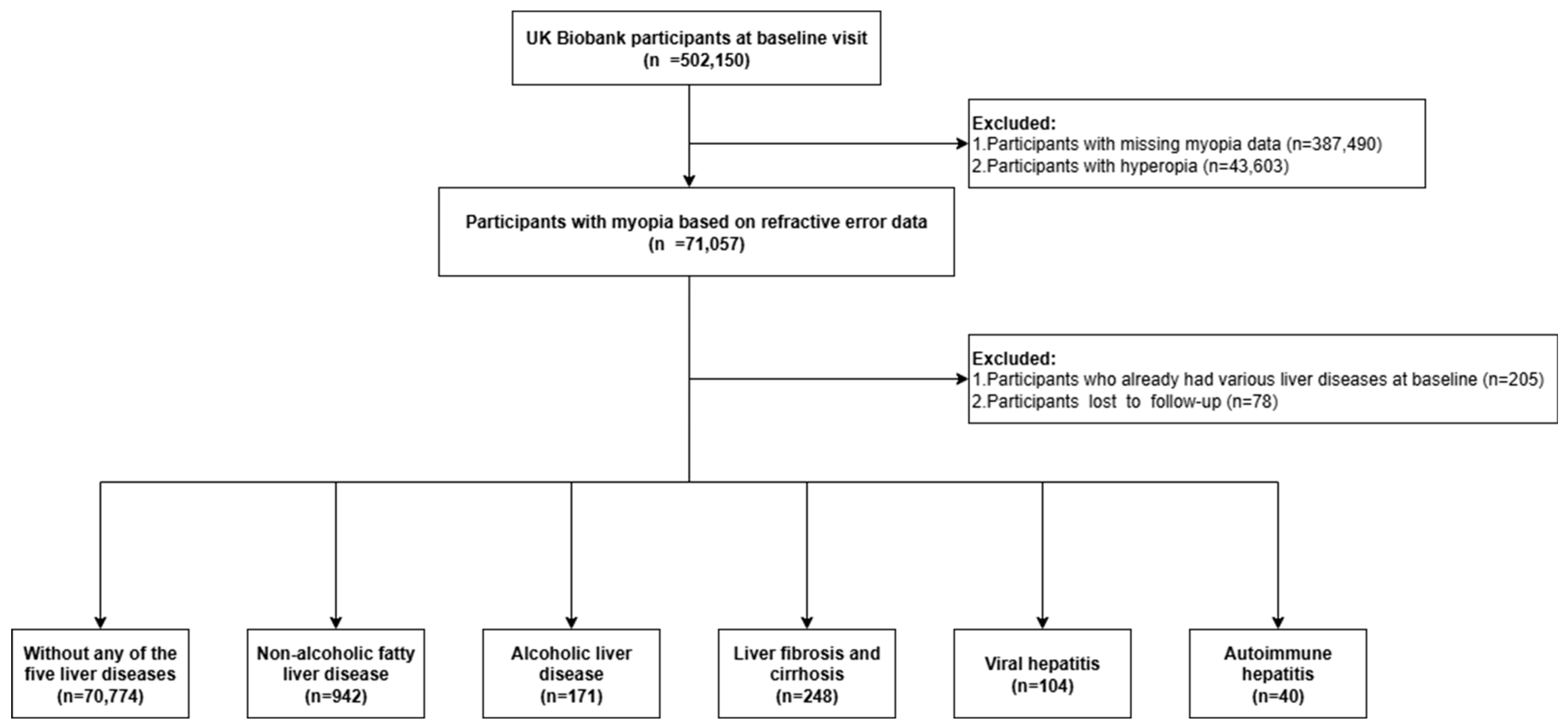
2. Materials and Methods
- Ethics
- Assessment of exposure
- Assessment of outcome
- Assessment of potential mediators
- Assessment of covariates
- Statistical analyses
- Role of the funding source
3. Results
3.1. Baseline Characteristics
3.2. Myopia Status and Risk of Incident Severe Liver Diseases
3.3. Subgroup Analyses in High-Risk Subgroups
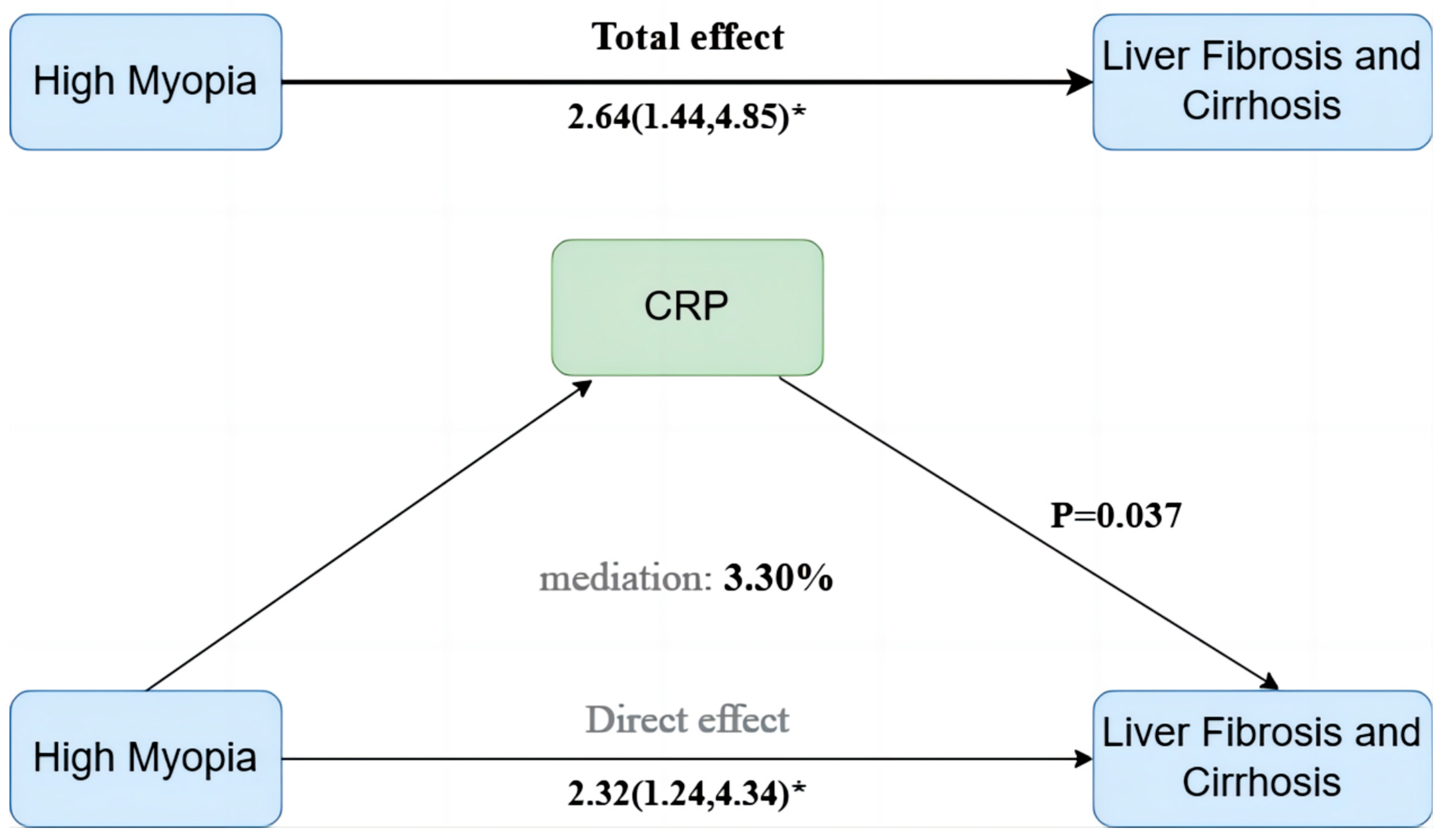
3.4. Mediation Effects of Inflammatory and Metabolic Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UKB | UK Biobank |
| AST | Aspartate aminotransferase |
| NAFLD | Non-alcoholic fatty liver disease |
| ALD | Alcoholic liver disease |
| AIH | Autoimmune hepatitis |
| avMSE | Mean spherical equivalent refractive error |
| CRP | C-reactive protein |
| NLR | Neutrophil-to-lymphocyte ratio |
| PLR | Platelet-to-lymphocyte ratio |
| MHR | Monocyte-to-high-density-lipoprotein-cholesterol ratio |
| LMR | Lymphocyte-to-monocyte ratio |
| WBC | White blood cell |
| INFLA | Low-grade chronic inflammation |
| TDI | Townsend deprivation index |
| IPAQ | International Physical Activity Questionnaire |
| BMI | Body mass index |
| ALT | Alanine aminotransferase |
| HDL | High-density lipoprotein |
| VLDL | Very-low-density lipoprotein |
References
- Myopia: Statistics, Trends, Causes, & Solutions. 2025. Available online: https://www.visioncenter.org/resources/myopia-prevalence-statistics/ (accessed on 1 March 2025).
- Tuo, Y.; Zhang, G.; Yi, H. Vision for the Future: Pioneering Strategies in China’s Battle against Myopia. Eye 2024, 38, 3042–3044. [Google Scholar] [CrossRef]
- Zhu, X.; Meng, J.; Han, C.; Wu, Q.; Du, Y.; Qi, J.; Wei, L.; Li, H.; He, W.; Zhang, K.; et al. CCL2-Mediated Inflammatory Pathogenesis Underlies High Myopia-Related Anxiety. Cell Discov. 2023, 9, 94. [Google Scholar] [CrossRef]
- Li, H.; Du, Y.; Cheng, K.; Chen, Y.; Wei, L.; Pei, Y.; Wang, X.; Wang, L.; Zhang, Y.; Hu, X.; et al. Gut Microbiota-Derived Indole-3-Acetic Acid Suppresses High Myopia Progression by Promoting Type I Collagen Synthesis. Cell Discov. 2024, 10, 89. [Google Scholar] [CrossRef]
- Du, Y.; Meng, J.; He, W.; Qi, J.; Lu, Y.; Zhu, X. Complications of High Myopia: An Update from Clinical Manifestations to Underlying Mechanisms. Adv. Ophthalmol. Pract. Res. 2024, 4, 156–163. [Google Scholar] [CrossRef]
- Huang, F.; Chen, Y.; Wu, J.; Zheng, S.; Huang, R.; Wan, W.; Hu, K. Comprehensive Bioinformatics Analysis of Metabolism-Related microRNAs in High Myopia in Young and Old Adults with Age-related Cataracts. Mol. Med. Rep. 2025, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Koh, J.H.Z.; Seah, S.H.Y.; Dan, Y.S.; Wang, Z.; Chan, X.; Zhou, L.; Barathi, V.A.; Hoang, Q.V. Key Role for Inflammation-Related Signaling in the Pathogenesis of Myopia Based on Evidence from Proteomics Analysis. Sci. Rep. 2024, 14, 23486. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, L.; Chen, S.; Xie, C.; Tong, J.; Shen, Y. The Potential Role of Amino Acids in Myopia: Inspiration from Metabolomics. Metabolomics 2025, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Qi, J.; Du, Y.; Zhao, C.; Liu, S.; Lu, Y.; Zhu, X. Tear Inflammatory Cytokines as Potential Biomarkers for Myopic Macular Degeneration. Exp. Eye Res. 2023, 235, 109648. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, K.; He, W.; Yang, J.; Sun, X.; Jiang, C.; Dai, J.; Lu, Y. Proinflammatory Status in the Aqueous Humor of High Myopic Cataract Eyes. Exp. Eye Res. 2016, 142, 13–18. [Google Scholar] [CrossRef]
- Francisco, B.-M.; Salvador, M.; Amparo, N. Oxidative Stress in Myopia. Oxid. Med. Cell. Longev. 2015, 2015, 750637. [Google Scholar] [CrossRef]
- Wu, J.; Duan, C.; Yang, Y.; Wang, Z.; Tan, C.; Han, C.; Hou, X. Insights into the Liver-Eyes Connections, from Epidemiological, Mechanical Studies to Clinical Translation. J. Transl. Med. 2023, 21, 712. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.C.; Zhang, L.; Ye, T.; Ding, B. Targeting Fibrosis: Mechanisms and Clinical Trials. Signal Transduct. Target. Ther. 2022, 7, 206. [Google Scholar] [CrossRef]
- Ezhilarasan, D.; Karthikeyan, S.; Najimi, M.; Vijayalakshmi, P.; Bhavani, G.; Rani, M.J. Preclinical Liver Toxicity Models: Advantages, Limitations and Recommendations. Toxicology 2025, 511, 154020. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lin, Y.; Song, J.; Song, M.; Nie, X.; Sun, H.; Xu, C.; Han, Z.; Cai, J. From Mechanisms to Medicine: Ferroptosis as a Therapeutic Target in Liver Disorders. Cell Commun. Signal. 2025, 23, 125. [Google Scholar] [CrossRef]
- Xie, K.; Chen, C.-H.; Tsai, S.-P.; Lu, P.-J.; Wu, H.; Zeng, Y.; Ye, Y.; Tu, H.; Wen, C.; Huang, M.; et al. Loss of Life Expectancy by 10 Years or More From Elevated Aspartate Aminotransferase: Finding Aspartate Aminotransferase a Better Mortality Predictor for All-Cause and Liver-Related than Alanine Aminotransferase. Am. J. Gastroenterol. 2019, 114, 1478–1487. [Google Scholar] [CrossRef]
- Hu, W.; Liu, B.-P.; Jia, C.-X. Association and Biological Pathways between Lung Function and Incident Depression: A Prospective Cohort Study of 280,032 Participants. BMC Med. 2024, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Cen, M.; Song, L.; Fu, X.; Gao, X.; Zuo, Q.; Wu, J. Associations between metabolic syndrome and anxiety, and the mediating role of inflammation: Findings from the UK Biobank. Brain Behav. Immun. 2024, 116, 1–9. [Google Scholar] [CrossRef]
- Hashemi, H.; Khabazkhoob, M.; Emamian, M.H.; Shariati, M.; Miraftab, M.; Yekta, A.; Ostadimoghaddam, H.; Fotouhi, A. Association between Refractive Errors and Ocular Biometry in Iranian Adults. J. Ophthalmic Vis. Res. 2015, 10, 214. [Google Scholar] [CrossRef]
- Wang, J.; Jost, R.M.; Birch, E.E. Ocular Biometric Components in Hyperopic Children and a Machine Learning-Based Model to Predict Axial Length. Transl. Vision Sci. Technol. 2024, 13, 25. [Google Scholar] [CrossRef]
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K.; Rahi, J.; Resnikoff, S.; Vitale, S.; Yannuzzi, L. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Invest. Ophthalmol. Vis. Sci. 2019, 60, M20. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, J.A.; Williams, C.; for the UK Biobank Eye and Vision Consortium. Role of Educational Exposure in the Association Between Myopia and Birth Order. JAMA Ophthalmol. 2015, 133, 1408. [Google Scholar] [CrossRef]
- Chen, C.; Wei, L.; He, W.; Zhang, Y.; Xiao, J.; Lu, Y.; Wang, F.; Zhu, X. Associations of Severe Liver Diseases with Cataract Using Data from UK Biobank: A Prospective Cohort Study. eClinicalMedicine 2024, 68, 102424. [Google Scholar] [CrossRef]
- Jensen, A.H.; Winther-Sørensen, M.; Burisch, J.; Bergquist, A.; Ytting, H.; Gluud, L.L.; Wewer Albrechtsen, N.J. Autoimmune Liver Diseases and Diabetes: A Propensity Score Matched Analysis and a Proportional Meta-analysis. Liver Int. 2023, 43, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Soininen, P.; Kangas, A.J.; Würtz, P.; Suna, T.; Ala-Korpela, M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Cardiovascular Epidemiology and Genetics. Circ.—Cardiovasc. Genet. 2015, 8, 192–206. [Google Scholar] [CrossRef]
- LeFort, K.R.; Rungratanawanich, W.; Song, B.-J. Contributing Roles of Mitochondrial Dysfunction and Hepatocyte Apoptosis in Liver Diseases through Oxidative Stress, Post-Translational Modifications, Inflammation, and Intestinal Barrier Dysfunction. Cell. Mol. Life Sci. 2024, 81, 34. Available online: https://pubmed.ncbi.nlm.nih.gov/38214802/ (accessed on 19 February 2025). [CrossRef]
- Tran, Q.-A.; Tran, G.V.; Velic, S.; Xiong, H.-M.; Kaur, J.; Moosavi, Z.; Nguyen, P.; Duong, N.; Luu, V.T.; Singh, G.; et al. Effects of Astragaloside IV and Formononetin on Oxidative Stress and Mitochondrial Biogenesis in Hepatocytes. Int. J. Mol. Sci. 2025, 26, 774. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, C.; Liu, Z.; Yue, Y.; Hsiao, Y.; Zhou, Q.; Zhou, J. Association between Inflammatory Cytokines and Oxidative Stress Levels in Aqueous Humor with Axial Length in Human Myopia. Exp. Eye Res. 2023, 237, 109670. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, D.; Zhou, Q.; Zhao, F.; He, M.; Yang, Z.; Su, Y.; Zhai, Y.; Yan, J.; Zhang, G.; et al. Scleral HIF-1α Is a Prominent Regulatory Candidate for Genetic and Environmental Interactions in Human Myopia Pathogenesis. eBioMedicine 2020, 57, 102878. [Google Scholar] [CrossRef]
- Wu, H.; Chen, W.; Zhao, F.; Zhou, Q.; Reinach, P.S.; Deng, L.; Ma, L.; Luo, S.; Srinivasalu, N.; Pan, M.; et al. Scleral Hypoxia Is a Target for Myopia Control. Proc. Natl. Acad. Sci. USA 2018, 115, E7091–E7100. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-Z.; Li, C.; Chen, Q.; Jing, Y.; Carpenter, R.; Jiang, Y.; Kung, H.-F.; Lai, L.; Jiang, B.-H. MiR-21 Induced Angiogenesis through AKT and ERK Activation and HIF-1α Expression. PLoS ONE 2011, 6, e19139. [Google Scholar] [CrossRef]
- Fu, R.-Q.; Hu, D.-P.; Hu, Y.-B.; Hong, L.; Sun, Q.-F.; Ding, J.-G. miR-21 Promotes α-SMA and Collagen I Expression in Hepatic Stellate Cells via the Smad7 Signaling Pathway. Mol. Med. Rep. 2017, 16, 4327–4333. [Google Scholar] [CrossRef]
- Wei, J.; Feng, L.; Li, Z.; Xu, G.; Fan, X. MicroRNA-21 Activates Hepatic Stellate Cells via PTEN/Akt Signaling. Biomed. Pharmacother. 2013, 67, 387–392. [Google Scholar] [CrossRef]
- Liang, B.; Qiu, X.; Huang, J.; Lu, Y.; Shen, H.; Ma, J.; Chen, Y. Nonlinear Associations of the Hs-CRP/HDL-C Index with Metabolic Dysfunction-Associated Steatotic Liver Disease and Advanced Liver Fibrosis in US Adults: Insights from NHANES 2017–2018. Sci. Rep. 2025, 15, 4029. [Google Scholar] [CrossRef]
- Mansouri, A.; Gattolliat, C.-H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Hu, Q.; Wu, J.; Wang, G.; Hong, Z.; Ren, J. Lab for Trauma and Surgical Infections Mitochondrial DNA in Liver Inflammation and Oxidative Stress. Life Sci. 2019, 236, 116464. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, J.; Ochiya, T. Extracellular microRNAs and Oxidative Stress in Liver Injury: A Systematic Mini Review. J. Clin. Biochem. Nutr. 2018, 63, 6–11. [Google Scholar] [CrossRef]
- Pezzoli, A.; Abenavoli, L.; Scarcella, M.; Rasetti, C.; Baroni, G.S.; Tack, J.; Scarpellini, E. The Management of Cardiometabolic Risk in MAFLD: Therapeutic Strategies to Modulate Deranged Metabolism and Cholesterol Levels. Medicina 2025, 61, 387. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, H.; Li, Y.; Hao, Y.; Wang, C.; Jia, P.; Chen, X.; Ma, S.; Xiao, Z. Crosstalk between Hepatic Stellate Cells and Surrounding Cells in Hepatic Fibrosis. Int. Immunopharmacol. 2021, 99, 108051. [Google Scholar] [CrossRef] [PubMed]
- Baghaei, K.; Mazhari, S.; Tokhanbigli, S.; Parsamanesh, G.; Alavifard, H.; Schaafsma, D.; Ghavami, S. Therapeutic Potential of Targeting Regulatory Mechanisms of Hepatic Stellate Cell Activation in Liver Fibrosis. Drug Discov. Today 2022, 27, 1044–1061. [Google Scholar] [CrossRef]
- Kim, D.; Konyn, P.; Sandhu, K.K.; Dennis, B.B.; Cheung, A.C.; Ahmed, A. Metabolic Dysfunction-Associated Fatty Liver Disease Is Associated with Increased All-Cause Mortality in the United States. J. Hepatol. 2021, 75, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Armandi, A.; Pellegrinelli, V.; Vidal-Puig, A.; Bugianesi, E. Μetabolic Dysfunction-Associated Steatotic Liver Disease: A Condition of Heterogeneous Metabolic Risk Factors, Mechanisms and Comorbidities Requiring Holistic Treatment. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Tilg, H.; Byrne, C.D.; Targher, G. Non-Alcoholic Fatty Liver Disease and Risk of Incident Diabetes Mellitus: An Updated Meta-Analysis of 501 022 Adult Individuals. Gut 2021, 70, 962–969. [Google Scholar] [CrossRef]
- Leal-Lassalle, H.; Estévez-Vázquez, O.; Cubero, F.J.; Nevzorova, Y.A. Metabolic and Alcohol-Associated Liver Disease (MetALD): A Representation of Duality. npj Gut Liver 2025, 2, 1. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, Y.; Zeng, G.; Huda, N.; Thoudam, T.; Yang, Z.; Liangpunsakul, S.; Ma, J. Cell-to-Cell and Organ-to-Organ Crosstalk in the Pathogenesis of Alcohol-Associated Liver Disease. eGastroenterology 2024, 2, e100104. [Google Scholar] [CrossRef]
- Ortega-Ribera, M.; Zhuang, Y.; Babuta, M.; Brezani, V.; Joshi, R.S.; Zsengeller, Z.; Nagesh, P.T.; Wang, Y.; Bronson, R.; Szabo, G.; et al. A Novel Multi-Organ Male Model of Alcohol-Induced Acute-on-Chronic Liver Failure Reveals NET-Mediated Hepatocellular Death, Which Is Prevented by RIPK3 Inhibition. Cell. Mol. Gastroenterol. Hepatol. 2025, 19, 101446. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total (n = 70,774) | Myopia Status | Statistic | p | ||
|---|---|---|---|---|---|---|
| Emmetropia (n = 36,180) | Low/Moderate Myopia (n = 30,022) | High Myopia (n = 4572) | ||||
| Age, M (Q1, Q3) | 56.00 (48.00, 62.00) | 54.00 (47.00, 62.00) | 57.00 (49.00, 63.00) | 56.00 (49.00, 62.00) | χ2 = 536.03 # | <0.001 |
| Sex, n (%) | χ2 = 50.94 | <0.001 | ||||
| Male | 32,968 (46.58) | 1917 (41.93) | 13,896 (46.29) | 17,155 (47.42) | ||
| Female | 37,806 (53.42) | 2655 (58.07) | 16,126 (53.71) | 19,025 (52.58) | ||
| Ethnicity, n (%) | χ2 = 82.88 | <0.001 | ||||
| Non-white | 13,944 (19.70) | 879 (19.23) | 5463 (18.20) | 7602 (21.01) | ||
| White | 56,830 (80.30) | 3693 (80.77) | 24,559 (81.80) | 28,578 (78.99) | ||
| Townsend deprivation index, M (Q1, Q3) | −1.52 (−3.25, 1.11) | −1.45 (−3.23, 1.30) | −1.62 (−3.31, 0.95) | −1.45 (−3.20, 0.98) | χ2 = 63.84 # | <0.001 |
| Education, n (%) | χ2 = 1418.00 | <0.001 | ||||
| Below high school | 27,715 (39.16) | 2446 (53.50) | 13,146 (43.79) | 12,123 (33.51) | ||
| High school | 8767 (12.39) | 610 (13.34) | 3916 (13.04) | 4241 (11.72) | ||
| College or above | 34,292 (48.45) | 1516 (33.16) | 12,960 (43.17) | 19,816 (54.77) | ||
| Physical activity, n (%) | χ2 = 166.82 | <0.001 | ||||
| Low | 12,174 (17.20) | 802 (17.54) | 5342 (17.79) | 6030 (16.67) | ||
| Moderate | 29,440 (41.60) | 2064 (45.14) | 12,958 (43.16) | 14,418 (39.85) | ||
| High | 29,160 (41.20) | 1706 (37.31) | 11,722 (39.04) | 15,732 (43.48) | ||
| BMI, M (Q1, Q3) | 26.60 (23.99, 29.77) | 26.74 (24.11, 29.91) | 26.53 (23.92, 29.66) | 26.03 (23.50, 29.19) | χ2 = 104.29 # | <0.001 |
| BMI group, n (%) | χ2 = 96.62 | <0.001 | ||||
| Low | 405 (0.57) | 47 (1.03) | 168 (0.56) | 190 (0.53) | ||
| Normal | 23,943 (33.83) | 1727 (37.77) | 10,402 (34.65) | 11,814 (32.65) | ||
| Overweight | 29,632 (41.87) | 1857 (40.62) | 12,486 (41.59) | 15,289 (42.26) | ||
| Obesity | 16,794 (23.73) | 941 (20.58) | 6966 (23.20) | 8887 (24.56) | ||
| Waist, M (Q1, Q3) | 90.00 (80.00, 99.00) | 90.00 (81.00, 99.00) | 90.00 (80.00, 99.00) | 88.00 (79.00, 97.00) | χ2 = 75.24 # | <0.001 |
| Alcohol Group, n (%) | χ2 = 105.30 | <0.001 | ||||
| 1–5 drinks/month | 17,066 (24.11) | 1141 (24.96) | 7163 (23.86) | 8762 (24.22) | ||
| 10+ drinks/month | 29,900 (42.25) | 1994 (43.61) | 13,169 (43.86) | 14,737 (40.73) | ||
| 5–10 drinks/month | 17,575 (24.83) | 1084 (23.71) | 7274 (24.23) | 9217 (25.48) | ||
| Non-drinker | 6233 (8.81) | 353 (7.72) | 2416 (8.05) | 3464 (9.57) | ||
| Smoke Group, n (%) | χ2 = 223.80 | <0.001 | ||||
| No | 41,177 (58.18) | 3019 (66.03) | 17,936 (59.74) | 20,222 (55.89) | ||
| Yes | 29,597 (41.82) | 1553 (33.97) | 12,086 (40.26) | 15,958 (44.11) | ||
| Hypertension Status, n (%) | χ2 = 29.39 | <0.001 | ||||
| No | 53,305 (75.32) | 3510 (76.77) | 22,312 (74.32) | 27,483 (75.96) | ||
| Yes | 17,469 (24.68) | 1062 (23.23) | 7710 (25.68) | 8697 (24.04) | ||
| Diabetes status, n (%) | χ2 = 19.83 | <0.001 | ||||
| No | 66,874 (94.49) | 4376 (95.71) | 28,272 (94.17) | 34,226 (94.60) | ||
| Yes | 3900 (5.51) | 196 (4.29) | 1750 (5.83) | 1954 (5.40) | ||
| HDL, M (Q1, Q3) | 1.30 (1.10, 1.54) | 1.29 (1.09, 1.54) | 1.30 (1.10, 1.55) | 1.32 (1.12, 1.57) | χ2 = 24.91 # | <0.001 |
| AST, M (Q1, Q3) | 24.50 (21.00, 29.00) | 24.50 (21.00, 29.10) | 24.50 (21.10, 29.00) | 24.20 (20.90, 28.50) | χ2 = 9.79 # | 0.007 |
| ALT, M (Q1, Q3) | 20.10 (15.27, 27.39) | 20.16 (15.24, 27.58) | 20.10 (15.35, 27.33) | 19.52 (14.98, 26.32) | χ2 = 19.02 # | <0.001 |
| Lymphocyte, M (Q1, Q3) | 1.93 (1.56, 2.36) | 1.94 (1.58, 2.38) | 1.91 (1.55, 2.34) | 1.89 (1.53, 2.31) | χ2 = 68.02 # | <0.001 |
| Neutrophil, M (Q1, Q3) | 4.09 (3.30, 5.02) | 4.10 (3.29, 5.03) | 4.08 (3.31, 5.01) | 4.07 (3.31, 5.03) | χ2 = 0.90 # | 0.637 |
| Platelet, M (Q1, Q3) | 236.30 (203.10, 272.60) | 236.80 (203.30, 273.20) | 235.90 (202.90, 271.90) | 235.25 (203.40, 272.70) | χ2 = 4.58 # | 0.101 |
| CRP, M (Q1, Q3) | 1.24 (0.61, 2.60) | 1.26 (0.62, 2.62) | 1.24 (0.61, 2.60) | 1.20 (0.58, 2.48) | χ2 = 12.25 # | 0.002 |
| Monocyte, M (Q1, Q3) | 0.46 (0.36, 0.57) | 0.45 (0.36, 0.57) | 0.46 (0.36, 0.57) | 0.45 (0.36, 0.55) | χ2 = 15.30 # | <0.001 |
| Leukocyte, M (Q1, Q3) | 6.77 (5.73, 7.98) | 6.79 (5.74, 8.02) | 6.75 (5.73, 7.95) | 6.74 (5.71, 7.92) | χ2 = 13.25 # | 0.001 |
| INFLA, M (Q1, Q3) | −1.00 (−5.00, 4.00) | −1.00 (−5.00, 4.00) | −1.00 (−5.00, 4.00) | −1.00 (−5.00, 4.00) | χ2 = 2.39 # | 0.303 |
| MHR, M (Q1, Q3) | 0.35 (0.26, 0.47) | 0.35 (0.26, 0.47) | 0.35 (0.26, 0.47) | 0.34 (0.25, 0.45) | χ2 = 23.29 # | <0.001 |
| NLR, M (Q1, Q3) | 2.11 (1.63, 2.73) | 2.10 (1.62, 2.72) | 2.12 (1.65, 2.75) | 2.14 (1.67, 2.79) | χ2 = 33.88 # | <0.001 |
| PLR, M (Q1, Q3) | 122.10 (97.37, 153.67) | 121.19 (96.65, 152.61) | 122.94 (98.06, 154.44) | 123.88 (99.30, 157.20) | χ2 = 43.01 # | <0.001 |
| LMR, M (Q1, Q3) | 4.25 (3.31, 5.45) | 4.30 (3.35, 5.51) | 4.20 (3.27, 5.39) | 4.23 (3.29, 5.47) | χ2 = 56.96 # | <0.001 |
| Liver Diseases | AST Level | Myopia Status | avMSE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Emmetropia | Low/Moderate Myopia | High Myopia | p for Trend | |||||||
| HR (95%CI) | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | ||||
| Liver fibrosis and cirrhosis | Model 1 a | <40 | 1 (Reference) | 1.24 (0.88~1.74) | 0.213 | 0.72 (0.31~1.65) | 0.483 | 0.291 | 1.01 (0.94~1.08) | 0.936 |
| ≥40 | 1 (Reference) | 1.28 (0.85~1.92) | 0.233 | 2.29 (1.26~4.15) | 0.006 | 0.014 | 0.92 (0.86~0.98) | 0.013 | ||
| Model 2 b | <40 | 1 (Reference) | 1.25 (0.89~1.76) | 0.195 | 0.74 (0.32~1.71) | 0.483 | 0.325 | 1.00 (0.94~1.08) | 0.936 | |
| ≥40 | 1 (Reference) | 1.29 (0.86~1.93) | 0.219 | 2.19 (1.21~3.98) | 0.010 | 0.022 | 0.93 (0.87~0.99) | 0.021 | ||
| Model 3 c | <40 | 1 (Reference) | 1.30 (0.93~1.83) | 0.130 | 0.80 (0.35~1.86) | 0.607 | 0.401 | 0.99 (0.93~1.06) | 0.848 | |
| ≥40 | 1 (Reference) | 1.21 (0.81~1.82) | 0.353 | 2.20 (1.21~4.01) | 0.010 | 0.016 | 0.92 (0.87~0.99) | 0.019 | ||
| Model 4 d | <40 | 1 (Reference) | 1.31 (0.93~1.84) | 0.126 | 0.88 (0.38~2.03) | 0.760 | 0.527 | 0.98 (0.92~1.06) | 0.669 | |
| ≥40 | 1 (Reference) | 1.32 (0.88~1.99) | 0.185 | 2.64 (1.44~4.85) | 0.002 | 0.004 | 0.90 (0.85~0.97) | 0.003 | ||
| Non-alcoholic fatty liver disease | Model 1 a | <40 | 1 (Reference) | 0.86 (0.74~1.00) | 0.055 | 0.82 (0.60~1.12) | 0.215 | 0.423 | 1.04 (1.01~1.07) | 0.024 |
| ≥40 | 1 (Reference) | 1.09 (0.81~1.47) | 0.572 | 1.03 (0.58~1.84) | 0.911 | 0.973 | 0.99 (0.93~1.05) | 0.704 | ||
| Model 2 b | <40 | 1 (Reference) | 0.88 (0.76~1.02) | 0.085 | 0.83 (0.61~1.14) | 0.257 | 0.461 | 1.04 (1.00~1.07) | 0.034 | |
| ≥40 | 1 (Reference) | 1.10 (0.81~1.48) | 0.541 | 1.00 (0.56~1.79) | 0.997 | 0.874 | 0.99 (0.94~1.05) | 0.802 | ||
| Model 3 c | <40 | 1 (Reference) | 0.90 (0.77~1.05) | 0.168 | 0.88 (0.64~1.21) | 0.433 | 0.641 | 1.03 (1.00~1.07) | 0.091 | |
| ≥40 | 1 (Reference) | 1.01 (0.75~1.36) | 0.963 | 0.98 (0.55~1.76) | 0.954 | 0.943 | 1.00 (0.94~1.06) | 0.909 | ||
| Model 4 d | <40 | 1 (Reference) | 0.92 (0.79~1.07) | 0.255 | 0.98 (0.72~1.35) | 0.911 | 0.871 | 1.02 (0.98~1.05) | 0.338 | |
| ≥40 | 1 (Reference) | 1.08 (0.80~1.46) | 0.608 | 1.14 (0.64~2.05) | 0.656 | 0.746 | 0.98 (0.92~1.04) | 0.459 | ||
| Alcoholic liver disease | Model 1 a | <40 | 1 (Reference) | 0.74 (0.47~1.18) | 0.206 | 0.67 (0.24~1.86) | 0.444 | 0.628 | 1.08 (0.97~1.21) | 0.153 |
| ≥40 | 1 (Reference) | 1.21 (0.79~1.85) | 0.370 | 0.86 (0.34~2.17) | 0.749 | 0.59 | 1.02 (0.94~1.12) | 0.598 | ||
| Model 2 b | <40 | 1 (Reference) | 0.75 (0.47~1.20) | 0.232 | 0.72 (0.26~2.01) | 0.536 | 0.725 | 1.07 (0.96~1.20) | 0.209 | |
| ≥40 | 1 (Reference) | 1.25 (0.82~1.91) | 0.304 | 0.86 (0.34~2.18) | 0.758 | 0.577 | 1.02 (0.93~1.12) | 0.630 | ||
| Model 3 c | <40 | 1 (Reference) | 0.76 (0.48~1.21) | 0.251 | 0.78 (0.28~2.16) | 0.627 | 0.819 | 1.06 (0.95~1.19) | 0.263 | |
| ≥40 | 1 (Reference) | 1.25 (0.82~1.92) | 0.302 | 0.84 (0.33~2.11) | 0.704 | 0.527 | 1.02 (0.93~1.12) | 0.626 | ||
| Model 4 d | <40 | 1 (Reference) | 0.81 (0.51~1.29) | 0.376 | 0.93 (0.33~2.60) | 0.886 | 0.953 | 1.04 (0.93~1.16) | 0.478 | |
| ≥40 | 1 (Reference) | 1.35 (0.88~2.08) | 0.168 | 0.99 (0.39~2.53) | 0.989 | 0.733 | 1.00 (0.92~1.10) | 0.933 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, L.; Huang, Z.; Du, Y.; Zhu, X. High Myopia as a Risk Factor for Severe Liver Disease in Individuals with Liver Dysfunction: Evidence from a Prospective Cohort. J. Clin. Med. 2025, 14, 5860. https://doi.org/10.3390/jcm14165860
Jian L, Huang Z, Du Y, Zhu X. High Myopia as a Risk Factor for Severe Liver Disease in Individuals with Liver Dysfunction: Evidence from a Prospective Cohort. Journal of Clinical Medicine. 2025; 14(16):5860. https://doi.org/10.3390/jcm14165860
Chicago/Turabian StyleJian, Linge, Zhiqian Huang, Yu Du, and Xiangjia Zhu. 2025. "High Myopia as a Risk Factor for Severe Liver Disease in Individuals with Liver Dysfunction: Evidence from a Prospective Cohort" Journal of Clinical Medicine 14, no. 16: 5860. https://doi.org/10.3390/jcm14165860
APA StyleJian, L., Huang, Z., Du, Y., & Zhu, X. (2025). High Myopia as a Risk Factor for Severe Liver Disease in Individuals with Liver Dysfunction: Evidence from a Prospective Cohort. Journal of Clinical Medicine, 14(16), 5860. https://doi.org/10.3390/jcm14165860






