Characterizing Stair Ambulation Kinetics and the Effects of Dual Tasking in Parkinson’s Disease
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Safe-Gait System
2.3. Experimental Protocol
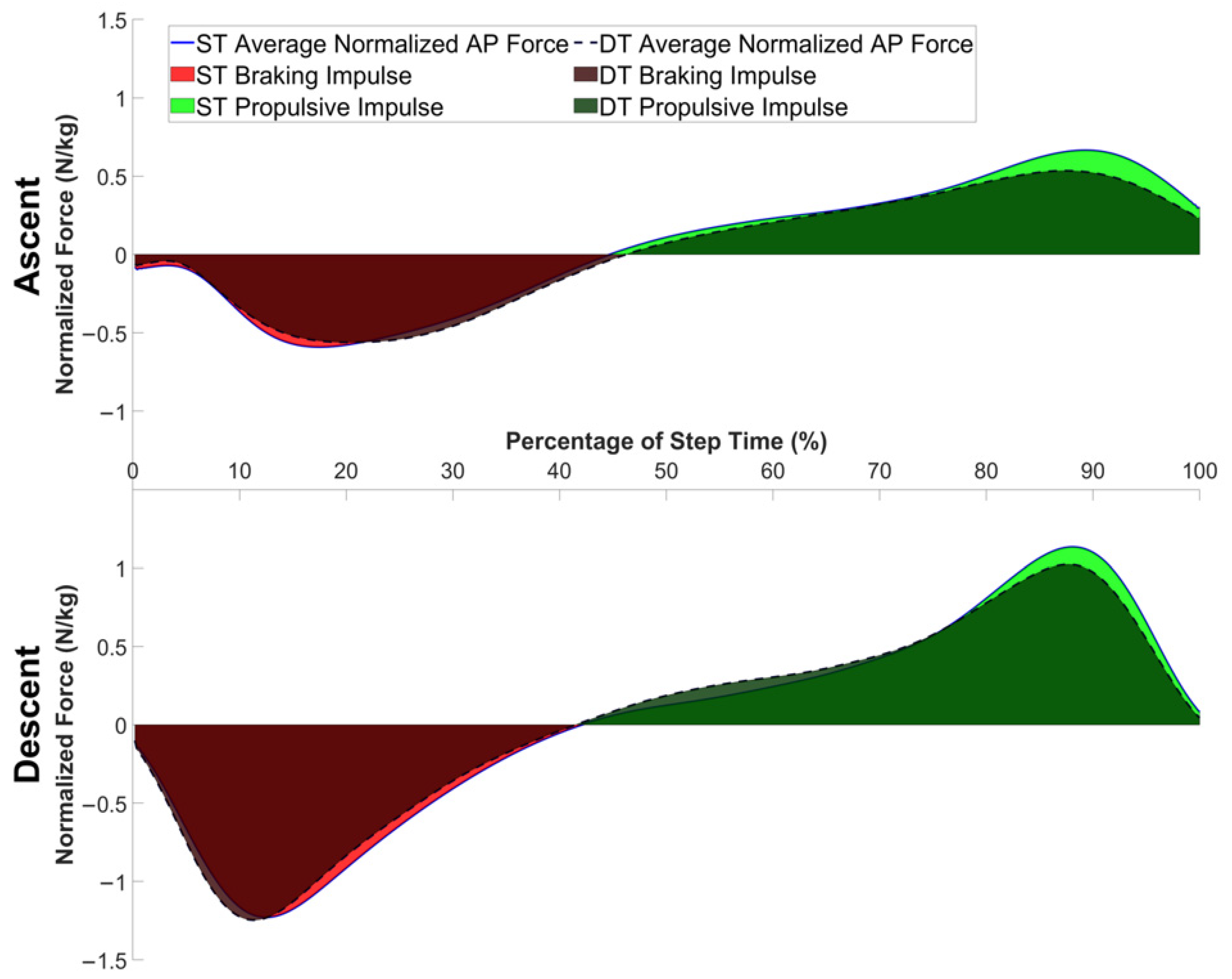
2.4. Kinetic Data
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PwPD | People with Parkinson’s disease |
| FOG | Freezing of gait |
| PIGD | Postural instability and gait difficulties |
| DT | Dual task |
| Safe-Gait | Stair Ambulation and Functional Evaluation of Gait |
| ST | Single task |
| CoP | Center of pressure |
| PD | Parkinson’s disease |
| AP | Anteroposterior |
| vGRF | Vertical ground reaction force |
| ML | Mediolateral |
| LMM | Linear mixed model |
| CI | Confidence interval |
| TUG | Timed Up and Go |
References
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Raffegeau, T.E.; Krehbiel, L.M.; Kang, N.; Thijs, F.J.; Altmann, L.J.P.; Cauraugh, J.H.; Hass, C.J. A meta-analysis: Parkinson’s disease and dual-task walking. Park. Relat. Disord. 2019, 62, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Allali, G.; Ayers, E.I.; Holtzer, R.; Verghese, J. The role of postural instability/gait difficulty and fear of falling in predicting falls in non-demented older adults. Arch. Gerontol. Geriatr. 2017, 69, 15–20. [Google Scholar] [CrossRef]
- Pelicioni, P.H.S.; Menant, J.C.; Latt, M.D.; Lord, S.R. Falls in Parkinson’s Disease Subtypes: Risk Factors, Locations and Circumstances. Int. J. Environ. Res. Public Health 2019, 16, 2216. [Google Scholar] [CrossRef]
- Monaghan, A.S.; Hooyman, A.; Dibble, L.E.; Mehta, S.H.; Peterson, D.S. Stability Changes in Fall-Prone Individuals with Parkinson Disease Following Reactive Step Training. J. Neurol. Phys. Ther. 2024, 48, 46–53. [Google Scholar] [CrossRef]
- Ashburn, A.; Stack, E.; Ballinger, C.; Fazakarley, L.; Fitton, C. The circumstances of falls among people with Parkinson’s disease and the use of Falls Diaries to facilitate reporting. Disabil. Rehabil. 2008, 30, 1205–1212. [Google Scholar] [CrossRef]
- Lin, Y.C.; Fok, L.A.; Schache, A.G.; Pandy, M.G. Muscle coordination of support, progression and balance during stair ambulation. J. Biomech. 2015, 48, 340–347. [Google Scholar] [CrossRef]
- Roth, N.; Ullrich, M.; Kuderle, A.; Gladow, T.; Marxreiter, F.; Gassner, H.; Kluge, F.; Klucken, J.; Eskofier, B.M. Real-World Stair Ambulation Characteristics Differ Between Prospective Fallers and Non-Fallers in Parkinson’s Disease. IEEE J. Biomed. Health Inform. 2022, 26, 4733–4742. [Google Scholar] [CrossRef]
- McGregor, M.M.; Nelson, A.B. Circuit Mechanisms of Parkinson’s Disease. Neuron 2019, 101, 1042–1056. [Google Scholar] [CrossRef]
- Wu, T.; Hallett, M.; Chan, P. Motor automaticity in Parkinson’s disease. Neurobiol. Dis. 2015, 82, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Lu, J.; Wang, J.; Shu, Z.; Cheng, Y.; Zhu, Z.; Liu, P.; Yu, Y.; Yu, N.; et al. Fronto-parietal cortex activation during walking in patients with Parkinson’s disease adopting different postural strategies. Front. Neurol. 2022, 13, 998243. [Google Scholar] [CrossRef]
- Frenkel-Toledo, S.; Giladi, N.; Peretz, C.; Herman, T.; Gruendlinger, L.; Hausdorff, J.M. Effect of gait speed on gait rhythmicity in Parkinson’s disease: Variability of stride time and swing time respond differently. J. Neuroeng. Rehabil. 2005, 2, 23. [Google Scholar] [CrossRef]
- Dubbioso, R.; Manganelli, F.; Siebner, H.R.; Di Lazzaro, V. Fast Intracortical Sensory-Motor Integration: A Window Into the Pathophysiology of Parkinson’s Disease. Front. Hum. Neurosci. 2019, 13, 111. [Google Scholar] [CrossRef]
- Vandenbossche, J.; Deroost, N.; Soetens, E.; Coomans, D.; Spildooren, J.; Vercruysse, S.; Nieuwboer, A.; Kerckhofs, E. Freezing of gait in Parkinson’s disease: Disturbances in automaticity and control. Front. Hum. Neurosci. 2012, 6, 356. [Google Scholar] [CrossRef]
- Yang, J.; Yan, S.; Cao, C. Different Dual-Task Paradigm Reduce Postural Control Ability and Dynamic Stability of Healthy Young Adults during Stair Descent. Appl. Bionics Biomech. 2024, 2024, 9942042. [Google Scholar] [CrossRef]
- Atrsaei, A.; Corra, M.F.; Dadashi, F.; Vila-Cha, N.; Maia, L.; Mariani, B.; Maetzler, W.; Aminian, K. Gait speed in clinical and daily living assessments in Parkinson’s disease patients: Performance versus capacity. NPJ Park. Dis. 2021, 7, 24. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, W.; Song, Q.; Gu, H.; Mao, D. Performance of older adults under dual task during stair descent. J. Exerc. Sci. Fit. 2018, 16, 99–105. [Google Scholar] [CrossRef]
- Clark, D.J. Automaticity of walking: Functional significance, mechanisms, measurement and rehabilitation strategies. Front. Hum. Neurosci. 2015, 9, 246. [Google Scholar] [CrossRef]
- Eltoukhy, M.; Kuenze, C.; Andersen, M.S.; Oh, J.; Signorile, J. Prediction of ground reaction forces for Parkinson’s disease patients using a kinect-driven musculoskeletal gait analysis model. Med. Eng. Phys. 2017, 50, 75–82. [Google Scholar] [CrossRef]
- Rochester, L.; Galna, B.; Lord, S.; Burn, D. The nature of dual-task interference during gait in incident Parkinson’s disease. Neuroscience 2014, 265, 83–94. [Google Scholar] [CrossRef]
- Salazar, R.D.; Ren, X.; Ellis, T.D.; Toraif, N.; Barthelemy, O.J.; Neargarder, S.; Cronin-Golomb, A. Dual tasking in Parkinson’s disease: Cognitive consequences while walking. Neuropsychology 2017, 31, 613–623. [Google Scholar] [CrossRef]
- Lu, C.F.; Liu, Y.C.; Yang, Y.R.; Wu, Y.T.; Wang, R.Y. Maintaining Gait Performance by Cortical Activation during Dual-Task Interference: A Functional Near-Infrared Spectroscopy Study. PLoS ONE 2015, 10, e0129390. [Google Scholar] [CrossRef]
- Kawami, Y.; Nikaido, Y.; Nose, S.; Unekawa, M.; Marumoto, K.; Kawami, M.; Matsugashita, S.; Kozuki, T.; Akisue, T. Mediolateral Postural Control during Gait in Parkinson’s Disease. Prog. Rehabil. Med. 2022, 7, 20220048. [Google Scholar] [CrossRef]
- Fernandes, A.; Sousa, A.S.; Couras, J.; Rocha, N.; Tavares, J.M. Influence of dual-task on sit-to-stand-to-sit postural control in Parkinson’s disease. Med. Eng. Phys. 2015, 37, 1070–1075. [Google Scholar] [CrossRef]
- Hwang, J.; Youm, C.; Park, H.; Kim, B.; Choi, H.; Cheon, S.M. Machine learning for early detection and severity classification in people with Parkinson’s disease. Sci. Rep. 2025, 15, 234. [Google Scholar] [CrossRef]
- Kelly, V.E.; Eusterbrock, A.J.; Shumway-Cook, A. A review of dual-task walking deficits in people with Parkinson’s disease: Motor and cognitive contributions, mechanisms, and clinical implications. Park. Dis. 2012, 2012, 918719. [Google Scholar] [CrossRef]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. 2008, 23, 329–342. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Horak, F.B. Cortical control of postural responses. J. Neural. Transm. 2007, 114, 1339–1348. [Google Scholar] [CrossRef]
- Pellecchia, G.L. Dual-task training reduces impact of cognitive task on postural sway. J. Mot. Behav. 2005, 37, 239–246. [Google Scholar] [CrossRef]
- Kim, S.D.; Allen, N.E.; Canning, C.G.; Fung, V.S. Postural instability in patients with Parkinson’s disease. Epidemiology, pathophysiology and management. CNS Drugs 2013, 27, 97–112. [Google Scholar] [CrossRef]
- Smulders, K.; Dale, M.L.; Carlson-Kuhta, P.; Nutt, J.G.; Horak, F.B. Pharmacological treatment in Parkinson’s disease: Effects on gait. Park. Relat. Disord. 2016, 31, 3–13. [Google Scholar] [CrossRef]
- Massot, C.; Simoneau, E.; Peron, D.; Barbier, F.; Kwiatkowski, A.; Donze, C.; Leteneur, S. Simplified stance limb kinetics patterns revealed during gait initiation in early stage of multiple sclerosis. Clin. Biomech. 2022, 91, 105549. [Google Scholar] [CrossRef]
- Ohta, M.; Tanabe, S.; Katsuhira, J.; Tamari, M. Kinetic and kinematic parameters associated with late braking force and effects on gait performance of stroke patients. Sci. Rep. 2023, 13, 7729. [Google Scholar] [CrossRef]
- Yamawaki, T. Diagnosis of MSA-P and PSP-P in Early Stage. Brain Nerve 2020, 72, 331–343. [Google Scholar] [PubMed]
- Amboni, M.; Ricciardi, C.; Picillo, M.; De Santis, C.; Ricciardelli, G.; Abate, F.; Tepedino, M.F.; D’Addio, G.; Cesarelli, G.; Volpe, G.; et al. Gait analysis may distinguish progressive supranuclear palsy and Parkinson disease since the earliest stages. Sci. Rep. 2021, 11, 9297. [Google Scholar] [CrossRef] [PubMed]
- Sidoroff, V.; Raccagni, C.; Kaindlstorfer, C.; Eschlboeck, S.; Fanciulli, A.; Granata, R.; Eskofier, B.; Seppi, K.; Poewe, W.; Willeit, J.; et al. Characterization of gait variability in multiple system atrophy and Parkinson’s disease. J. Neurol. 2021, 268, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Janssen Daalen, J.M.; Selvaraj, A.; Arnts, H.; Bloem, B.R.; Bartels, R.H.; Georgiev, D.; Esselink, R.A.J.; Vinke, R.S. Gait and balance worsening after bilateral deep brain stimulation of the subthalamic nucleus (STN-DBS) for Parkinson’s disease: A systematic review. BMJ Neurol. Open 2025, 7, e000898. [Google Scholar] [CrossRef]
- Cabanes-Martinez, L.; Villadoniga, M.; Millan, A.S.; Del Alamo, M.; Regidor, I. Effects of deep brain stimulation on the kinematics of gait and balance in patients with idiopathic Parkinson’s disease. Clin. Biomech. 2022, 98, 105737. [Google Scholar] [CrossRef]
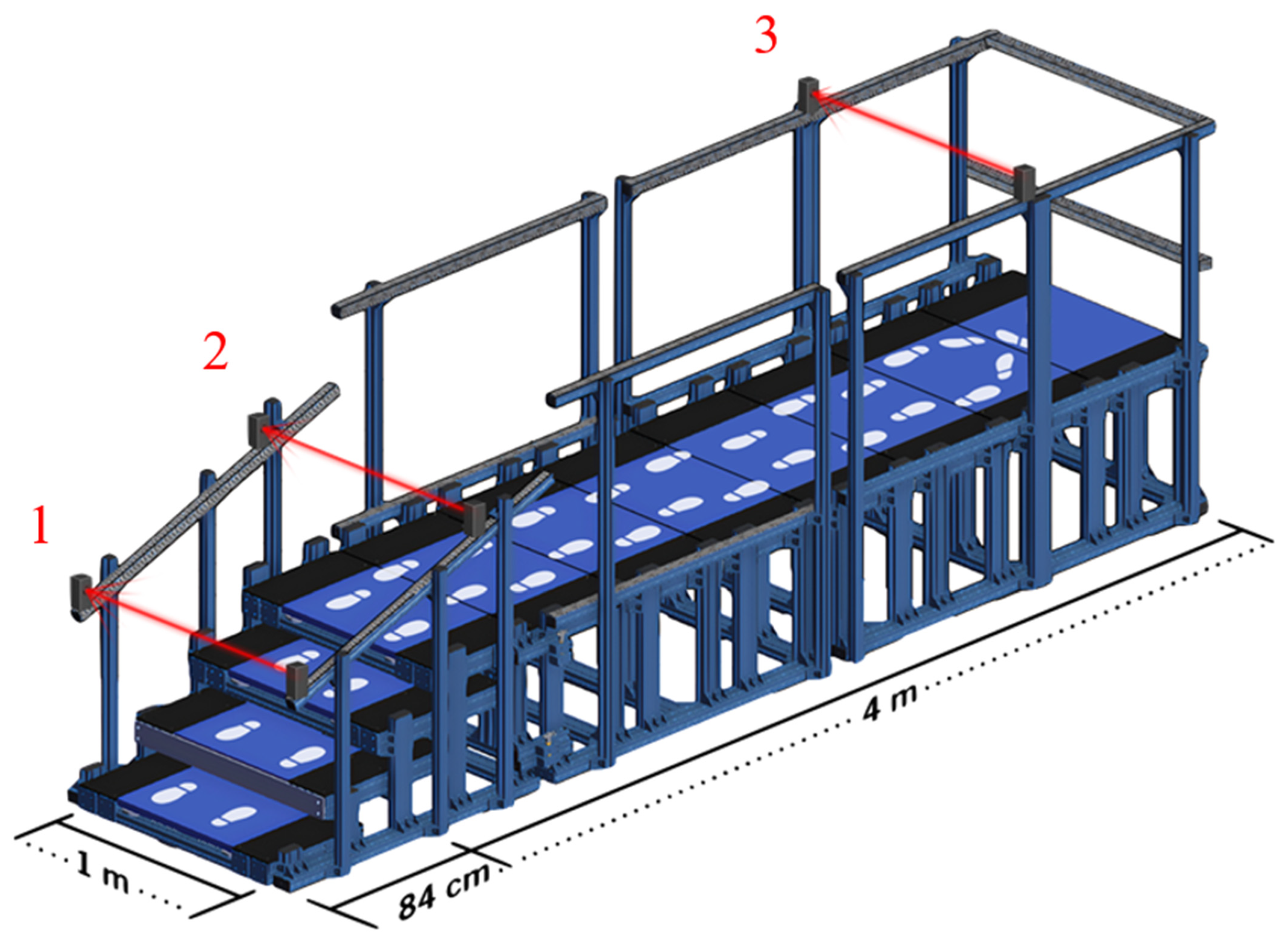
| Demographics | Overall (N = 17) |
|---|---|
| Age | 68.2 (7.8) |
| Race | |
| Black | 3 (17.6%) |
| White | 14 (82.4%) |
| Sex | |
| Female | 5 (29.4%) |
| Male | 12 (70.6%) |
| Body Mass Index | 27.4 (4.4) |
| Years of Education | 16.5 (1.8) |
| Hoehn and Yahr stage | |
| I | 1 (5.9%) |
| II | 10 (58.8%) |
| III | 6 (35.3%) |
| UPDRS Total Score | 41.7 (16.3) |
| Metric | ST | DT | Hedges’ g | p-Value |
|---|---|---|---|---|
| Average Step Time (s) A | 0.51, (0.44, 0.59) | 0.60, (0.52, 0.69) | 0.52 | 0.00015 |
| Normalized Braking Impulse (N·s/kg) | 0.17, (0.15, 0.20) | 0.20, (0.17, 0.22) | 0.48 | 0.0062 |
| Normalized Propulsive Impulse (N·s/kg) | 0.20, (0.18, 0.23) | 0.22, (0.19, 0.24) | 0.25 | 0.13 |
| AP range (cm) | 10.8, (9.4, 12.2) | 11.7, (10.3, 13.1) | 0.32 | 0.017 |
| Average CoP Speed ML (m/s) A | 1.07, (0.90, 1.26) | 0.95, (0.02, 1.12) | −0.35 | 0.0070 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, S.V.; Waltz, C.; Zimmerman, E.; Miller Koop, M.; Hastilow, K.; Alberts, J.L. Characterizing Stair Ambulation Kinetics and the Effects of Dual Tasking in Parkinson’s Disease. J. Clin. Med. 2025, 14, 5830. https://doi.org/10.3390/jcm14165830
Jones SV, Waltz C, Zimmerman E, Miller Koop M, Hastilow K, Alberts JL. Characterizing Stair Ambulation Kinetics and the Effects of Dual Tasking in Parkinson’s Disease. Journal of Clinical Medicine. 2025; 14(16):5830. https://doi.org/10.3390/jcm14165830
Chicago/Turabian StyleJones, Sumner V., Colin Waltz, Eric Zimmerman, Mandy Miller Koop, Karissa Hastilow, and Jay L. Alberts. 2025. "Characterizing Stair Ambulation Kinetics and the Effects of Dual Tasking in Parkinson’s Disease" Journal of Clinical Medicine 14, no. 16: 5830. https://doi.org/10.3390/jcm14165830
APA StyleJones, S. V., Waltz, C., Zimmerman, E., Miller Koop, M., Hastilow, K., & Alberts, J. L. (2025). Characterizing Stair Ambulation Kinetics and the Effects of Dual Tasking in Parkinson’s Disease. Journal of Clinical Medicine, 14(16), 5830. https://doi.org/10.3390/jcm14165830






