Integrated Multimodal Strategy to Reduce Healthcare-Associated Infections in a Trauma ICU: Impact of a Quality Improvement Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Aim
2.2. Study Design and Population
2.3. Data Collection and Variables
2.4. Quality Improvement Intervention
2.5. Statistical Analysis
3. Results
3.1. Patient Population Overview
3.2. Multivariable Logistic Regression Analysis
3.3. Impact of QIP on ICU Length Stay
3.4. Impact of QIP on Nosocomial Infection Rates
3.5. Effect of QIP on Antibiotic Use
3.6. Effects of QIP in Mechanical Ventilation Days
3.7. Subgroup Analysis
4. Discussion
5. Sustainability, Limitations, and the Romanian Context
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Report on Infection Prevention and Control; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC). Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals—2023; ECDC: Stockholm, Sweden, 2023.
- Despotovic, A.; Milosevic, B.; Milosevic, I.; Mitrovic, N.; Cirkovic, A.; Jovanovic, S.; Stevanovic, G. Hospital-acquired infections in the adult intensive care unit—Epidemiology, antimicrobial resistance patterns, and risk factors for acquisition and mortality. Am. J. Infect. Control 2020, 48, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Monegro, A.F.; Muppidi, V.; Regunath, H. Hospital Acquired Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441857/ (accessed on 27 June 2025).
- Rosenthal, V.D.; Al-Abdely, H.M.; El-Kholy, A.A.; AlKhawaja, S.A.A.; Leblebicioglu, H.; Mehta, Y.; Rai, V.; Hung, N.V.; Kanj, S.S.; Salama, M.F.; et al. International Nosocomial Infection Control Consortium Report, Data Summary of 50 Countries for 2010–2015: Device-Associated Module. Am. J. Infect. Control 2016, 44, 1495–1504. [Google Scholar] [CrossRef]
- Vincent, J.L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Murray, E.; Holmes, A.; Marra, A.R. The Politics and Ethics of Hospital Infection Prevention and Control: A Qualitative Case Study of Senior Clinicians’ Perceptions of Professional and Cultural Challenges. BMC Health Serv. Res. 2019, 19, 547. [Google Scholar]
- Gilbert, G.L.; Cheung, P.Y.; Kerridge, I.B. Infection control, ethics and accountability. Med. J. Aust. 2009, 190, 696–698. [Google Scholar] [CrossRef]
- Lynn, J.; Baily, M.A.; Bottrell, M.; Jennings, B.; Levine, R.J.; Davidoff, F.; Casarett, D.; Corrigan, J.; Fox, E.; Wynia, M.K.; et al. The ethics of using quality improvement methods in health care. Ann. Intern. Med. 2007, 146, 666–673. [Google Scholar] [CrossRef]
- Borgert, M.J.; Goossens, A.; Dongelmans, D.A. What Are Effective Strategies for the Implementation of Care Bundles on ICUs: A Systematic Review. Implement. Sci. 2015, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, V.D.; Bat-Erdene, I.; Gupta, D.; Belkebir, S.; Rajhans, P.; Zand, F.; Myatra, S.N.; Afeef, M.; Tanzi, V.L.; Muralidharan, S.; et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012–2017: Device-associated module. Am. J. Infect. Control 2020, 48, 423–432. [Google Scholar] [CrossRef]
- Bion, J.; Richardson, A.; Hibbert, P.; Beer, J.; Abrusci, T.; McCutcheon, M.; Cassidy, J.; Eddleston, J.; Gunning, K.; Bellingan, G.; et al. ‘Matching Michigan’: A 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual. Saf. 2013, 22, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Resar, R.; Griffin, F.A.; Haraden, C.; Nolan, T.W. Using Care Bundles to Improve Health Care Quality; Institute for Healthcare Improvement: Cambridge, MA, USA, 2012. [Google Scholar]
- Klompas, M.; Branson, R.; Eichenwald, E.C.; Greene, L.R.; Howell, M.D.; Lee, G.; Magill, S.S.; Maragakis, L.L.; Priebe, G.P.; Speck, K.; et al. Strategies to Prevent Ventilator-Associated Pneumonia in Acute Care Hospitals: 2014 Update. Infect. Control Hosp. Epidemiol. 2014, 35, 915–936. [Google Scholar] [CrossRef]
- Rosenthal, V.D.; Ramachandran, B.; Dueñas, L.; Alvarez-Moreno, C.; Navoa-Ng, J.A.; Armas-Ruiz, A.; Ersoz, G.; Matta-Cortés, L.; Pawar, M.; Nevzat-Yalcin, A.; et al. Findings of the International Nosocomial Infection Control Consortium (INICC), Part I: Effectiveness of a multidimensional infection control approach on catheter-associated urinary tract infection rates in pediatric intensive care units of 6 developing countries. Infect. Control Hosp. Epidemiol. 2012, 33, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Mouajou, V.; Adams, K.; DeLisle, G.; Quach, C. Hand hygiene compliance in the prevention of hospital-acquired infections: A systematic review. J. Hosp. Infect. 2022, 119, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Issa, M.; Dunne, S.S.; Dunne, C.P. Hand Hygiene Practices for Prevention of Health Care-Associated Infections Associated with Admitted Infectious Patients in the Emergency Department: A Systematic Review. Ir. J. Med. Sci. 2023, 192, 871–899. [Google Scholar] [CrossRef]
- Khalish, G.; Gautama, M.S.N. Hand Hygiene Compliance among Hospital Visitors: A Systematic Review and Meta-Analysis of Observational Studies. J. Infect. Prev. 2024, 25. in press. [Google Scholar] [CrossRef]
- Boyce, J.M. Current Issues in Hand Hygiene. Am. J. Infect. Control 2001, 29 (Suppl. S11), A35–A43. [Google Scholar]
- Alshagrawi, S.; Alhodaithy, N. Determinants of Hand Hygiene Compliance among Healthcare Workers in Intensive Care Units: A Qualitative Study. BMC Public Health 2024, 24, 2333. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.J.; Rogawski, E.; Thom, K.A.; Johnson, J.K.; Perencevich, E.N.; Shardell, M.; Leekha, S.; Harris, A.D. Transfer of multidrug-resistant bacteria to healthcare workers’ gloves and gowns after patient contact increases with environmental contamination. Crit. Care Med. 2012, 40, 1045–1051. [Google Scholar] [CrossRef]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2007.
- Morgan, D.J.; Murthy, R.; Munoz-Price, L.S.; Barnden, M.; Camins, B.C.; Johnston, B.L.; Rubin, Z.; Sullivan, K.V.; Shane, A.L.; Dellinger, E.P.; et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect. Control Hosp. Epidemiol. 2015, 36, 1163–1172. [Google Scholar] [CrossRef]
- Needleman, J.; Buerhaus, P.; Mattke, S.; Stewart, M.; Zelevinsky, K. Nurse-Staffing Levels and the Quality of Care in Hospitals. N. Engl. J. Med. 2002, 346, 1715–1722. [Google Scholar] [CrossRef]
- Tencic, M.; Roche, M.A. Nurse–Patient Ratios and Infection Control Practices: A Cross-Sectional Study. Collegian 2023, 30, 828–834. [Google Scholar] [CrossRef]
- Lake, E.T.; Sanders, J.; Duan, R.; Riman, K.A.; Schoenauer, K.M.; Chen, Y. A Meta-Analysis of the Associations Between the Nurse Work Environment in Hospitals and 4 Sets of Outcomes. Med. Care 2019, 57, 353–361. [Google Scholar] [CrossRef]
- Rogowski, J.A.; Staiger, D.; Patrick, T.; Horbar, J.; Kenny, M.; Lake, E.T. Nurse staffing and NICU infection rates. JAMA Pediatr. 2013, 167, 444–450. [Google Scholar] [CrossRef]
- Ball, J.E.; Bruyneel, L.; Aiken, L.H.; Sermeus, W.; Sloane, D.M.; Rafferty, A.M.; Lindqvist, R.; Tishelman, C.; Griffiths, P.; RN4Cast Consortium. Post-operative mortality, missed care and nurse staffing in nine countries: A cross-sectional study. Int. J. Nurs. Stud. 2018, 78, 10–15. [Google Scholar] [CrossRef]
- World Health Organization. Antibiotic Stewardship: Principles and Practice; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Mokrani, D.; Chommeloux, J.; Pineton de Chambrun, M.; Hékimian, G.; Luyt, C.-E. Antibiotic stewardship in the ICU: Time to shift into overdrive. Ann. Intensive Care 2023, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Schouten, J.; Hulscher, M.; Prins, J.M. Antimicrobial Stewardship in the ICU in COVID-19 Times: The Known Unknowns. Int. J. Antimicrob. Agents 2021, 58, 106409. [Google Scholar] [CrossRef]
- Timsit, J.-F.; Bassetti, M.; Cremer, O.; Daikos, G.; de Waele, J.; Kallil, A.; Kipnis, E.; Kollef, M.; Laupland, K.; Paiva, J.-A.; et al. Rationalizing antimicrobial therapy in the ICU: A narrative review. Intensive Care Med. 2019, 45, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, P.J.; Rohailla, S.; Taggart, L.R.; Lightfoot, D.; Havey, T.; Daneman, N.; Lowe, C.; Muller, M.P. Antimicrobial stewardship and intensive care unit mortality: A systematic review. Clin. Infect. Dis. 2019, 68, 748–756. [Google Scholar] [CrossRef]
- Vicentini, C.; Bussolino, R.; Gastaldo, C.; Castagnotto, M.; D’Ancona, F.P.; Zotti, C.M.; Working group “Unità Prevenzione Rischio Infettivo (UPRI); Regione Piemonte”. Level of implementation of multimodal strategies for infection prevention and control interventions and prevalence of healthcare-associated infections in Northern Italy. Antimicrob. Resist. Infect. Control 2024, 13, 39. [Google Scholar] [CrossRef]
- Moro, M.L. Multimodal Approach to Implement Infection Prevention and Control in Surgery. In Infections in Surgery; Springer: Cham, Switzerland, 2023; pp. 47–54. [Google Scholar]
- Pittet, D.; Hugonnet, S.; Harbarth, S.; Mourouga, P.; Sauvan, V.; Touveneau, S.; Perneger, T.V. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet 2000, 356, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Sonpar, A.; Hundal, C.O.; Totté, J.E.E.; Wang, J.; Klein, S.D.; Twyman, A.; Allegranzi, B.; Zingg, W. Multimodal strategies for the implementation of infection prevention and control interventions—Update of a systematic review for the WHO guidelines on core components of infection prevention and control programmes at the facility level. Clin. Microbiol. Infect. 2025, 31, 948–957. [Google Scholar] [CrossRef]
- Rinaldi, M.; Gatti, M.; Tonetti, T.; Nocera, D.; Ambretti, S.; Berlingeri, A.; Nigrisoli, G.; Pierucci, E.; Siniscalchi, A.; Pea, F.; et al. Impact of a multidisciplinary management team on clinical outcome in ICU patients affected by Gram-negative bloodstream infections: A pre-post quasi-experimental study. Ann. Intensive Care 2024, 14, 36. [Google Scholar] [CrossRef]
- World Health Organization, Country Office Romania. Country Assessment on Infection Prevention and Control Programmes and WASH in Healthcare Facilities; WHO Regional Office for Europe: Copenhagen, Denmark, 2023. [Google Scholar]
- Rosenthal, V.D.; Guzman, S.; Crnich, C.J. Effectiveness of a Multidimensional Infection Control Approach. Am. J. Infect. Control 2013, 41, 507–512. [Google Scholar]
- Sopirala, M.; Smyer, J.; Fawley, L.; Mangino, J.; Lustberg, M.; Lu, J.; Chucta, S.; Crouser, E. Sustained reduction of central line–associated bloodstream infections in an intensive care unit using a top-down and bottom-up approach. Am. J. Infect. Control 2013, 41, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.R.; Gonen, M.; Usiak, S.; Pottinger, T.; Samedy, P.; Patel, D.; Seo, S.; Smith, J.J.; Guillem, J.G.; Temple, L.; et al. Effectiveness of a multidisciplinary patient care bundle for reducing surgical-site infections. Br. J. Surg. 2018, 105, 1680–1687. [Google Scholar] [CrossRef]
- Furuya, E.Y.; Dick, A.; Perencevich, E.N.; Pogorzelska, M.; Goldmann, D.; Stone, P.W. Central line–associated bloodstream infection reduction and bundle compliance in intensive care units: A national study. Infect. Control Hosp. Epidemiol. 2016, 37, 805–810. [Google Scholar] [CrossRef]
- Timsit, J.F.; Ruppé, E.; Barbier, F.; Tabah, A.; Bassetti, M. Bloodstream infections in critically ill patients: An expert statement. Intensive Care Med. 2020, 46, 266–284. [Google Scholar] [CrossRef]
- Potugari, B.R.; Umukoro, P.E.; Vedre, J.G. Multimodal intervention approach reduces catheter-associated urinary tract infections in a rural tertiary care center. Clin. Med. Res. 2020, 18, 140–144. [Google Scholar] [CrossRef]
- Aiken, L.H.; Sloane, D.M.; Bruyneel, L.; Van den Heede, K.; Griffiths, P.; Busse, R.; Diomidous, M.; Kinnunen, J.; Kózka, M.; Lesaffre, E.; et al. Nurse Staffing and Education and Hospital Mortality in Nine European Countries: A Retrospective Observational Study. Lancet 2014, 383, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Roberts, D.; Wood, K.E. Duration of Inappropriate Antimicrobial Therapy Is Associated with Increased Mortality in Septic Shock. Chest 2009, 136, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Ministerul Sănătății. Ordinul Nr. 1500/2009 Privind Aprobarea Regulamentului de Organizare și Funcționare a Secțiilor și Compartimentelor ATI. Monitorul Oficial al României. 2009. Available online: https://legislatie.just.ro/Public/DetaliiDocumentAfis/209803 (accessed on 11 August 2025).
- British Association of Critical Care Nurses. BACCN Standards for Nurse Staffing in Critical Care; British Association of Critical Care Nurses: Newcastle upon Tyne, UK, 2010. [Google Scholar]
- American Association of Critical-Care Nurses. Standards Published for Critical Care Nurse Staffing; American Association of Critical-Care Nurses: Aliso Viejo, CA, USA, 2024. [Google Scholar]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of Adverse Events with Antimicrobial Therapy in Hospitalized Patients. Clin. Infect. Dis. 2017, 177, 1308–1315. [Google Scholar]
- Bell, M.M.; Johnson, C.C.; Zomorodi, M. Preventing CAUTIs via Multidisciplinary Education. Care Manag. J. 2020, 21, 1533. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and using data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
| Category | Specific Measures Implemented |
|---|---|
| Standard IPC Strategies | Hand hygiene per WHO’s 5 moments (training and monitoring) |
| Mandatory PPE use: gloves, gown, cap for all patient contact | |
| Surface disinfection: 3× weekly (surfaces), 2–3× daily (floors) | |
| Use of sterile gloves for invasive/aseptic procedures | |
| Targeted IPC Strategies | Ventilator-Associated Pneumonia (VAP): |
| Daily oral care with chlorhexidine | |
| Daily sedation break, cuff pressure monitoring (20–30 cm H2O) | |
| Closed suction systems, subglottic suction ETT, passive humidification Sterile suctioning via orotracheal tube using sterile gloves and sterile suction catheter | |
| Central Line-Associated BSI (CLABSI): | |
| Maximal sterility during insertion, antimicrobial-impregnated catheters | |
| Dressing change every 7 days, port disinfection, daily IV set change | |
| Catheter-Associated UTI (CAUTI): | |
| Aseptic insertion, closed drainage system | |
| Catheter secured above thigh, bag below bladder, replaced every 14 days or sooner if needed | |
| Staffing Optimization | Nurse-to-patient ratio improved from 1:4 (pre-QIP) to 1:2 (post-QIP) |
| Antibiotic Stewardship | Local antibiogram-guided de-escalation protocols |
| Avoiding treatment solely based on positive cultures | |
| Regular screening for MDR colonization | |
| Monitoring and reduction of total antibiotic consumption |
| Parameter | Pre-QIP Group (n = 35) | Post-QIP Group (n = 43) | p-Value |
|---|---|---|---|
| Age (years) | 47.54 ± 18.29 | 48.49 ± 19.35 | 0.8 |
| Male/Female (%) | 85.71/14.29 | 60.47/39.53 | 0.02 |
| ICU (days) | 16.51 ± 22.12 | 15.35 ± 15.00 | 0.79 |
| ISS Score | 34.45 ± 14.80 | 31.29 ± 12.80 | 0.39 |
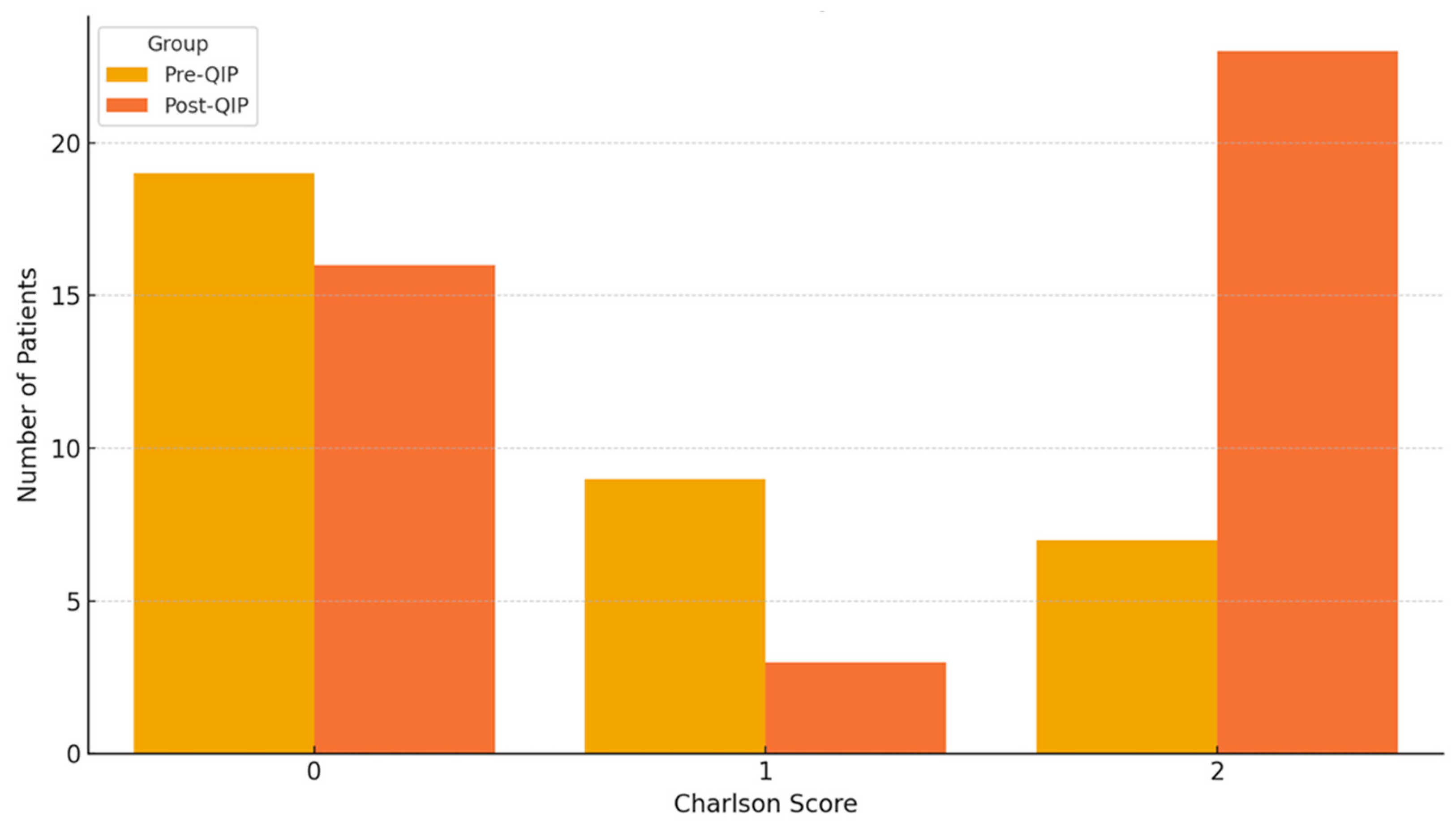
| Charlson Comorbidity Index | 0.76 ± 0.89 | 1.14 ± 0.91 | 0.07 |
| Antibiotic Use | 11.99 ± 17.89 | 9.00 ± 958 | 0.38 |
| Mechanical Ventilation (days) | 14.03 ± 21.59 | 12.35 ± 12.71 | 0.68 |
| Nosocomial infection (number) | 1.41 ± 1.97 | 0.60 ± 0.95 | 0.03 |
| Mortality | 0.34 | 0.19 | 0.18 |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| Pre-QIP group (vs. post-QIP) | 11.06 | 0.98–124.70 | 0.05 |
| Male (vs. Female) | 37.38 | 1.27–1104.56 | 0.03 |
| Age (per year) | 1.07 | 1.00–1.14 | 0.04 |
| ISS score | 1.09 | 1.03–1.16 | 0.006 |
| Variable | Coefficient | 95% CI | p-Value |
|---|---|---|---|
| Pre-QIP group (vs. post-QIP) | −4.37 | −13.18 to 4.44 | 0.32 |
| Male (vs. female) | 3.38 | −6.64 to 13.39 | 0.5 |
| Age (per year) | −0.25 | −0.56 to 0.06 | 0.10 |
| ISS score | 0.31 | −0.01 to 0.63 | 0.05 |
| Charlson Comorbidity Index | 8.60 | 2.28 to 14.93 | 0.008 |
| Variable | IRR | 95% CI | p-Value |
|---|---|---|---|
| Pre-QIP group (vs. post-QIP) | 1.22 | 0.69–2.17 | 0.49 |
| Male (vs. female) | 3.94 | 1.39–11.19 | 0.009 |
| Age (per year) | 0.99 | 0.97–1.01 | 0.49 |
| ISS score | 1.03 | 1.01–1.05 | 0.005 |
| Charlson Comorbidity Index | 1.71 | 1.14–2.55 | 0.008 |
| Variable | Coefficient | 95% CI | p-Value |
|---|---|---|---|
| Pre-QIP group (vs. post-QIP) | −0.79 | −7.24 to 5.65 | 0.80 |
| Male (vs. female) | 5.36 | −1.96 to 12.68 | 0.14 |
| Age (per year) | −0.03 | −0.26 to 0.19 | 0.75 |
| ISS score | 0.28 | 0.05 to 0.51 | 0.01 |
| Charlson Comorbidity Index | 4.57 | −0.05 to 9.20 | 0.05 |
| Variable | Coefficient | 95% CI | p-Value |
|---|---|---|---|
| Pre-QIP group (vs. post-QIP) | −3.34 | −11.51 to 4.84 | 0.41 |
| Male (vs. female) | 2.70 | −6.58 to 11.99 | 0.55 |
| Age (per year) | −0.15 | −0.44 to 0.13 | 0.28 |
| ISS score | 0.31 | 0.02 to 0.61 | 0.03 |
| Charlson Comorbidity Index | 6.97 | 0.08 to 1.55 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, D.; Păpurică, M.; Rogobete, A.; Ghenciu, L.A.; Băloi, A.; Bârsac, C.R.; Bedreag, O.H.; Gizea, C.A.; Haţegan, O.A.; Săndesc, D. Integrated Multimodal Strategy to Reduce Healthcare-Associated Infections in a Trauma ICU: Impact of a Quality Improvement Project. J. Clin. Med. 2025, 14, 5826. https://doi.org/10.3390/jcm14165826
Toma D, Păpurică M, Rogobete A, Ghenciu LA, Băloi A, Bârsac CR, Bedreag OH, Gizea CA, Haţegan OA, Săndesc D. Integrated Multimodal Strategy to Reduce Healthcare-Associated Infections in a Trauma ICU: Impact of a Quality Improvement Project. Journal of Clinical Medicine. 2025; 14(16):5826. https://doi.org/10.3390/jcm14165826
Chicago/Turabian StyleToma, Daiana, Marius Păpurică, Alexandru Rogobete, Laura Andreea Ghenciu, Adelina Băloi, Claudiu Rafael Bârsac, Ovidiu Horea Bedreag, Carmen Alina Gizea, Ovidiu Alin Haţegan, and Dorel Săndesc. 2025. "Integrated Multimodal Strategy to Reduce Healthcare-Associated Infections in a Trauma ICU: Impact of a Quality Improvement Project" Journal of Clinical Medicine 14, no. 16: 5826. https://doi.org/10.3390/jcm14165826
APA StyleToma, D., Păpurică, M., Rogobete, A., Ghenciu, L. A., Băloi, A., Bârsac, C. R., Bedreag, O. H., Gizea, C. A., Haţegan, O. A., & Săndesc, D. (2025). Integrated Multimodal Strategy to Reduce Healthcare-Associated Infections in a Trauma ICU: Impact of a Quality Improvement Project. Journal of Clinical Medicine, 14(16), 5826. https://doi.org/10.3390/jcm14165826







