Effect of Food Intake on Vortex Formation Time as a Measurement of Diastolic Left Ventricular Function
Abstract
1. Introduction
2. Material and Method
2.1. Study Population
2.2. Procedure
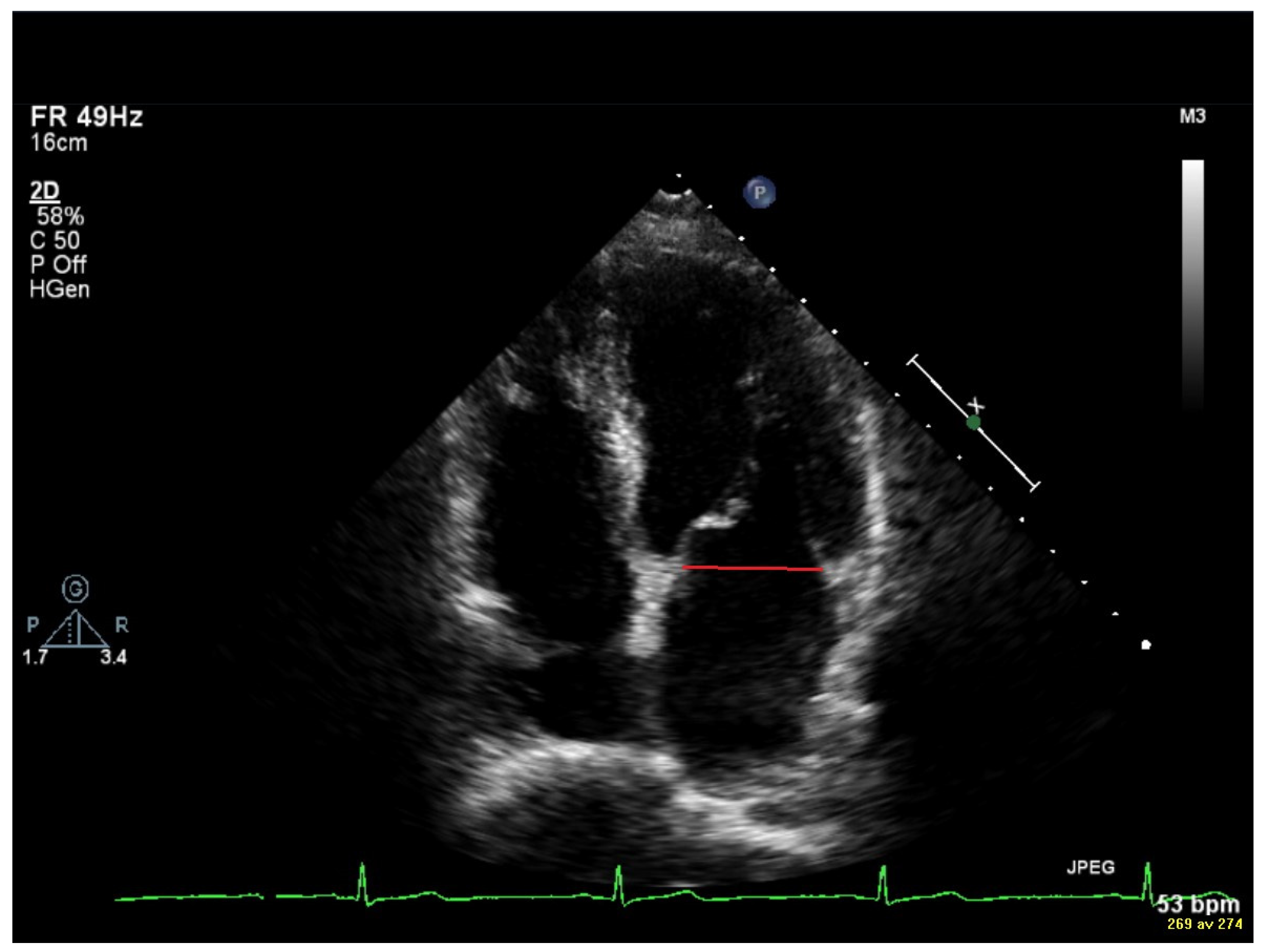
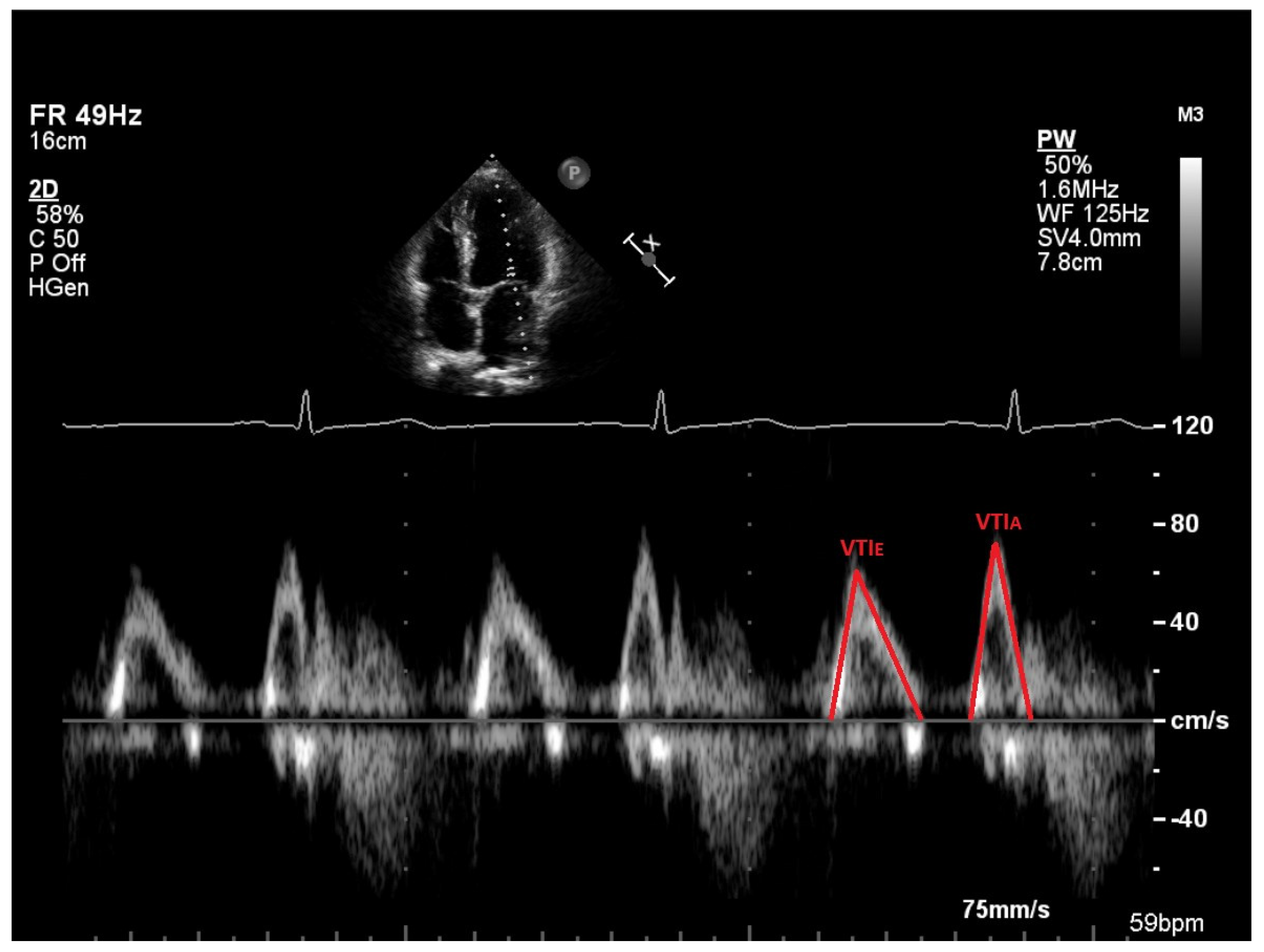
2.3. Vortex Formation and Vortex Formation Time
2.4. Transthoracic Echocardiography (TTE)
2.5. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waaler, B.A.; Hisdal, J.; Eriksen, M. Circulatory Responses to a Meal in Patients with a Newly Transplanted Heart. Acta Physiol. Scand. 2002, 174, 101–108. [Google Scholar] [CrossRef]
- Fagan, T.C.; Sawyer, P.R.; Gourley, L.A.; Lee, J.T.; Gaffney, T.E. Postprandial Alterations in Hemodynamics and Blood Pressure in Normal Subjects. Am. J. Cardiol. 1986, 58, 636–641. [Google Scholar] [CrossRef]
- Gårdinger, Y.; Malmgren, A.; Hlebowicz, J.; Dencker, M. Effect of Food Intake on Echocardiographic Measurements in Healthy Elderly. Echocardiography 2022, 39, 811–818. [Google Scholar] [CrossRef]
- Dencker, M.; Björgell, O.; Hlebowicz, J. Effect of Food Intake on Commonly Used Pulsed Doppler and Tissue Doppler Measurements. Echocardiography 2011, 28, 843–847. [Google Scholar] [CrossRef]
- Dieden, A.; Gårdinger, Y.; Hlebowicz, J.; Björgell, O.; Dencker, M. Effect of Food Intake on Left and Right Ventricular Systolic Tissue Doppler Measurements. Clin. Physiol. Funct. Imaging 2016, 36, 396–400. [Google Scholar] [CrossRef]
- Visby, L.; Møgelvang, R.; Grund, F.F.; Myhr, K.A.; Hassager, C.; Vejlstrup, N.; Mattu, R.; Kristensen, C.B. The Influence of Food Intake and Preload Augmentation on Cardiac Functional Parameters: A Study Using Both Cardiac Magnetic Resonance and Echocardiography. J. Clin. Med. 2023, 12, 6781. [Google Scholar] [CrossRef] [PubMed]
- Waaler, B.A.; Hisdal, J.; Ihlen, H.; Kjekshus, J. Mechanisms behind the Postprandial Increase in Cardiac Output: A Clue Obtained from Transplanted Hearts. Eur. J. Appl. Physiol. 2006, 97, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, D.M.; Chan, W.L.; Ang, E.L.; Oakley, C.M. Effects of a Meal on Hemodynamic Function at Rest and during Exercise in Patients with Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 1991, 18, 429–436. [Google Scholar] [CrossRef][Green Version]
- Evangelista, A.; Flachskampf, F.; Lancellotti, P.; Badano, L.; Aguilar, R.; Monaghan, M.; Zamorano, J.; Nihoyannopoulos, P.; on behalf of the European Association of Echocardiography. European Association of Echocardiography Recommendations for Standardization of Performance, Digital Storage and Reporting of Echocardiographic Studies. Eur. J. Echocardiogr. 2008, 9, 438–448. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Carlhäll, C.J.; Bolger, A. Passing Strange: Flow in the Failing Ventricle. Circ. Heart Fail. 2010, 3, 326–331. [Google Scholar] [CrossRef]
- Ghosh, E.; Kovács, S.J. The Vortex Formation Time to Diastolic Function Relation: Assessment of Pseudonormalized versus Normal Filling. Physiol. Rep. 2013, 1, e00170. [Google Scholar] [CrossRef] [PubMed]
- Poh, K.K.; Lee, L.C.; Shen, L.; Chong, E.; Tan, Y.L.; Chai, P.; Yeo, T.C.; Wood, M.J. Left Ventricular Fluid Dynamics in Heart Failure: Echocardiographic Measurement and Utilities of Vortex Formation Time. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 385–393. [Google Scholar] [CrossRef]
- Gharib, M.; Rambod, E.; Kheradvar, A.; Sahn, D.J.; Dabiri, J.O. Optimal Vortex Formation as an Index of Cardiac Health. Proc. Natl. Acad. Sci. USA 2006, 103, 6305–6308. [Google Scholar] [CrossRef] [PubMed]
- Cirovic, S.; Malmgren, A.; Kurdie, R.; Bilal, D.; Dencker, M.; Gudmundsson, P. Vortex Formation Time in Female Athletes. Int. J. Cardiovasc. Imaging 2024, 40, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kheradvar, A.; Assadi, R.; Falahatpisheh, A.; Sengupta, P.P. Assessment of Transmitral Vortex Formation in Patients with Diastolic Dysfunction. J. Am. Soc. Echocardiogr. 2012, 25, 220–227. [Google Scholar] [CrossRef]
- Dencker, M.; Stagmo, M. Reported Normal Values and Weighted Means for Commonly Used Echocardiography Pulsed Doppler and Tissue Doppler Measurements. Clin. Physiol. Funct. Imaging 2018, 38, 341–350. [Google Scholar] [CrossRef]
- Pugh, K.G.; Wei, J.Y. Clinical Implications of Physiological Changes in the Aging Heart. Drugs Aging 2001, 18, 263–276. [Google Scholar] [CrossRef]
- Strait, J.B.; Lakatta, E.G. Aging-Associated Cardiovascular Changes and Their Relationship to Heart Failure. Heart Fail. Clin. 2012, 8, 143–164. [Google Scholar] [CrossRef]
- Du Bois, D.; Du Bois, E.F. A Formula to Estimate the Approximate Surface Area If Height and Weight Be Known. 1916. Nutr. Burbank Los Angel. Cty. Calif 1989, 5, 303–313. [Google Scholar]
- Faludi, R.; Szulik, M.; D’hooge, J.; Herijgers, P.; Rademakers, F.; Pedrizzetti, G.; Voigt, J.-U. Left Ventricular Flow Patterns in Healthy Subjects and Patients with Prosthetic Mitral Valves: An In Vivo Study Using Echocardiographic Particle Image Velocimetry. J. Thorac. Cardiovasc. Surg. 2010, 139, 1501–1510. [Google Scholar] [CrossRef]
- Gharib, M.; Rambod, E.; Shariff, K. A Universal Time Scale for Vortex Ring Formation. J. Fluid Mech. 1998, 360, 121–140. [Google Scholar] [CrossRef]
- Dabiri, J.O.; Gharib, M. Delay of Vortex Ring Pinchoff by an Imposed Bulk Counterflow. Phys. Fluids 2004, 16, 28–30. [Google Scholar] [CrossRef]
- Mohseni, K.; Gharib, M. A Model for Universal Time Scale of Vortex Ring Formation. Phys. Fluids 1998, 10, 2436–2438. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, J.O.; Gharib, M. The Role of Optimal Vortex Formation in Biological Fluid Transport. Proc. R. Soc. B Biol. Sci. 2005, 272, 1557–1560. [Google Scholar] [CrossRef]
- Pasipoularides, A.; Vlachos, P.P.; Little, W.C. Vortex Formation Time Is Not an Index of Ventricular Function. J Cardiovasc. Transl. Res. 2015, 8, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Stewart, K.C.; Charonko, J.C.; Niebel, C.L.; Little, W.C.; Vlachos, P.P. Left Ventricular Vortex Formation Is Unaffected by Diastolic Impairment. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Gårdinger, Y.; Hlebowicz, J.; Björgell, O.; Dencker, M. Effect of Food Intake on Left Ventricular Wall Stress. Cardiovasc. Ultrasound 2014, 12, 2. [Google Scholar] [CrossRef]
- Gårdinger, Y.; Dieden, A.; Hlebowicz, J.; Björgell, O.; Dencker, M. Effect of Food Intake on Myocardial Performance Index. Cardiovasc. Ultrasound 2017, 15, 10. [Google Scholar] [CrossRef]
- Nuzzi, V.; Raafs, A.; Manca, P.; Henkens, M.T.H.M.; Gregorio, C.; Boscutti, A.; Verdonschot, J.; Hazebroek, M.; Knackstedt, C.; Merlo, M.; et al. Left Atrial Reverse Remodeling in Dilated Cardiomyopathy. J. Am. Soc. Echocardiogr. 2023, 36, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Katbamna, B.; Wu, L.; Rodriguez, M.; King, P.; Schilling, J.; Mahar, J.; Nair, A.P.; Jneid, H.; Klings, E.S.; Weinhouse, G.L.; et al. The Uses of Right Heart Catheterization in Cardio-Pulmonary Disease: State-of-the-Art. Am. Heart J. Plus Cardiol. Res. Pract. 2025, 49, 100488. [Google Scholar] [CrossRef]
- Jiamsripong, P.; Calleja, A.M.; Alharthi, M.S.; Dzsinich, M.; McMahon, E.M.; Heys, J.J.; Milano, M.; Sengupta, P.P.; Khandheria, B.K.; Belohlavek, M. Impact of Acute Moderate Elevation in Left Ventricular Afterload on Diastolic Transmitral Flow Efficiency: Analysis by Vortex Formation Time. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2009, 22, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Jiamsripong, P.; Alharthi, M.S.; Calleja, A.M.; McMahon, E.M.; Katayama, M.; Westerdale, J.; Milano, M.; Heys, J.J.; Mookadam, F.; Belohlavek, M. Impact of Pericardial Adhesions on Diastolic Function as Assessed by Vortex Formation Time, a Parameter of Transmitral Flow Efficiency. Cardiovasc. Ultrasound 2010, 8, 42. [Google Scholar] [CrossRef]
- Jiamsripong, P.; Calleja, A.M.; Alharthi, M.S.; Cho, E.J.; McMahon, E.M.; Heys, J.J.; Milano, M.; Sengupta, P.P.; Khandheria, B.K.; Belohlavek, M. Increase in the Late Diastolic Filling Force Is Associated With Impaired Transmitral Flow Efficiency in Acute Moderate Elevation of Left Ventricular Afterload. J. Ultrasound Med. 2009, 28, 175–182. [Google Scholar] [CrossRef]
- Belohlavek, M.; Jiamsripong, P.; Calleja, A.M.; McMahon, E.M.; Maarouf, C.L.; Kokjohn, T.A.; Chaffin, T.L.; Vedders, L.J.; Garami, Z.; Beach, T.G.; et al. Patients With Alzheimer Disease Have Altered Transmitral Flow. J. Ultrasound Med. 2009, 28, 1493–1500. [Google Scholar] [CrossRef]
- Ricci, F.; Aung, N.; Gallina, S.; Zemrak, F.; Fung, K.; Bisaccia, G.; Paiva, J.M.; Khanji, M.Y.; Mantini, C.; Palermi, S.; et al. Cardiovascular Magnetic Resonance Reference Values of Mitral and Tricuspid Annular Dimensions: The UK Biobank Cohort. J. Cardiovasc. Magn. Reson. 2021, 23, 5. [Google Scholar] [CrossRef] [PubMed]
| Median (IQR) | ||
|---|---|---|
| Number of participants | 23 (12 females, 11 males) | 30 (15 females, 15 males) |
| Age (years) | 25 (24–28) | 68 (66–69) |
| Height (cm) | 177 (168–184) | 172 (163–181) |
| Weight (kg) | 65 (58–80) | 75 (59–82) |
| BSA (m2) | 1.8 (1.7–2.0) | 1.9 (1.6–2.0) |
| BMI (kg/m2) | 21.6 (20.2–23.1) | 24.3 (22.1–26.0) |
| Variable | Fasting Median (IQR) | 30 min After Food Intake Median (IQR) | p-Value | Percent Change Fasting Versus 30 min (%) |
|---|---|---|---|---|
| SBP (mm Hg) | 103 (97–110) | 103 (93–110) | 0.760 | 0 |
| DBP (mm Hg) | 65 (62–70) | 58 (52–62) | <0.001 | −11 |
| HR (bpm) | 60 (51–68) | 64 (55–72) | 0.009 | 7 |
| LA volume (mL) | 48.0 (43.5–58.1) | 52.3 (47.8–61.0) | 0.010 | 8.9 |
| E/A ratio | 1.8 (1.6–2.2) | 1.7 (1.5–2.0) | 0.104 | −5.6 |
| E/é ratio (average) | 5.1 (4.5–5.7) | 5.2 (4.7–5.8) | 0.456 | 2.0 |
| VTI E (cm) | 11.8 (10.8–13.5) | 12.1 (11.2–13.3) | 0.761 | 2.5 |
| VTI A (cm) | 4.2 (3.4–5.0) | 4.9 (4.2–6.1) | 0.002 | 16.7 |
| MAD (cm) | 3.0 (2.8–3.2) | 3.0 (2.8–3.2) | 0.878 | 0 |
| EDV (mL) | 130.7 (104.3–151.4) | 131.0 (112.8–153.4) | 0.101 | 0.2 |
| ESV (mL) | 55.7 (49.0–70.7) | 54.0 (49.0–62.3) | 0.153 | −3.1 |
| LVEF (%) | 54 (52.5–57.3) | 56.4 (54.2–59.9) | <0.001 | 4.4 |
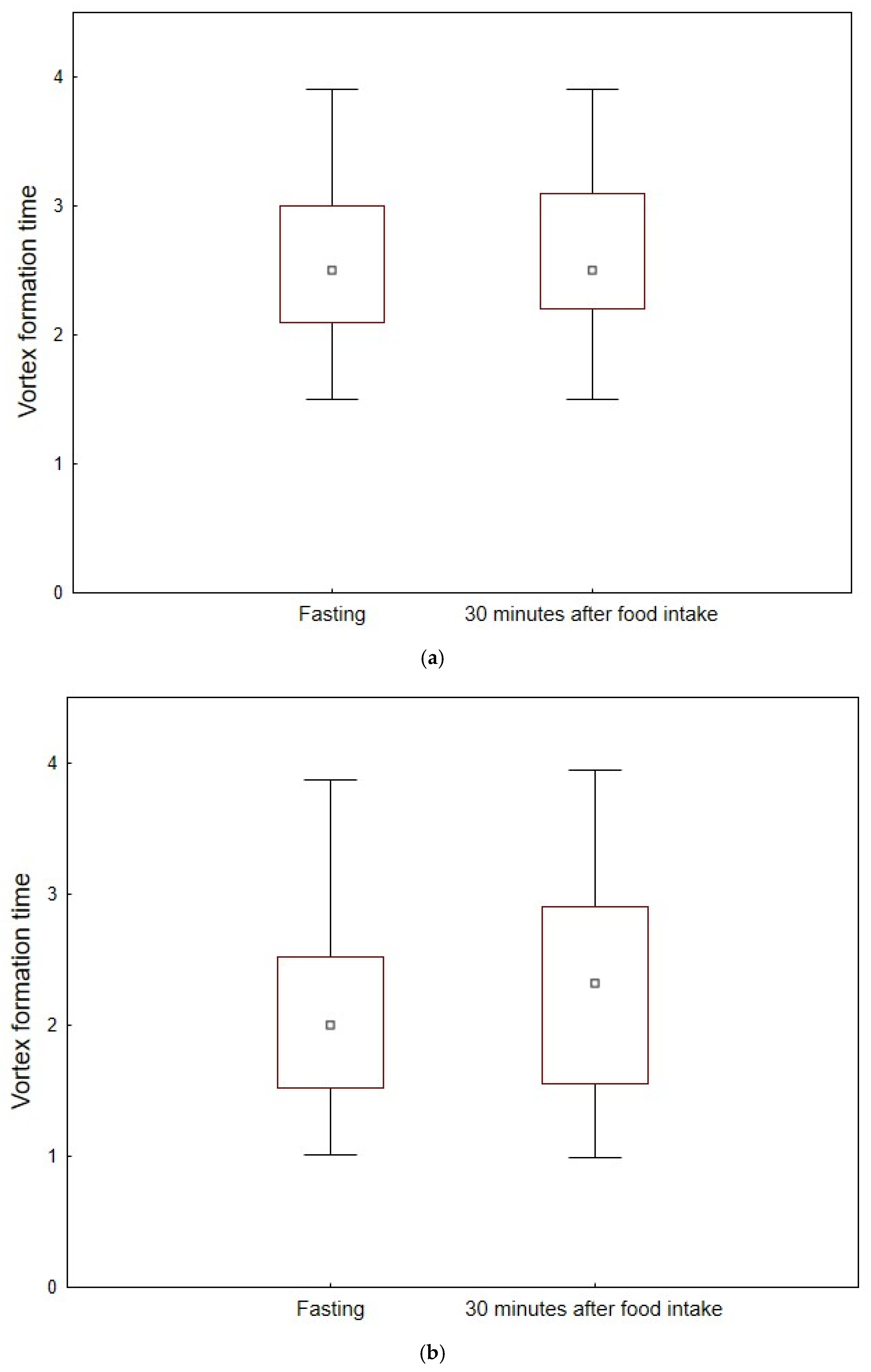
| VFT | 2.5 (2.1–3.0) | 2.5 (2.2–3.1) | 0.369 | 0 |
| Variable | Fasting Median (IQR) | 30 min After Food Intake Median (IQR) | p-Value | Percent Change Fasting Versus 30 min (%) |
|---|---|---|---|---|
| SBP (mm Hg) | 127 (116–137) | 124 (112–130) | 0.021 | −2 |
| DBP (mm Hg) | 78 (74–84) | 72 (66–75) | <0.001 | −8 |
| HR (bpm) | 61 (57–67) | 65 (60–68) | 0.009 | 7 |
| LA volume (mL) | 53.7 (44.6–65.8) | 54.2 (49.4–67.2) | 0.012 | 1 |
| E/A ratio | 1.0 (0.8–1.1) | 1.0 (0.8–1.2) | 0.039 | 0 |
| E/é ratio (average) | 7.6 (6.5–8.9) | 7.8 (6.7–8.6) | 0.371 | 2.6 |
| VTI E (cm) | 10.5 (9.0–11.7) | 12.3 (10.6–14.8) | <0.001 | 17.1 |
| VTI A (cm) | 7.1 (6.2–7.5) | 7.5 (6.8–8.3) | <0.001 | 5.6 |
| MAD (cm) | 2.9 (2.7–3.1) | 2.9 (2.7–3.1) | 0.935 | 0 |
| EDV (mL) | 90.4 (81.6–111.5) | 91.1 (77.3–113.6) | 0.453 | 0.8 |
| ESV (mL) | 32.5 (27.7–41.0) | 29.6 (22.1–36.4) | <0.001 | −9.0 |
| LVEF (%) | 64.9 (62.3–66.3) | 68.9 (66.6–72.0) | <0.001 | 6.2 |
| VFT | 2.0 (1.5–2.5) | 2.3 (1.5–2.9) | <0.001 | 15 |
| Variable | Fasting | 30 min After Food Intake | ||||
|---|---|---|---|---|---|---|
| Younger Population Median (IQR) | Older Population Median (IQR) | p-Value | Younger Population Median (IQR) | Older Population Median (IQR) | p-Value | |
| MAD (cm) | 3.0 (2.8–3.2) | 2.9 (2.7–3.1) | 0.193 | 3.0 (2.8–3.2) | 2.9 (2.7–3.1) | 0.122 |
| VTI E (cm) | 11.8 (10.8–13.5) | 10.5 (9.0–11.7) | 0.005 | 12.1 (11.2–13.3) | 12.3 (10.6–14.8) | 0.851 |
| VTI A(cm) | 4.2 (3.4–5.0) | 7.1 (6.2–7.5) | <0.001 | 4.9 (4.2–6.1) | 7.5 (6.8–8.3) | <0.001 |
| EDV (mL) | 130.7 (104.3–151.4) | 90.4 (81.6–111.5) | <0.001 | 131.0 (112.8–153.4) | 91.1 (77.3–113.6) | <0.001 |
| LVEF (%) | 54 (52.5–57.3) | 64.9 (62.3–66.3) | <0.001 | 56.4 (54.2–59.9) | 68.9 (66.6–72.0) | <0.001 |
| VFT | 2.5 (2.1–3.0) | 2.0 (1.5–2.5) | 0.011 | 2.5 (2.2–3.1) | 2.3 (1.5–2.9) | 0.172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, S.; Malmgren, A.; Gårdinger, Y.; Hlebowicz, J.; Dencker, M. Effect of Food Intake on Vortex Formation Time as a Measurement of Diastolic Left Ventricular Function. J. Clin. Med. 2025, 14, 5783. https://doi.org/10.3390/jcm14165783
Smith S, Malmgren A, Gårdinger Y, Hlebowicz J, Dencker M. Effect of Food Intake on Vortex Formation Time as a Measurement of Diastolic Left Ventricular Function. Journal of Clinical Medicine. 2025; 14(16):5783. https://doi.org/10.3390/jcm14165783
Chicago/Turabian StyleSmith, Sarah, Andreas Malmgren, Ylva Gårdinger, Joanna Hlebowicz, and Magnus Dencker. 2025. "Effect of Food Intake on Vortex Formation Time as a Measurement of Diastolic Left Ventricular Function" Journal of Clinical Medicine 14, no. 16: 5783. https://doi.org/10.3390/jcm14165783
APA StyleSmith, S., Malmgren, A., Gårdinger, Y., Hlebowicz, J., & Dencker, M. (2025). Effect of Food Intake on Vortex Formation Time as a Measurement of Diastolic Left Ventricular Function. Journal of Clinical Medicine, 14(16), 5783. https://doi.org/10.3390/jcm14165783






