Long COVID in Healthcare Workers from a Pediatric Hospital in Romania: A Cross-Sectional Study of Prevalence, Symptom Burden, and the Role of Vaccination and Reinfection
Abstract
1. Introduction
2. Materials and Methods
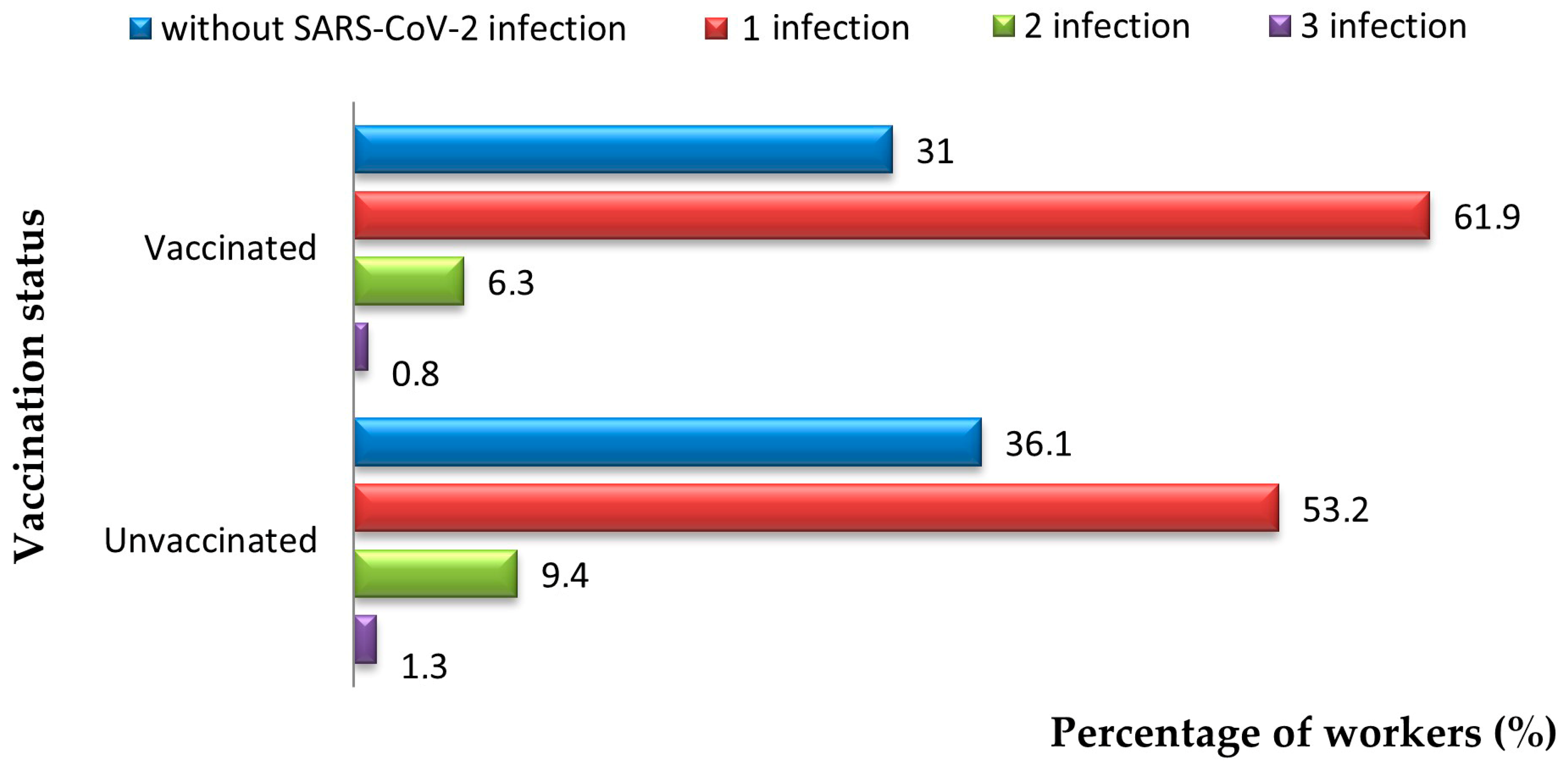
3. Results
- -
- Moderate fatigue (31%), with an average duration of approximately 11 weeks, and severe fatigue (22.5%), with an average duration of approximately 15 weeks;
- -
- Moderate headache (12%), with an average duration of approximately 7 weeks;
- -
- Mild loss of smell in 32.9% of patients, with an average duration of approximately 12 weeks;
- -
- Mild loss of taste in 17.1% of patients, with an average duration of approximately 12 weeks;
- -
- Moderate joint pain (21.3%), with an average duration of approximately 13 weeks;
- -
- Moderate myalgia (14.7%), with an average duration of approximately 13 weeks;
- -
- Moderate balance disorders (8.1%), with an average duration of over 11 weeks;
- -
- Moderate dizziness (11.6%), with an average duration of approximately 10 weeks;
- -
- Moderate dyspnea (11.2%), with an average duration of approximately 12 weeks;
- -
- Persistent cough of moderate intensity (20.5%), with an average duration of approximately 10 weeks;
- -
- Moderate diarrhea (3.1%), with an average duration of over 3 weeks.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| % | Percentage |
| ANOVA | Analysis of Variance |
| AUC | Area Under the Curve |
| CI | Confidence Interval |
| COVID-19 | Coronavirus Disease 2019 |
| HCWs | Healthcare Workers |
| p-value | Probability Value (in statistical significance testing) |
| n | variable |
| r | Pearson’s correlation coefficient |
| ROC curve | Receiver Operating Characteristic curve |
| SD | Standard Deviation |
| SPSS | Statistical Package for the Social Sciences |
References
- Davis, H.; McCorkell, L.; Vogel, J.; Topol, E. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef]
- Mehandru, S.; Merad, M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022, 23, 194–202. [Google Scholar] [CrossRef]
- Montani, D.; Savale, L.; Noel, N.; Meyrignac, O.; Colle, R.; Gasnier, M.; Corruble, E.; Beurnier, A.; Jutant, E.M.; Pham, T.; et al. Post-Acute COVID-19 Syndrome. Eur. Respir. Rev. 2022, 31, 210185. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Mohan, A.; Upadhyay, V. Long COVID Syndrome: An unfolding enigma. Indian J. Med. Res. 2024, 159, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Nittas, V.; Gao, M.; West, E.A.; Ballouz, T.; Menges, D.; Wulf Hanson, S.; Puhan, M.A. Long COVID through a public health lens: An umbrella review. Public Health Rev. 2022, 43, 1604501. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Ayuzo Del Valle, N.C.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef]
- Nehme, M.; Vieux, L.; Kaiser, L.; Chappuis, F.; Chenaud, C.; HealthCo Study Team; Guessous, I. The longitudinal study of subjective wellbeing and absenteeism of healthcare workers considering post-COVID condition and the covid-19 pandemic toll. Sci. Rep. 2023, 13, 10759. [Google Scholar] [CrossRef]
- Prazeres, F.; Romualdo, A.P.; Campos Pinto, I.; Silva, J.; Oliveira, A.M. The impact of long COVID on quality of life and work performance among healthcare workers in portugal. PeerJ 2025, 13, e19089. [Google Scholar] [CrossRef]
- Mendola, M.; Leoni, M.; Cozzi, Y.; Manzari, A.; Tonelli, F.; Metruccio, F.; Tosti, L.; Battini, V.; Cucchi, I.; Costa, M.C.; et al. Long-term COVID symptoms, work ability and fitness to work in healthcare workers hospitalized for SARS-CoV-2 infection. Med. Lav. 2022, 113, e2022040. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or Post-COVID-19 Syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Anaya, J.M.; Rojas, M.; Salinas, M.L.; Rodríguez, Y.; Roa, G.; Lozano, M.; Rodríguez-Jiménez, M.; Montoya, N.; Zapata, E. Post-COVID Syndrome: A case series and comprehensive review. Autoimmun. Rev. 2021, 20, 102947. [Google Scholar] [CrossRef]
- Sandler, C.X.; Wyller, V.B.B.; Moss-Morris, R.; Buchwald, D.; Crawley, E.; Hautvast, J.; Katz, B.Z.; Knoop, H.; Little, P.; Taylor, R.; et al. Long COVID and post-infective fatigue syndrome: A review. Open Forum Infect. Dis. 2021, 8, ofab440. [Google Scholar] [CrossRef]
- Raveendran, A.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Stefanou, M.I.; Palaiodimou, L.; Bakola, E.; Smyrnis, N.; Papadopoulou, M.; Paraskevas, G.P.; Rizos, E.; Boutati, E.; Grigoriadis, N.; Krogias, C.; et al. Neurological manifestations of long-COVID syndrome: A narrative review. Ther. Adv. Chronic Dis. 2022, 13, 20406223221076890. [Google Scholar] [CrossRef]
- Luchian, M.L.; Higny, J.; Benoit, M.; Robaye, B.; Berners, Y.; Henry, J.P.; Colle, B.; Xhaët, O.; Blommaert, D.; Droogmans, S.; et al. Unmasking pandemic echoes: An in-depth review of long COVID’s unabated cardiovascular consequences beyond 2020. Diagnostics 2023, 13, 3368. [Google Scholar] [CrossRef] [PubMed]
- Wanga, V.; Chevinsky, J.R.; Dimitrov, L.V.; Gerdes, M.E.; Whitfield, G.P.; Bonacci, R.A.; Nji, M.A.M.; Hernandez-Romieu, A.C.; Rogers-Brown, J.S.; McLeod, T.; et al. Long-Term symptoms among adults tested for SARS-CoV-2—United States, January 2020–April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R.; Caravita, S. Phenotyping Long COVID. Eur. Respir. J. 2021, 58, 2101763. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Iwagami, M.; Yasuhara, J.; Takagi, H.; Kuno, T. Protective Effect of COVID-19 Vaccination against Long COVID Syndrome: A Systematic Review and Meta-Analysis. Vaccine 2023, 41, 1783–1790. [Google Scholar] [CrossRef]
- Català, M.; Mercadé-Besora, N.; Kolde, R.; Trinh, N.; Roel, E.; Burn, E.; Rathod-Mistry, T.; Kostka, K.; Man, W.; Delmestri, A.; et al. The effectiveness of COVID-19 vaccines to prevent long COVID symptoms: Staggered cohort study of data from the UK, Spain, and Estonia. Lancet Respir. Med. 2024, 12, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Liu, J.; Liu, M. Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12422. [Google Scholar] [CrossRef]
- Abu Hamdh, B.; Nazzal, Z. A Prospective Cohort Study Assessing the Relationship between Long-COVID Symptom Incidence in COVID-19 Patients and COVID-19 Vaccination. Sci. Rep. 2023, 13, 4896. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.; Catahay, J.; Velasco, J.; Pastrana, A.; Ver, A.; Pangilinan, F.; Peligro, P.; Casimiro, M.; Guerrero, J.; Gellaco, M.; et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. eClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef]
- Ceban, F.; Kulzhabayeva, D.; Rodrigues, N.; Di Vincenzo, J.; Gill, H.; Subramaniapillai, M.; Lui, L.; Cao, B.; Mansur, R.; Ho, R.; et al. COVID-19 vaccination for the prevention and treatment of long COVID: A systematic review and meta-analysis. Brain Behav. Immun. 2023, 111, 211–229. [Google Scholar] [CrossRef]
- Chow, N.K.N.; Tsang, C.Y.W.; Chan, Y.H.; Telaga, S.A.; Ng, L.Y.A.; Chung, C.M.; Yip, Y.M.; Cheung, P.P. The Effect of Pre-COVID and Post-COVID Vaccination on Long COVID: A Systematic Review and Meta-Analysis. J. Infect. 2024, 89, 106358. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Nemati, M.; Shahisavandi, M.; Nemati, H.; Karimi, A.; Jafari, A.; Nasiri, S.; Mohammadi, S.S.; Rahimian, Z.; Bayat, H.; et al. How Does COVID-19 Vaccination Affect Long-COVID Symptoms? PLoS ONE 2024, 19, e0296680. [Google Scholar] [CrossRef]
- Mumtaz, A.; Sheikh, A.A.E.; Khan, A.M.; Khalid, S.N.; Khan, J.; Nasrullah, A.; Sagheer, S.; Sheikh, A.B. COVID-19 Vaccine and Long COVID: A Scoping Review. Life 2022, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- Byambasuren, O.; Stehlik, P.; Clark, J.; Alcorn, K.; Glasziou, P. Effect of COVID-19 vaccination on long COVID: Systematic review. BMJ Med. 2023, 2, e000385. [Google Scholar] [CrossRef]
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.B.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-Term Effectiveness of COVID-19 Vaccines against Infections, Hospitalisations, and Mortality in Adults: Findings from a Rapid Living Systematic Evidence Synthesis and Meta-Analysis up to December, 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef]
- Nys, E.; Pauwels, S.; Ádám, B.; Amaro, J.; Athanasiou, A.; Bashkin, O.; Bric, T.K.; Bulat, P.; Caglayan, C.; Guseva Canu, I.; et al. Recognition of COVID-19 with Occupational Origin: A Comparison between European Countries. Occup. Environ. Med. 2023, 80, 694–701. [Google Scholar] [CrossRef]
- Ma, Y.; Ni, X.; Shi, Y.; Yan, C.; Shi, L.; Li, Z.; Gao, X.; Wang, D.; Yang, X.; Fan, L.; et al. Epidemic Characteristics and Related Risk Factors of Occupational Exposure for Pediatric Health Care Workers in Chinese Public Hospitals: A Cross-Sectional Study. BMC Public Health 2019, 19, 1453. [Google Scholar] [CrossRef] [PubMed]
- Hemida, E.A.; Mo’men, M.A.T.; Boulos, D.N.K.; Aboalfotouh, A.M. Assessment of implementation of occupational infection prevention of the employee wellness program among a group of healthcare workers in pediatrics hospital of Ain Shams University, Cairo, Egypt. QJM Int. J. Med. 2020, 113, hcaa045.001. [Google Scholar] [CrossRef]
- Ferrari, C.; Somma, G.; Ippoliti, L.; Magrini, A.; Di Giampaolo, L.; Coppeta, L. Global Policy to Reduce the Incidence of Infection Spreading in Non-Vaccinated Healthcare Workers: A Literature Review. Vaccines 2022, 10, 2058. [Google Scholar] [CrossRef]
- Al-Oraibi, A.; Woolf, K.; Naidu, J.; Nellums, L.B.; Pan, D.; Sze, S.; Tarrant, C.; Martin, C.A.; Gogoi, M.; Nazareth, J.; et al. Global prevalence of long COVID and its most common symptoms among healthcare workers: A systematic review and meta-analysis. BMJ Public Health 2025, 3, e000269. [Google Scholar] [CrossRef]
- Popa, M.V.; Bogdan Goroftei, E.R.; Guțu, C.; Duceac (Covrig), M.; Marcu, C.; Popescu, M.R.; Druguș, D.; Duceac, L.D. Observational Study of Post-COVID-19 Syndrome in Health Care Workers Infected with SARS-CoV-2 Virus: General and Oral Cavity Complications. Rom. J. Oral Rehabil. 2023, 15, 198–207. [Google Scholar]
- Smith, C. The structural vulnerability of healthcare workers during COVID-19: Observations on the social context of risk and the equitable distribution of resources. Soc. Sci. Med. 2020, 258, 113119. [Google Scholar] [CrossRef]
- Zhang, S.X.; Miller, S.O.; Xu, W.; Yin, A.; Chen, B.Z.; Delios, A.; Dong, R.K.; Chen, R.Z.; McIntyre, R.S.; Wan, X.; et al. Meta-analytic evidence of depression and anxiety in Eastern Europe during the COVID-19 pandemic. Eur. J. Psychotraumatol. 2022, 13, 2000132. [Google Scholar] [CrossRef] [PubMed]
- Botan, E.; Uyar, E.; Öztürk, Z.; Şevketoğlu, E.; Sarı, Y.; Dursun, O.; Sincar, Ş.; Duyu, M.; Oto, A.; Celegen, M.; et al. COVID-19 Transmission and clinical features in pediatric intensive care health care workers. Turk. Arch. Pediatr. 2022, 57, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, P.; Attanasi, M.; Dodi, G.; Porreca, A.; Raso, M.; Di Pillo, S.; Chiarelli, F. Evaluation of sleep quality and anxiety in Italian pediatric healthcare workers during the first wave of COVID-19 pandemic. BMC Res. Notes 2021, 14, 219. [Google Scholar] [CrossRef]
- Rigas, N.; Antoniou, E.; Orovou, E.; Kourakos, M.; Papatrehas, A.; Kyritsis, Z.; Tzitiridou-Chatzopoulou, M. Work satisfaction and post-traumatic stress disorder in pediatric healthcare workers during the COVID-19 Pandemic Era. Cureus 2025, 17, e76726. [Google Scholar] [CrossRef] [PubMed]
- Bahmer, T.; Borzikowsky, C.; Lieb, W.; Horn, A.; Krist, L.; Fricke, J.; Scheibenbogen, C.; Rabe, K.; Maetzler, W.; Maetzler, C.; et al. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: A prospective, multi-centre, population-based cohort study. eClinicalMedicine 2022, 51, 101549. [Google Scholar] [CrossRef]
- Sivan, M.; Parkin, A.; Makower, S.; Greenwood, D. Post-COVID syndrome symptoms, functional disability, and clinical severity phenotypes in hospitalized and nonhospitalized individuals: A cross-sectional evaluation from a community COVID rehabilitation service. J. Med. Virol. 2021, 94, 1419–1427. [Google Scholar] [CrossRef]
- Ballhausen, S.; Ruß, A.-K.; Lieb, W.; Horn, A.; Krist, L.; Fricke, J.; Scheibenbogen, C.; Rabe, K.F.; Maetzler, W.; Maetzler, C.; et al. Subdomains of post-COVID-syndrome (PCS)—A population-based study. arXiv 2025. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Bull. World Health Organ. 2007, 85, 867–872. [Google Scholar] [CrossRef]
- La Torre, G.; Paglione, G.; Barone, L.C.; Cammalleri, V.; Faticoni, A.; Marte, M.; Pocino, R.N.; Previte, C.M.; Bongiovanni, A.; Colaprico, C.; et al. Evaluation of the factors associated with reinfections towards SARS-CoV-2 using a case control design. J. Clin. Med. 2023, 12, 3861. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Z.; Chen, Y.; Li, Z. The symptoms and interval of Omicron SARS-CoV-2 reinfection among healthcare workers in a hospital of Southern China: A cross-sectional study. BMC Infect. Dis. 2024, 24, 354. [Google Scholar] [CrossRef]
- Würtz, A.M.; Kinnerup, M.B.; Pugdahl, K.; Schlünssen, V.; Vestergaard, J.M.; Nielsen, K.; Cramer, C.; Bonde, J.P.; Biering, K.; Carstensen, O.; et al. Healthcare workers’ SARS-CoV-2 infection rates during the second wave of the pandemic: Follow-up study. Scand. J. Work Environ. Health 2022, 48, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, X.; Mendoza-Cano, O.; Ríos-Silva, M.; Huerta, M.; Guzmán-Esquivel, J.; Benites-Godínez, V.; Lugo-Radillo, A.; Bricio-Barrios, J.A.; Cárdenas-Rojas, M.I.; Ríos-Bracamontes, E.F.; et al. Predictors of recurrent laboratory-confirmed symptomatic SARS-CoV-2 infections in a cohort of healthcare workers. Vaccines 2023, 11, 626. [Google Scholar] [CrossRef]
- Mochizuki, T.; Hori, T.; Yano, K.; Ikari, K.; Okazaki, K. Factors associated with change in SARS-CoV-2 antibody titers from three to six months after the administration of the BNT162b2 mRNA COVID-19 vaccine among healthcare workers in Japan: A prospective study. Intern. Med. 2022, 61, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.R.; Sampaio, V.S.; Ozahata, M.C.; Lopes, R.; Brito, A.F.; Bragatte, M.; Kalil, J.; Miraglia, J.L.; Malheiro, D.T.; Guozhang, Y.; et al. Risk factors for long coronavirus disease 2019 (long COVID) among healthcare personnel, Brazil, 2020–2022. Infect. Control Hosp. Epidemiol. 2023, 44, 1972–1978. [Google Scholar] [CrossRef]
- Mallett, P.; Thompson, A.; Bourke, T. Addressing recruitment and retention in paediatrics: A pipeline to a brighter future. Arch. Dis. Child. 2021, 107, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, M.; Brown, B.; Schults, J. Factors Influencing Pediatric Hematology/Oncology Nurse Retention: A Scoping Review. J. Pediatr. Hematol./Oncol. Nurs. 2022, 39, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Păcurari, N.; De Clercq, E.; Dragomir, M.; Colita, A.; Wangmo, T.; Elger, B. Challenges of paediatric palliative care in Romania: A focus groups study. BMC Palliat. Care 2021, 20, 178. [Google Scholar] [CrossRef]
- Petre, I.; Barna, F.; Gurguş, D.; Tomescu, L.; Apostol, A.; Petre, I.; Furău, C.; Năchescu, M.; Bordianu, A. Analysis of the Healthcare System in Romania: A Brief Review. Healthcare 2023, 11, 2069. [Google Scholar] [CrossRef]
- Rusu, R.E.; Hanganu, B.; Iorga, M.; Rusu, V.C.; Coman, A.E.; Ioan, B.G. Workplace Verbal Violence Toward Romanian Doctors and Nurses: Prevalence, Contributing Factors, and Psychological Correlates. Healthcare 2025, 13, 786. [Google Scholar] [CrossRef]
- Secosan, I.; Virga, D.; Crainiceanu, Z.P.; Bratu, L.M.; Bratu, T. Infodemia: Another Enemy for Romanian Frontline Healthcare Workers to Fight during the COVID-19 Outbreak. Medicina 2020, 56, 679. [Google Scholar] [CrossRef]
- Reisz, D.; Crișan, I.; Reisz, A.; Tudor, R.; Georgescu, D. Stress and Bio-Ethical Issues Perceived by Romanian Healthcare Practitioners in the COVID-19 Era. Int. J. Environ. Res. Public Health 2021, 18, 12749. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.; Gunșahin, D.; Filimon, C.; Bar, G.; Craiu, M. Pediatric Emergencies and Hospital Admissions in the First Six Months of the COVID-19 Pandemic in a Tertiary Children’s Hospital in Romania. Children 2022, 9, 513. [Google Scholar] [CrossRef]
- Brătucu, G.; Tudor, A.I.M.; Litră, A.V.; Nichifor, E.; Chițu, I.B.; Brătucu, T.O. Designing the Well-Being of Romanians by Achieving Mental Health with Digital Methods and Public Health Promotion. Int. J. Environ. Res. Public Health 2022, 19, 7868. [Google Scholar] [CrossRef]
- Al-Oraibi, A.; Martin, C.A.A.; Woolf, K.; Bryant, L.; Nellums, L.B.; Tarrant, C.; Khunti, K.; Pareek, M. Long COVID prevalence and factors among ethnically diverse UK healthcare workers. Eur. J. Public Health 2024, 34 (Suppl. S3), ckae144.2081. [Google Scholar] [CrossRef]
- Mirza, S.; Arvinden, V.R.; Rophina, M.; Bhawalkar, J.; Khan, U.; Chothani, B.; Singh, S.; Sharma, T.; Dwivedi, A.; Pandey, E.; et al. Impact of COVID-19 outbreak on healthcare workers in a tertiary healthcare center in India: A cross-sectional study. Sci. Rep. 2024, 14, 1504. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Mauro, M.; Sansone, D.; Tassinari, A.; Gobba, F.M.; Modenese, A.; Casolari, L.; Liviero, F.; Pavanello, S.; Scapellato, M.L.; et al. A multi-center study investigating long COVID-19 in healthcare workers from north-eastern Italy: Prevalence, risk factors and the impact of pre-existing humoral immunity—ORCHESTRA Project. Vaccines 2023, 11, 1769. [Google Scholar] [CrossRef] [PubMed]
- Doleman, G.; Leo, A.; Bloxsome, D. The impact of the COVID-19 pandemic on nurses’ and junior doctors’ workloads. J. Adv. Nurs. 2025, 81, 3729–3738. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Padierna, Á.; Villanueva, A.; Quintana, J. Caring for patients in the COVID era: Are the quality of life the same for doctors and nursing staff? Arch. Psychiatr. Nurs. 2024, 49, 126–132. [Google Scholar] [CrossRef]
- Dragioti, E.; Tsartsalis, D.; Mentis, M.; Mantzoukas, S.; Gouva, M. Impact of the COVID-19 pandemic on the mental health of hospital staff: An umbrella review of 44 meta-analyses. Int. J. Nurs. Stud. 2022, 131, 104272. [Google Scholar] [CrossRef]
- Popa, M.V.; Mîndru, D.E.; Hizanu Dumitrache, M.; Gurzu, I.L.; Anton-Păduraru, D.T.; Ștreangă, V.; Gurzu, B.; Guțu, C.; Elkan, E.M.; Duceac, L.D. Stress Factors for the Paediatric and Adult Palliative Care Multidisciplinary Team and Workplace Wellbeing Solutions. Healthcare 2024, 12, 868. [Google Scholar] [CrossRef]
- Uphoff, E.P.; Lombardo, C.; Johnston, G.; Weeks, L.; Rodgers, M.; Dawson, S.; Seymour, C.; Kousoulis, A.A.; Churchill, R. Mental health among healthcare workers and other vulnerable groups during the COVID-19 pandemic and other coronavirus outbreaks: A rapid systematic review. PLoS ONE 2021, 16, e0254821. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, X.; Xing, C.; Zheng, J. How healthcare workers respond to COVID-19: The role of vulnerability and social support in a close relationships defense mechanism. Acta Psychol. 2021, 221, 103442. [Google Scholar] [CrossRef]
- Cruickshank, M.; Brazzelli, M.; Manson, P.; Torrance, N.; Grant, A. What is the impact of long-term COVID-19 on workers in healthcare settings? A rapid systematic review of current evidence. PLoS ONE 2024, 19, e0299743. [Google Scholar] [CrossRef]
- Dempsey, B.; Madan, I.; Stevelink, S.; Lamb, D. Long COVID among healthcare workers: A narrative review of definitions, prevalence, symptoms, risk factors and impacts. Br. Med. Bull. 2024, 151, 16–35. [Google Scholar] [CrossRef]
- Romero-Rodríguez, E.; Vélez-Santamaría, R.; Pérula-de-Torres, L.Á.; González-Lama, J.; Castro-Jiménez, R.Á.; Simón-Vicente, L.; Jiménez-García, C.; González-Bernal, J.J.; Santamaría-Peláez, M.; Fernández-Solana, J.; et al. Clinical and Epidemiological Profiles of Primary Healthcare Professionals with COVID-19 Infection and Long COVID: An Observational Study. Healthcare 2023, 11, 1677. [Google Scholar] [CrossRef]
- Qi, G.; Yuan, P.; Qi, M.; Hu, X.; Shi, S.; Shi, X. Influencing Factors of High PTSD Among Medical Staff During COVID-19: Evidences From Both Meta-analysis and Subgroup Analysis. Saf. Health Work 2022, 13, 269–278. [Google Scholar] [CrossRef]
- Salari, N.; Khazaie, H.; Hosseinian-Far, A.; Ghasemi, H.; Mohammadi, M.; Shohaimi, S.; Daneshkhah, A.; Khaledi-Paveh, B.; Hosseinian-Far, M. The prevalence of sleep disturbances among physicians and nurses facing the COVID-19 patients: A systematic review and meta-analysis. Glob. Health 2020, 16, 92. [Google Scholar] [CrossRef]
- White, E.; Wetle, T.; Reddy, A.; Baier, R. Front-line Nursing Home Staff Experiences During the COVID-19 Pandemic. J. Am. Med. Dir. Assoc. 2020, 22, 199–203. [Google Scholar] [CrossRef]
- Popa, M.V.; Gurzu, I.L.; Handra, C.M.; Gurzu, B.; Pleșea Condratovici, A.; Duceac, M.; Elkan, E.M.; Mîndru, D.E.; Dabija, V.A.; Duceac, L.D. Impact of the COVID-19 Pandemic on Musculoskeletal Disorder-Related Absenteeism Among Pediatric Healthcare Workers. Healthcare 2025, 13, 1116. [Google Scholar] [CrossRef]
- Schaufeli, W.B.; Taris, T.W. A critical review of the job demands-resources model: Implications for improving work and health. In Bridging Occupational, Organizational and Public Health; Bauer, G., Hammig, O., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 43–68. Available online: https://www.wilmarschaufeli.nl/publications/Schaufeli/411.pdf (accessed on 10 August 2025). [CrossRef]
- Bakker, A.B.; Demerouti, E. The Job Demands–Resources model: State of the art. J. Manag. Psychol. 2007, 22, 309–328. [Google Scholar] [CrossRef]
- Britt, T.W.; Shen, W.; Sinclair, R.R.; Grossman, M.R. How much do we really know about employee resilience? Ind. Organ. Psychol. 2016, 9, 378–404. [Google Scholar] [CrossRef]
- Gonçalves, L.; Sala, R.; Navarro, J.B. Resilience and occupational health of health care workers: A moderator analysis of organizational resilience and sociodemographic attributes. Int. Arch. Occup. Environ. Health 2022, 95, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Ustuner Top, F.; Kulakaç, N.; Cam, H.H. Prevalence and Determinants of Workplace Violence Against Pediatric Emergency Healthcare Workers and Its Effect on Their Psychological Resilience. Clin. Pediatr. 2024, 63, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lu, G.; Gan, L.; Nie, Y.; Liu, Z.; Feng, Z.; Xu, Z.; Yu, X.; Xia, O. Career adaptability and job burnout of pediatric residents: The role of psychological resilience and insomnia. Med. Educ. Online 2025, 30, 2525180. [Google Scholar] [CrossRef]
- Olson, K.; Kemper, K.J.; Mahan, J.D. What factors promote resilience and protect against burnout in first-year pediatric and medicine-pediatric residents? J. Evid.-Based Complement. Altern. Med. 2015, 20, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Rigas, N.; Kyritsis, Z.; Dagla, M.; Soldatou, A.; Orovou, E.; Tzitiridou-Chatzopoulou, M.; Eskitzis, P.; Antoniou, E. Post-Traumatic Stress Disorder Symptoms among Pediatric Healthcare Workers. Nurs. Rep. 2024, 14, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Munn, L.T.; Liu, T.L.; Swick, M.; Rose, R.; Broyhill, B.; New, L.; Gibbs, M. Original Research: Well-Being and Resilience Among Health Care Workers During the COVID-19 Pandemic: A Cross-Sectional Study. Am. J. Nurs. 2021, 121, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, J.; Hiebel, N.; Kriegsmann-Rabe, M.; Schneider, J.; Matthias, J.K.; Erim, Y.; Morawa, E.; Jerg-Bretzke, L.; Beschoner, P.; Albus, C.; et al. Individual Stress Burden and Mental Health in Health Care Workers during the COVID-19 Pandemic: Moderating and Mediating Effects of Resilience. Int. J. Environ. Res. Public Health 2022, 19, 6545. [Google Scholar] [CrossRef]
- Strahm, C.; Seneghini, M.; Güsewell, S.; Egger, T.; Leal-Neto, O.; Brucher, A.; Lemmenmeier, E.; Meier Kleeb, D.; Möller, J.C.; Rieder, P.; et al. Symptoms compatible with long coronavirus disease (COVID) in healthcare workers with and without severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection—Results of a prospective multicenter cohort. Clin. Infect. Dis. 2022, 75, e1011–e1019. [Google Scholar] [CrossRef]
- Matula, Z.; Király, V.; Bekő, G.; Gönczi, M.; Zóka, A.; Steinhauser, R.; Uher, F.; Vályi-Nagy, I. High prevalence of long COVID in anti-TPO positive euthyroid individuals with strongly elevated SARS-CoV-2-specific T cell responses and moderately raised anti-spike IgG levels 23 months post-infection. Front. Immunol. 2024, 15, 1448659. [Google Scholar] [CrossRef]
- Lai, C.C.; Hsu, C.K.; Yen, M.Y.; Lee, P.I.; Ko, W.C.; Hsueh, P.R. Long COVID: An inevitable sequela of SARS-CoV-2 infection. J. Microbiol. Immunol. Infect. 2023, 56, 1–9. [Google Scholar] [CrossRef]
- Proal, A.; VanElzakker, M. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Saidara, E.; Maes, M. Persistent SARS-CoV-2 Infection, EBV, HHV-6 and Other Factors May Contribute to Inflammation and Autoimmunity in Long COVID. Viruses 2023, 15, 400. [Google Scholar] [CrossRef]
- Pavli, A.; Theodoridou, M.; Maltezou, H. Post-COVID Syndrome: Incidence, Clinical Spectrum, and Challenges for Primary Healthcare Professionals. Arch. Med. Res. 2021, 52, 575–581. [Google Scholar] [CrossRef]
- Gaber, T.A.K.; Ashish, A.; Unsworth, A. Persistent post-COVID symptoms in healthcare workers. Occup. Med. 2021, 71, 144–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K.; et al. Symptoms, complications and management of long COVID: A review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katz, A.; Kosowan, L.; Sanchez-Ramirez, D. Understanding symptoms suggestive of long COVID syndrome and healthcare use among community-based populations in Manitoba, Canada: An observational cross-sectional survey. BMJ Open 2024, 14, e075301. [Google Scholar] [CrossRef]
- Duceac, M.; Guțu, C.; Pleșea-Condratovici, A.; Duceac, L.D.; Eva, L.; Dabija, M.G.; Elkan, E.-M.; Miftode, A.M.; Stefanache, A.; Dabija, V.-A.; et al. A Retrospective Study of Lumbar Disk Herniation: An Analysis of Clinical Cases and Treatment Plans. J. Clin. Med. 2025, 14, 3952. [Google Scholar] [CrossRef]
- Duceac (Covrig), M.; Gutu, C.; Eva, L.; Dabija, M.G.; Calin, G.; Duceac, L.D.; Ciupilan, C.; Voinescu, D.C. Retrospective study of lumbar disc herniation in a hospital in north-eastern Romania. Balneo PRM Res. J. 2024, 15, 666. [Google Scholar] [CrossRef]
- Popa, M.V.; Gurzu, I.L.; Mîndru, D.E.; Gurzu, B.; Handra, C.M.; Eva-Maria, E.; Olaru, I.; Anton-Păduraru, D.T.; Warter, C.; Duceac, L.D. Dynamics of Absences Due to Respiratory Infections, Including COVID-19, Among Medical Staff in a Regional Pediatric Hospital. Healthcare 2025, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Nopp, S.; Moik, F.; Klok, F.A.; Gattinger, D.; Petrovic, M.; Vonbank, K.; Koczulla, A.R.; Ay, C.; Zwick, R.H. Outpatient Pulmonary Rehabilitation in Patients with Long COVID Improves Exercise Capacity, Functional Status, Dyspnea, Fatigue, and Quality of Life. Respir. Int. Rev. Thorac. Dis. 2022, 101, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dai, B.; Hou, Y.; Zhang, L.; Liu, J.; Hou, H.; Song, D.; Wang, S.; Li, X.; Zhao, H.; et al. Effect of pulmonary rehabilitation for patients with long COVID-19: A systematic review and meta-analysis of randomized controlled trials. Ther. Adv. Respir. Dis. 2025, 19, 17534666251323482. [Google Scholar] [CrossRef]
- Morales-Camino, J. SPL07 long COVID impact on workers and its monitoring in occupational health. Occup. Med. 2024, 74. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Calabria, M.; García-Sánchez, C.; Grunden, N.; Pons, C.; Arroyo, J.A.; Gómez-Anson, B.; Estévez García, M.D.C.; Belvís, R.; Morollón, N.; Vera Igual, J.; et al. Post-COVID-19 fatigue: The contribution of cognitive and neuropsychiatric symptoms. J. Neurol. 2022, 269, 3990–3999. [Google Scholar] [CrossRef]
- Venkatesh, A.K.; Yu, H.; Malicki, C.; Gottlieb, M.; Elmore, J.G.; Hill, M.J.; Idris, A.H.; Montoy, J.C.C.; O’Laughlin, K.N.; Rising, K.L.; et al. The association between prolonged SARS-CoV-2 symptoms and work outcomes. PLoS ONE 2024, 19, e0300947. [Google Scholar] [CrossRef] [PubMed]
- Ayoubkhani, D.; Zaccardi, F.; Pouwels, K.B.; Walker, A.S.; Houston, D.; Alwan, N.A.; Martin, J.; Khunti, K.; Nafilyan, V. Employment outcomes of people with Long COVID symptoms: Community-based cohort study. Eur. J. Public Health 2024, 34, 489–496. [Google Scholar] [CrossRef]
- Naik, H.; Zhu, B.; Er, L.; Sbihi, H.; Janjua, N.Z.; Smith, P.M.; Tran, K.C.; Levin, A.; Zhang, W. Work productivity loss in people living with long COVID symptoms over 2 years from infection. J. Occup. Environ. Med. 2025, 67, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Yoshimura, N.; Ishizuka, K.; Katayama, K.; Inoue, Y.; Hirose, M.; Nakagama, Y.; Kido, Y.; Sugimori, H.; Matsuda, T.; et al. Five cluster classifications of long COVID and their background factors: A cross-sectional study in Japan. Clin. Exp. Med. 2023, 23, 3663–3670. [Google Scholar] [CrossRef]
- Galgut, O.; Ashford, F.; Deeks, A.; Ghataure, A.; Islam, M.; Sambhi, T.; Ker, Y.W.; Duncan, C.J.A.; de Silva, T.I.; Hopkins, S.; et al. COVID-19 vaccines are effective at preventing symptomatic and severe infection among healthcare workers: A clinical review. Vaccine X 2024, 20, 100546. [Google Scholar] [CrossRef]
- Hasan, T.; Lynch, M.; King, C.; Wehbe, C.; Plymoth, M.; Islam, M.S.; Iannuzzi, T.; Dao, A.; Lai, J.; Martiniuk, A.; et al. Vaccine-preventable disease outbreaks among healthcare workers: A scoping review. Clin. Infect. Dis. 2024, 79, 555–561. [Google Scholar] [CrossRef]
- Duceac, L.D.; Eva, L.; Dabija, M.G.; Ciuhodaru, T.; Gutu, C.; Romila, L.; Nazarie, S. Prevention and limitation of coronavirus SARS-COV-2 cases in hospitals and dental medicine offices. Int. J. Med. Dent. 2020, 2, 149–156. [Google Scholar]
- Kurmangaliyeva, S.S.; Madenbayeva, A.M.; Urazayeva, S.T.; Bazargaliyev, Y.S.; Kudabayeva, K.I.; Kurmangaliyev, K.B. The role of memory T-cell mediated immunity in long-term COVID-19: Effects of vaccination status. Iran. J. Med. Sci. 2025, 50, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Spinardi, J.R.; Srivastava, A. Hybrid Immunity to SARS-CoV-2 from Infection and Vaccination—Evidence Synthesis and Implications for New COVID-19 Vaccines. Biomedicines 2023, 11, 370. [Google Scholar] [CrossRef]
- Man, M.A.; Rosca, D.; Bratosin, F.; Fira-Mladinescu, O.; Ilie, A.C.; Burtic, S.R.; Fildan, A.P.; Fizedean, C.M.; Jianu, A.M.; Negrean, R.A.; et al. Impact of pre-infection COVID-19 vaccination on the incidence and severity of post-COVID syndrome: A systematic review and meta-analysis. Vaccines 2024, 12, 189. [Google Scholar] [CrossRef]
- Efrati, S.; Catalogna, M.; Abu Hamad, R.; Hadanny, A.; Bar-Chaim, A.; Benveniste-Levkovitz, P.; Levtzion-Korach, O. Safety and humoral responses to BNT162b2 mRNA vaccination of SARS-CoV-2 previously infected and naive populations. Sci. Rep. 2021, 11, 16543. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bermingham, C.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Trajectory of long COVID symptoms after COVID-19 vaccination: Community based cohort study. BMJ 2022, 377, e069676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, Y.; Shen, Q.; Zang, Y.; Miao, T.; Yang, M.; Liu, Y.; Zheng, X.; Shen, S.; Wu, W. COVID-19 vaccination and long COVID among 50 years older and above European: The role of chronic multimorbidity. Arch. Gerontol. Geriatr. 2024, 126, 105554. [Google Scholar] [CrossRef] [PubMed]
- Al Namat, R.; Duceac, L.D.; Chelaru, L.; Dabija, M.G.; Guțu, C.; Marcu, C.; Popa, M.V.; Popa, F.; Bogdan Goroftei, E.R.; Țarcă, E. Post-Coronary Artery Bypass Grafting Outcomes of Patients with/without Type-2 Diabetes Mellitus and Chronic Kidney Disease Treated with SGLT2 Inhibitor Dapagliflozin: A Single-Center Experience Analysis. Diagnostics 2024, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Duceac, M.; Buzea, C.G.; Pleșea-Condratovici, A.; Eva, L.; Duceac, L.D.; Dabija, M.G.; Costăchescu, B.; Elkan, E.M.; Guțu, C.; Voinescu, D.C. A Hybrid Ensemble Learning Framework for Predicting Lumbar Disc Herniation Recurrence: Integrating Supervised Models, Anomaly Detection, and Threshold Optimization. Diagnostics 2025, 15, 1628. [Google Scholar] [CrossRef]
- .Berber, E.; Ross, T.M. Factors Predicting COVID-19 Vaccine Effectiveness and Longevity of Humoral Immune Responses. Vaccines 2024, 12, 1284. [Google Scholar] [CrossRef] [PubMed]
- Al Namat, R.; Duceac, L.D.; Chelaru, L.; Dimitriu, C.; Bazyani, A.; Tarus, A.; Bacusca, A.; Roșca, A.; Al Namat, D.; Livanu, L.I.; et al. The impact of COVID-19 vaccination on oxidative stress and cardiac fibrosis biomarkers in patients with acute myocardial infarction (STEMI), a single-center experience analysis. Life 2024, 14, 1350. [Google Scholar] [CrossRef]
- Brehon, K.; Niemeläinen, R.; Hall, M.; Bostick, G.P.; Brown, C.A.; Wieler, M.; Gross, D.P. Return-to-Work Following Occupational Rehabilitation for Long COVID: Descriptive Cohort Study. JMIR Rehabil. Assist. Technol. 2022, 9, e39883. [Google Scholar] [CrossRef]
- Onik, G.; Knapik, K.; Dąbrowska-Galas, M.; Sieroń, K. Health resort treatment improves functioning and physical performance in long COVID patients: A retrospective study. Healthcare 2024, 12, 2344. [Google Scholar] [CrossRef]
- Howard, J.; Cloeren, M.; Vanichkachorn, G. Long COVID and occupational medicine practice. J. Occup. Environ. Med. 2024, 66, 1–5. [Google Scholar] [CrossRef]
- Zhu, Z.; Zheng, W.; Tang, N.; Zhong, W. Review of Manpower Management in Healthcare System: Strategies, Challenges, and Innovations. J. Multidiscip. Healthc. 2024, 17, 5341–5351. [Google Scholar] [CrossRef]
- Băcescu Ene, G.V.; Stoia, M.A.; Cojocaru, C.; Todea, D.A. SMART Multi-Criteria Decision Analysis (MCDA)-One of the Keys to Future Pandemic Strategies. J. Clin. Med. 2025, 14, 1943. [Google Scholar] [CrossRef]
- Adler, A.L.; Kociolek, L.K.; Glomb, N.; Ramírez-Àvila, L.; Kest, H.; Niescierenko, M.; West, D.; Zerr, D.M. Strategies Used by Children’s Hospitals During the 2022 Viral Respiratory Surge. Hosp. Pediatr. 2025, 15, e105–e107. [Google Scholar] [CrossRef] [PubMed]
- Ayyala, R.S.; Baird, G.L.; Sze, R.W.; Brown, B.P.; Taylor, G.A. The growing issue of burnout in radiology—A survey-based evaluation of driving factors and potential impacts in pediatric radiologists. Pediatr. Radiol. 2020, 50, 1071–1077. [Google Scholar] [CrossRef]
- Moerdler, S.; Steinberg, D.M.; Jin, Z.; Cole, P.D.; Levy, A.S.; Rosenthal, S.L. Well-Being of Pediatric Hematology Oncology Providers and Staff During the COVID-19 Pandemic in the New York and New Jersey Epicenter. JCO Oncol. Pract. 2021, 17, e925–e935. [Google Scholar] [CrossRef]
- Thapa, D.R.; Stengård, J.; Ekström-Bergström, A.; Areskoug Josefsson, K.; Krettek, A.; Nyberg, A. Job demands, job resources, and health outcomes among nursing professionals in private and public healthcare sectors in Sweden—A prospective study. BMC Nurs. 2022, 21, 140. [Google Scholar] [CrossRef]
- Raspanti, A.; Provenzi, L.; Acampora, M.; Borgatti, R.; Millepiedi, S.; Mariani Wigley, I.L.C.; Barello, S. Job Demands and Resources Shape the Risk of Burnout in Italian Child Neuropsychiatrists. Healthcare 2024, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Limungi, G.M.; Amer, M.; Elmadani, M.; Simon, K.; Hamad, O.; Horvath, E.; Livia, P.; Orsolya, M. Understanding key factors contributing to mental health challenges among pediatric nurses: A systematic review. Front. Public Health 2025, 13, 1480024. [Google Scholar] [CrossRef] [PubMed]
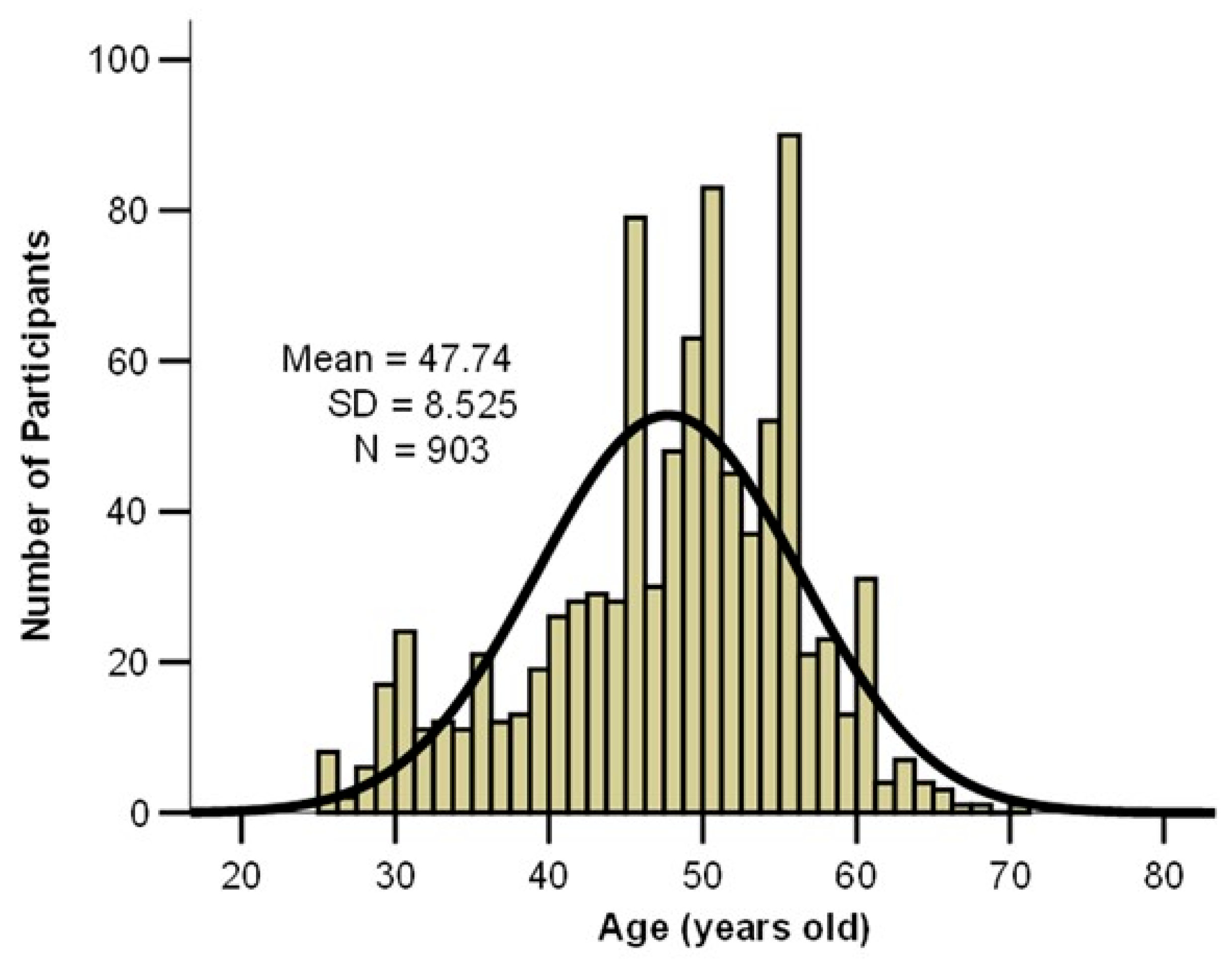
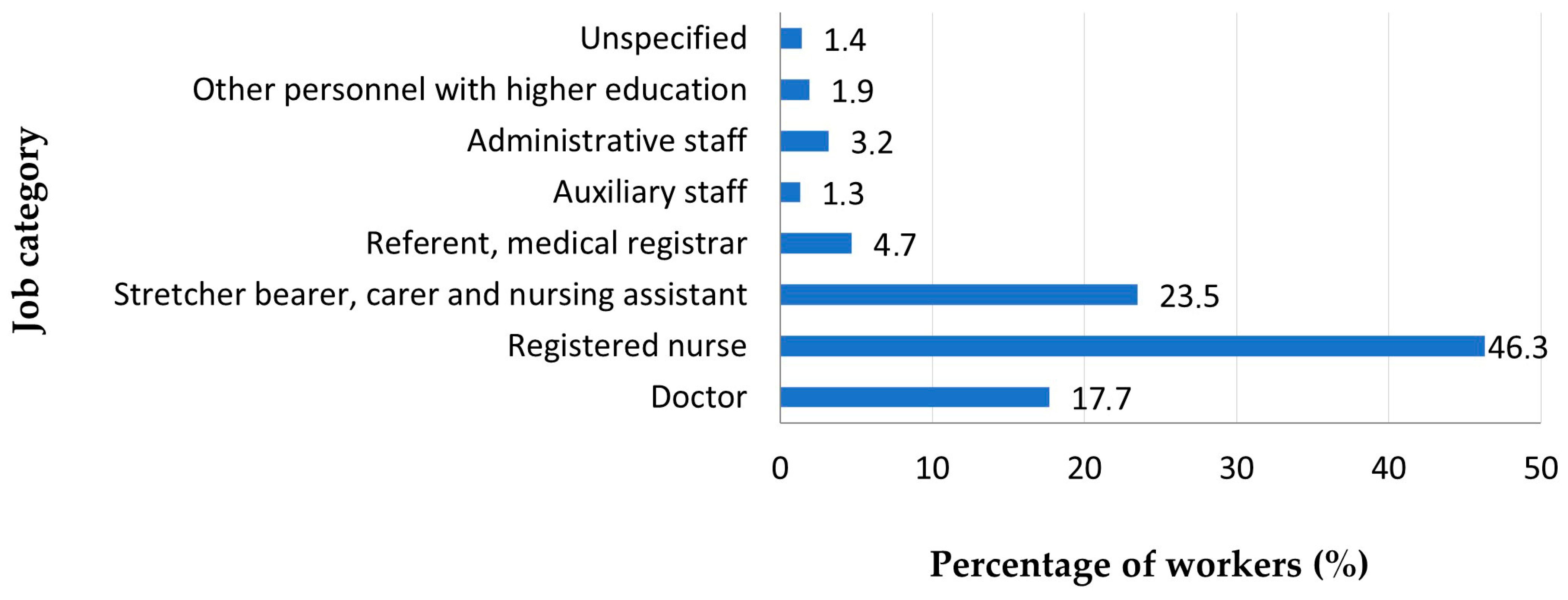

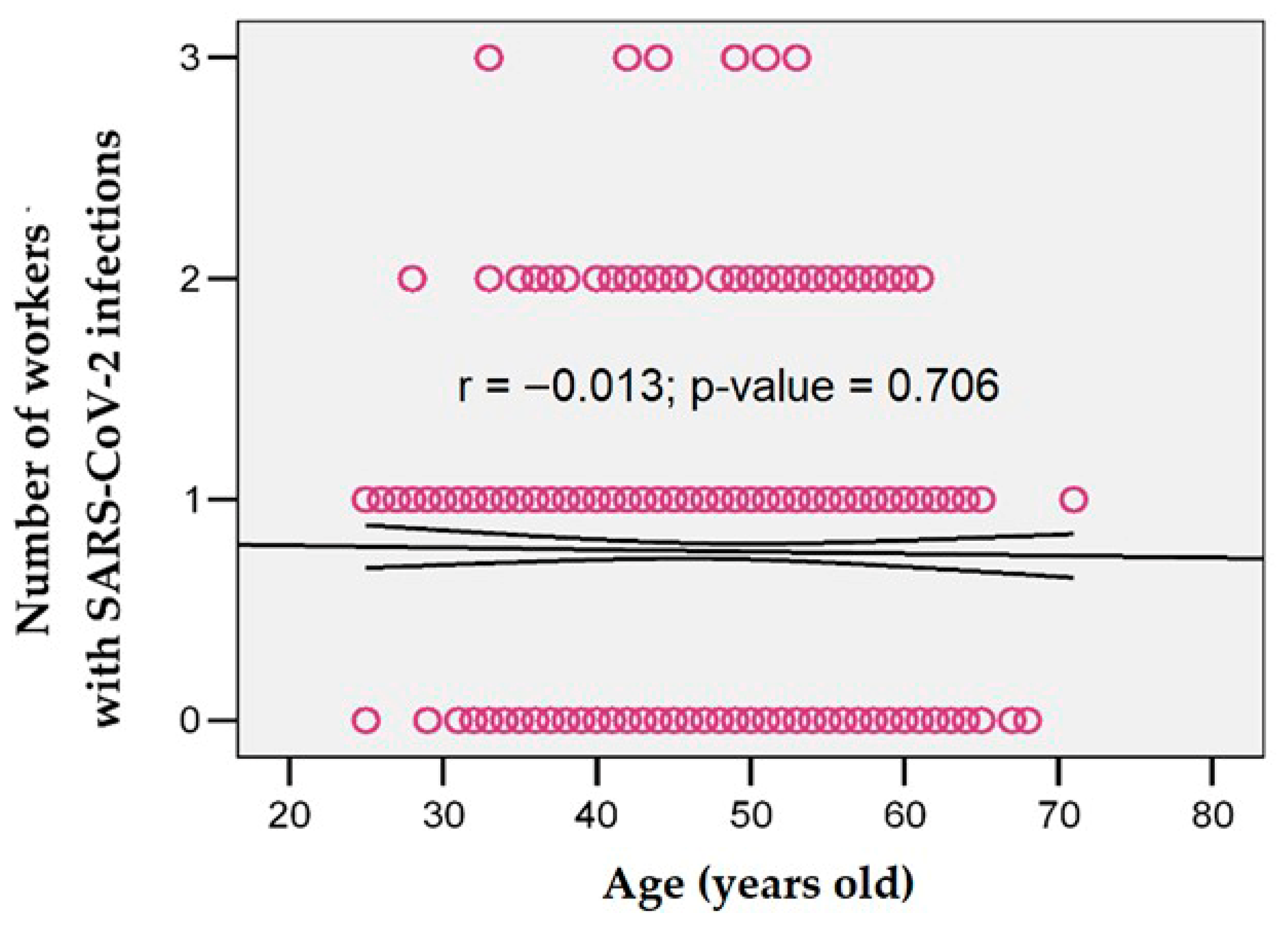
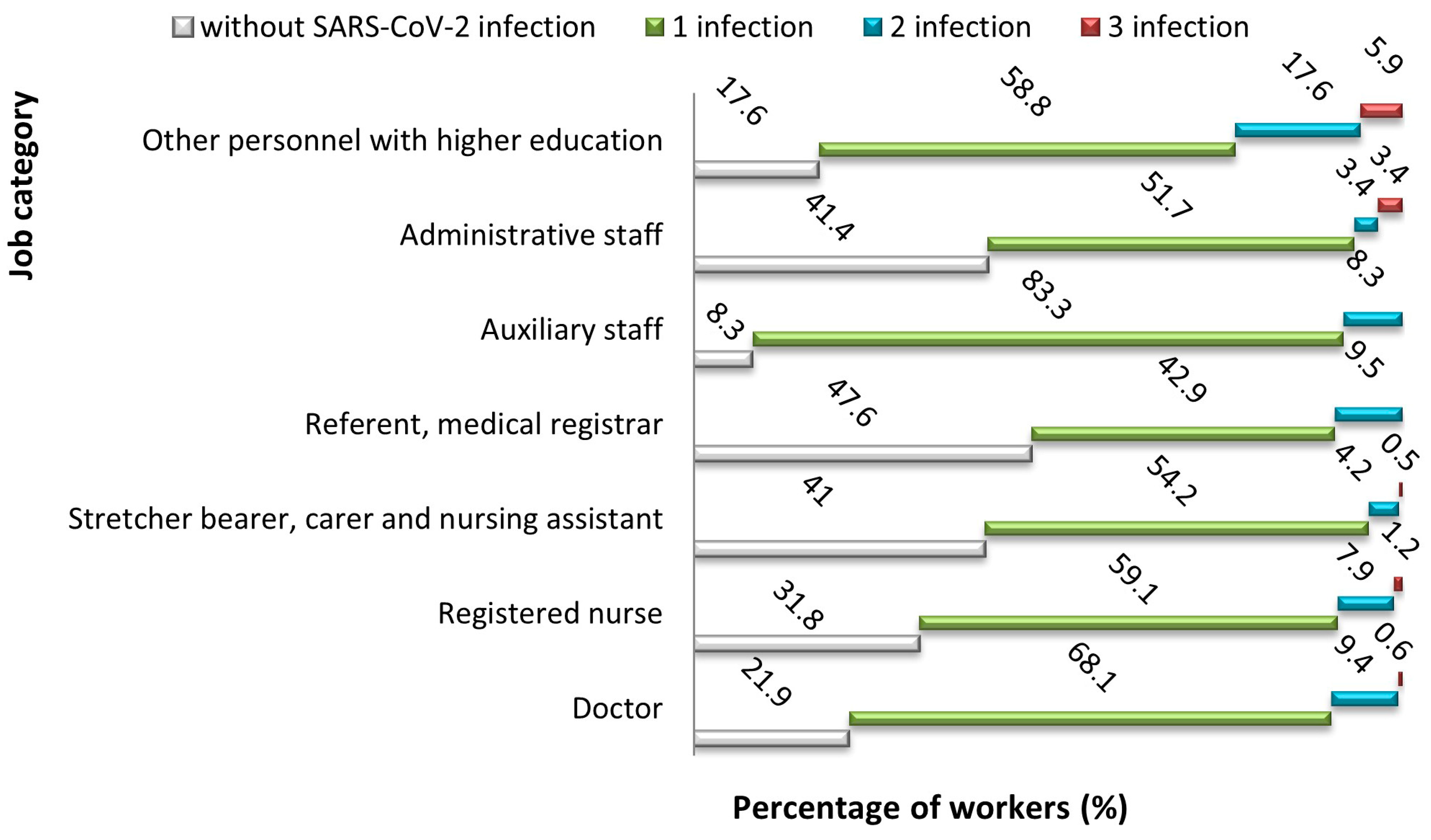
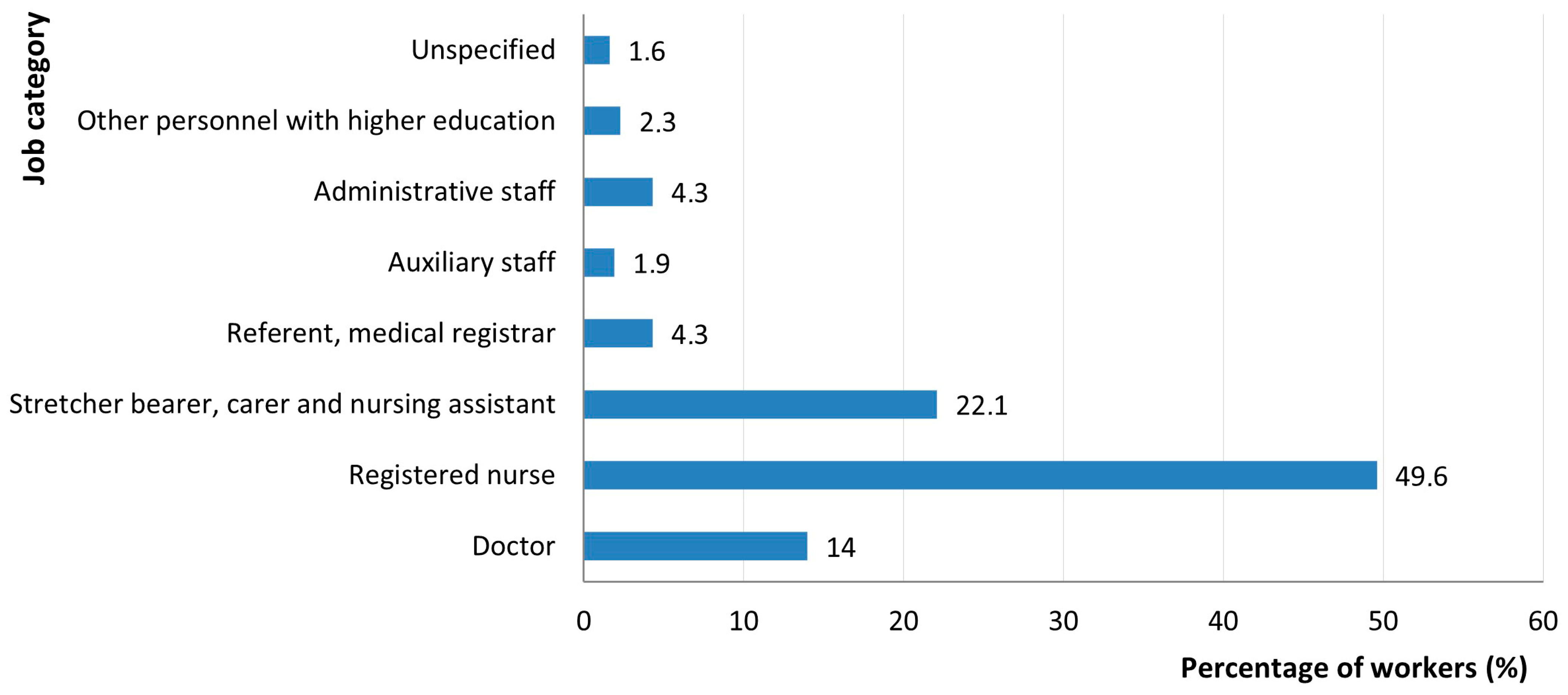

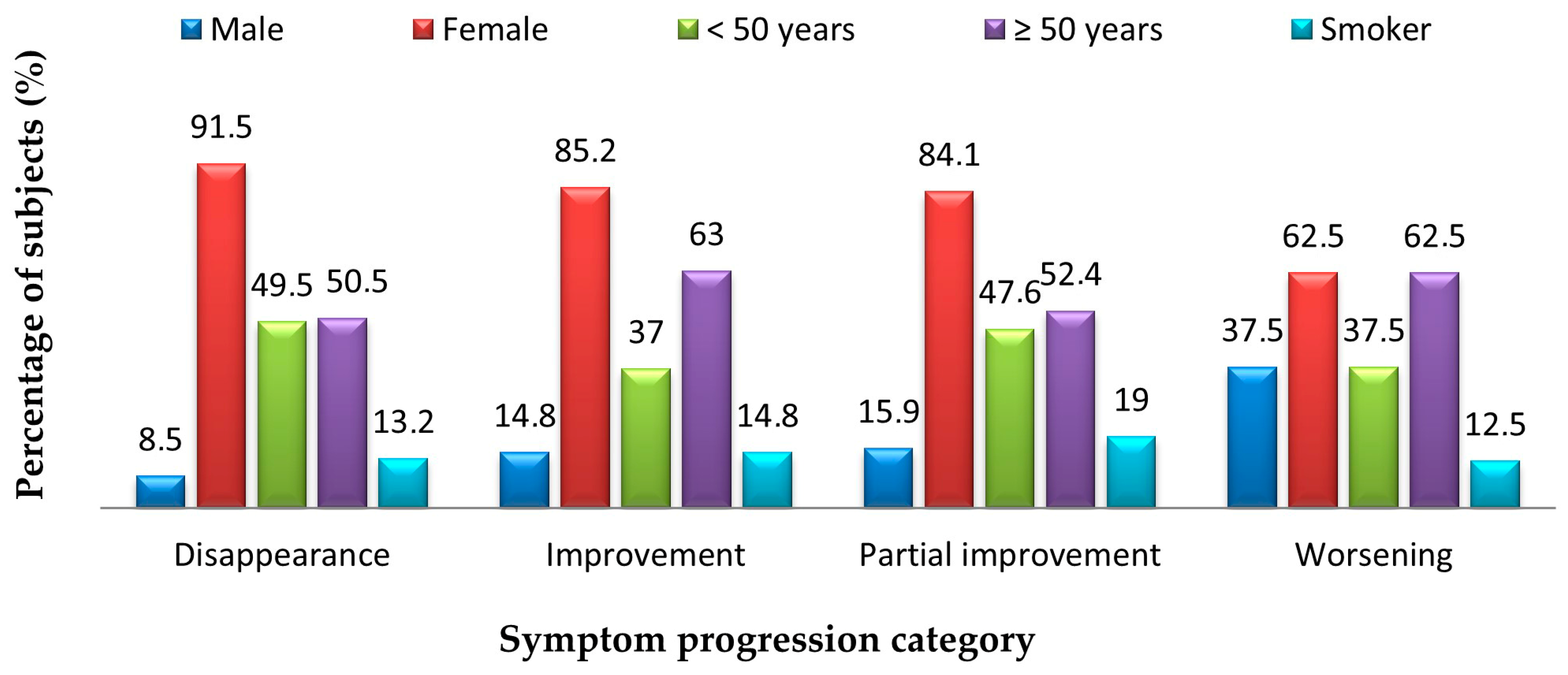
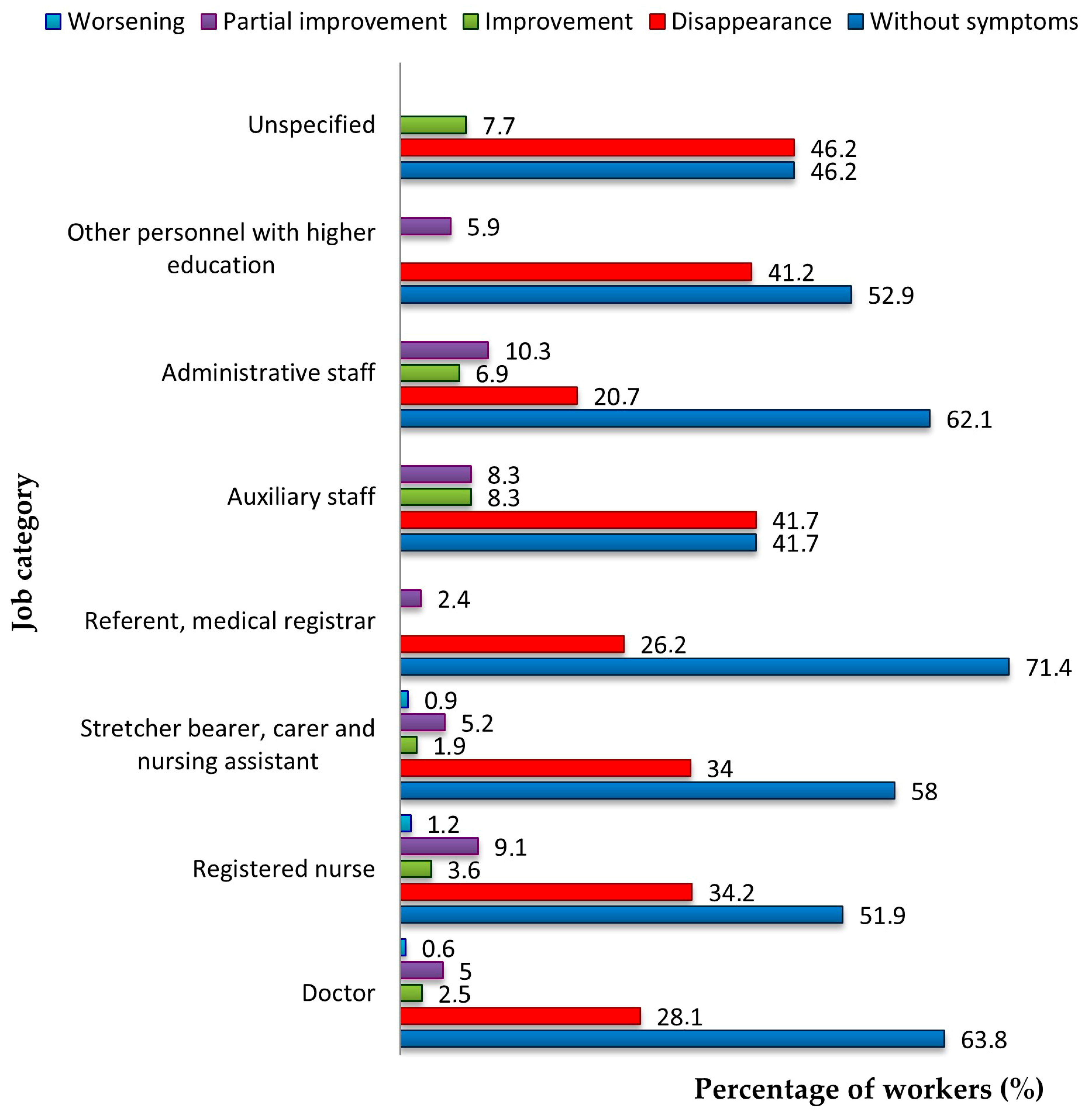
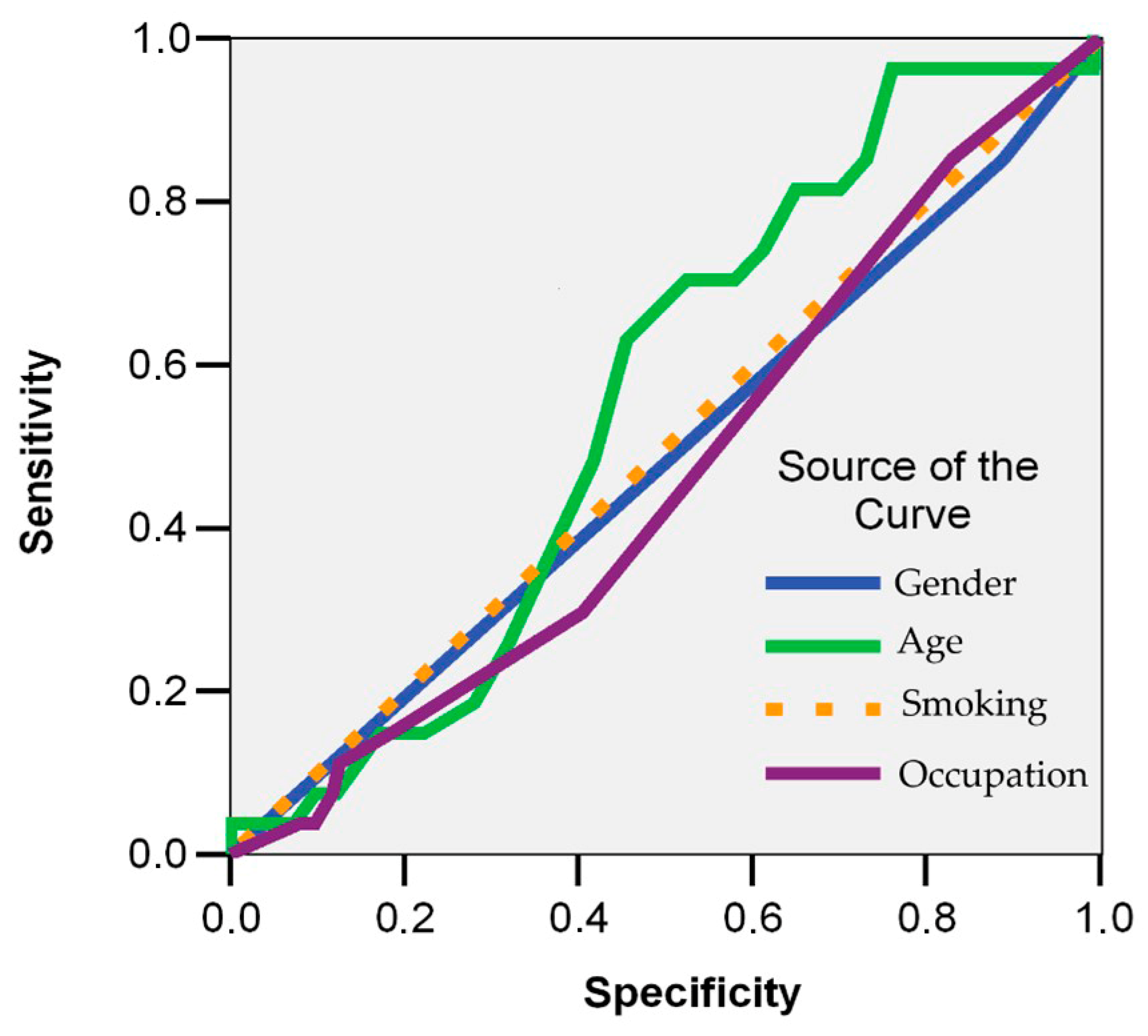


| Symptom | Symptom Severity in Total Long COVID Cases (n = 258) | p Chi Square Test | |||||
|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||||
| n | % | n | % | n | % | ||
| Excessive fatigue Duration, weeks | 24 | 9.3 | 80 | 31.0 | 58 | 22.5 | 0.001 |
| 8.67 ± 5.83 | 10.83 ± 7.87 | 15.09 ± 11.57 | 0.005 | ||||
| Memory impairment Duration, weeks | 26 | 10.1 | 21 | 8.1 | 6 | 2.3 | 0.314 |
| 7.60 ± 5.32 | 12.95 ± 6.77 | 9.67 ± 2.66 | 0.011 | ||||
| Mental exhaustion Duration, weeks | 19 | 7.4 | 36 | 14.0 | 13 | 5.0 | 0.651 |
| 7.33 ± 4.50 | 13.03 ± 8.70 | 16.15 ± 10.66 | 0.026 | ||||
| Concentration difficulties Duration, weeks | 42 | 16.3 | 51 | 19.8 | 8 | 3.1 | 0.497 |
| 10.57 ± 8.20 | 11.44 ± 6.35 | 19.50 ± 18.94 | 0.030 | ||||
| Insomnia Duration, weeks | 17 | 6.6 | 35 | 13.6 | 15 | 5.8 | 0.395 |
| 9.63 ± 7.05 | 7.47 ± 5.73 | 12.93 ± 10.50 | 0.064 | ||||
| Stress Duration, weeks | 12 | 4.7 | 40 | 15.6 | 21 | 8.1 | 0.598 |
| 9.67 ± 6.97 | 11.41 ± 11.39 | 14.10 ± 9.90 | 0.458 | ||||
| Depression Duration, weeks | 13 | 5.0 | 19 | 7.4 | 9 | 3.5 | 0.126 |
| 11.83 ± 6.74 | 10.82 ± 6.56 | 9.78 ± 3.83 | 0.743 | ||||
| Anxiety Duration, weeks | 18 | 7.0 | 23 | 8.9 | 11 | 4.3 | 0.821 |
| 10.59 ± 5.86 | 10.62 ± 6.76 | 12.18 ± 4.77 | 0.751 | ||||
| Headache Duration, weeks | 19 | 7.4 | 31 | 12.0 | 23 | 8.9 | 0.001 |
| 8.44 ± 6.42 | 7.17 ± 4.70 | 8.82 ± 4.57 | 0.483 | ||||
| Change in smell Duration, weeks | 25 | 9.7 | 33 | 14.0 | 15 | 5.8 | 0.113 |
| 11.00 ± 7.08 | 12.66 ± 9.85 | 15.00 ± 14.59 | 0.497 | ||||
| Loss of smell Duration, weeks | 85 | 32.9 | 0 | 0.0 | 0 | 0.0 | 0.001 |
| 11.92 ± 9.62 | - | - | - | ||||
| Change in taste Duration, weeks | 16 | 6.2 | 17 | 6.6 | 13 | 5.0 | 0.611 |
| 10.20 ± 6.46 | 11.94 ± 5.39 | 14.00 ± 14.12 | 0.546 | ||||
| Loss of taste Duration, weeks | 44 | 17.1 | 0 | 0.0 | 0 | 0.0 | 0.001 |
| 11.75 ± 2.21 | - | - | - | ||||
| Joint pain Duration, weeks | 28 | 10.9 | 55 | 21.3 | 40 | 15.5 | 0.012 |
| 10.48 ± 5.46 | 13.19 ± 14.18 | 13.76 ± 2.49 | 0.581 | ||||
| Myalgia (muscle pain) Duration, weeks | 19 | 7.4 | 38 | 14.7 | 26 | 10.1 | 0.001 |
| 7.56 ± 4.16 | 12.84 ± 2.68 | 15.08 ± 3.60 | 0.037 | ||||
| Generalized pain Duration, weeks | 14 | 5.4 | 36 | 14.0 | 12 | 4.7 | 0.902 |
| 9.38 ± 3.86 | 11.03 ± 8.01 | 9.75 ± 5.46 | 0.710 | ||||
| Balance disorders Duration, weeks | 12 | 4.7 | 21 | 8.1 | 11 | 4.3 | 0.050 |
| 11.83 ± 3.75 | 11.45 ± 9.29 | 10.73 ± 3.84 | 0.972 | ||||
| Dizziness Duration, weeks | 25 | 9.7 | 30 | 11.6 | 10 | 3.9 | 0.050 |
| 10.33 ± 5.95 | 9.90 ± 7.11 | 6.80 ± 3.43 | 0.308 | ||||
| Paresthesia (tingling/numbness) Duration, weeks | 10 | 3.9 | 13 | 5.0 | 4 | 1.6 | 0.251 |
| 11.40 ± 6.26 | 17.38 ± 15.15 | 8.50 ± 4.12 | 0.005 | ||||
| Cold extremities Duration, weeks | 14 | 5.4 | 13 | 5.0 | 9 | 3.5 | 0.165 |
| 10.43 ± 4.59 | 8.33 ± 6.20 | 8.67 ± 2.83 | 0.511 | ||||
| Shortness of breath Duration, weeks | 20 | 7.8 | 29 | 11.2 | 24 | 9.3 | 0.001 |
| 10.84 ± 3.18 | 11.69 ± 9.25 | 12.25 ± 7.40 | 0.903 | ||||
| Persistent cough Duration, weeks | 22 | 8.5 | 53 | 20.5 | 26 | 10.1 | 0.001 |
| 14.67 ± 6.83 | 9.50 ± 5.86 | 9.41 ± 5.99 | 0.165 | ||||
| Palpitations Duration, weeks | 16 | 6.2 | 22 | 8.5 | 13 | 5.0 | 0.484 |
| 7.47 ± 5.87 | 11.90 ± 3.10 | 10.62 ± 5.68 | 0.445 | ||||
| Chest pain Duration, weeks | 11 | 4.3 | 18 | 7.0 | 14 | 5.4 | 0.309 |
| 7.10 ± 4.38 | 8.22 ± 6.10 | 8.64 ± 4.60 | 0.733 | ||||
| Sore throat Duration, weeks | 19 | 7.4 | 15 | 5.8 | 9 | 3.5 | 0.777 |
| 5.37 ± 4.13 | 4.80 ± 2.91 | 7.44 ± 4.28 | 0.248 | ||||
| Diarrhea Duration, weeks | 5 | 1.9 | 8 | 3.1 | 5 | 1.9 | 0.045 |
| 3.00 ± 3.08 | 3.25 ± 2.25 | 4.60 ± 3.29 | 0.618 | ||||
| Nausea Duration, weeks | 10 | 3.9 | 9 | 3.5 | 6 | 2.3 | 0.689 |
| 5.00 ± 2.65 | 4.78 ± 1.86 | 6.50 ± 4.18 | 0.493 | ||||
| Vomiting Duration, weeks | 6 | 2.3 | 2 | 0.8 | 4 | 1.6 | 0.344 |
| 2.17 ± 1.42 | 2.50 ± 2.12 | 5.00 ± 3.46 | 0.223 | ||||
| Loss of appetite Duration, weeks | 21 | 8.1 | 31 | 12.0 | 18 | 7.0 | 0.843 |
| 8.71 ± 10.00 | 10.06 ± 6.42 | 8.00 ± 5.93 | 0.628 | ||||
| Constipation Duration, weeks | 2 | 0.8 | 5 | 1.9 | 1 | 0.4 | 0.292 |
| 4.50 ± 4.95 | 6.00 ± 4.32 | 8.00 ± 0.00 | 0.821 | ||||
| Abdominal pain Duration, weeks | 15 | 5.8 | 10 | 3.9 | 7 | 2.7 | 0.813 |
| 5.73 ± 3.83 | 5.00 ± 2.75 | 6.29 ± 3.73 | 0.750 | ||||
| Hair loss Duration, weeks | 7 | 2.7 | 24 | 9.3 | 12 | 4.7 | 0.157 |
| 14.86 ± 8.86 | 20.13 ± 14.89 | 22.33 ± 12.59 | 0.509 | ||||
| Skin rashes Duration, weeks | 17 | 6.6 | 9 | 3.5 | 1 | 0.4 | 0.460 |
| 9.76 ± 10.49 | 10.25 ± 5.99 | 8.00 ± 0.00 | 0.973 | ||||
| Itching (pruritus) Duration, weeks | 8 | 3.1 | 9 | 3.5 | 0 | 0.0 | 0.500 |
| 8.67 ± 2.73 | 8.75 ± 4.53 | - | 0.969 | ||||
| Eye discomfort Duration, weeks | 22 | 8.5 | 17 | 6.6 | 3 | 1.2 | 0.589 |
| 10.55 ± 9.90 | 8.93 ± 3.77 | 8.00 ± 0.00 | 0.764 | ||||
| Fever > 38 °C Duration, weeks | 25 | 9.7 | 0 | 0.0 | 0 | 0.0 | 0.901 |
| 1.40 ± 1.41 | - | - | - | ||||
| Post-COVID Symptom | Vaccinated (n = 604) | Unvaccinated (n = 299) | Chi2 Test p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Excessive fatigue | 154 | 25.5 | 54 | 18.1 | 0.013 |
| Memory disorders | 48 | 7.9 | 19 | 6.4 | 0.390 |
| Mental exhaustion | 60 | 9.9 | 19 | 6.4 | 0.073 |
| Difficulty concentrating | 85 | 14.1 | 33 | 19.0 | 0.203 |
| Insomnia | 69 | 11.4 | 20 | 6.7 | 0.025 |
| Stress | 67 | 11.1 | 21 | 7.0 | 0.050 |
| Depression | 39 | 6.5 | 11 | 3.7 | 0.086 |
| Anxiety | 46 | 7.6 | 20 | 6.7 | 0.615 |
| Headache | 79 | 13.1 | 27 | 9.0 | 0.076 |
| Change in smell | 62 | 10.3 | 21 | 7.0 | 0.962 |
| Loss of smell | 91 | 15.1 | 33 | 11.0 | 0.050 |
| Myalgia | 87 | 14.4 | 31 | 10.4 | 0.091 |
| Generalized pain | 50 | 8.3 | 20 | 6.7 | 0.401 |
| Balance disorders | 36 | 6.0 | 16 | 6.4 | 0.712 |
| Dizziness | 59 | 9.8 | 23 | 7.7 | 0.307 |
| Paresthesia | 24 | 4.0 | 9 | 3.0 | 0.468 |
| Cold extremities | 33 | 5.5 | 9 | 3.0 | 0.100 |
| Shortness of breath | 69 | 11.4 | 25 | 8.4 | 0.156 |
| Persistent cough | 105 | 17.4 | 37 | 12.4 | 0.050 |
| Palpitations | 46 | 7.6 | 18 | 6.0 | 0.379 |
| Sore throat | 47 | 7.8 | 15 | 5.0 | 0.122 |
| Diarrhea | 23 | 3.8 | 7 | 2.3 | 0.247 |
| Nausea | 30 | 5.0 | 8 | 2.7 | 0.107 |
| Vomiting | 15 | 2.5 | 2 | 0.7 | 0.059 |
| Loss of appetite | 61 | 10.1 | 29 | 9.7 | 0.850 |
| Constipation | 7 | 1.2 | 2 | 0.7 | 0.486 |
| Abdominal pain | 31 | 5.1 | 8 | 2.7 | 0.088 |
| Hair loss | 32 | 5.3 | 16 | 5.4 | 0.974 |
| Skin rashes | 23 | 3.8 | 9 | 3.0 | 0.541 |
| Itching (pruritus) | 13 | 2.2 | 5 | 1.7 | 0.627 |
| Eye discomfort | 41 | 6.8 | 7 | 2.3 | 0.005 |
| Fever > 38 °C | 37 | 6.1 | 12 | 4.0 | 0.121 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, M.V.; Gurzu, I.L.; Handra, C.M.; Mandanach, C.; Gurzu, B.; Mîndru, D.E.; Duceac, M.; Ciuhodaru, M.I.; Duceac, L.D. Long COVID in Healthcare Workers from a Pediatric Hospital in Romania: A Cross-Sectional Study of Prevalence, Symptom Burden, and the Role of Vaccination and Reinfection. J. Clin. Med. 2025, 14, 5782. https://doi.org/10.3390/jcm14165782
Popa MV, Gurzu IL, Handra CM, Mandanach C, Gurzu B, Mîndru DE, Duceac M, Ciuhodaru MI, Duceac LD. Long COVID in Healthcare Workers from a Pediatric Hospital in Romania: A Cross-Sectional Study of Prevalence, Symptom Burden, and the Role of Vaccination and Reinfection. Journal of Clinical Medicine. 2025; 14(16):5782. https://doi.org/10.3390/jcm14165782
Chicago/Turabian StylePopa, Maria Valentina, Irina Luciana Gurzu, Claudia Mariana Handra, Cristina Mandanach, Bogdan Gurzu, Dana Elena Mîndru, Mădălina Duceac (Covrig), Mădălina Irina Ciuhodaru, and Letiția Doina Duceac. 2025. "Long COVID in Healthcare Workers from a Pediatric Hospital in Romania: A Cross-Sectional Study of Prevalence, Symptom Burden, and the Role of Vaccination and Reinfection" Journal of Clinical Medicine 14, no. 16: 5782. https://doi.org/10.3390/jcm14165782
APA StylePopa, M. V., Gurzu, I. L., Handra, C. M., Mandanach, C., Gurzu, B., Mîndru, D. E., Duceac, M., Ciuhodaru, M. I., & Duceac, L. D. (2025). Long COVID in Healthcare Workers from a Pediatric Hospital in Romania: A Cross-Sectional Study of Prevalence, Symptom Burden, and the Role of Vaccination and Reinfection. Journal of Clinical Medicine, 14(16), 5782. https://doi.org/10.3390/jcm14165782






