Aortic Valve Annular Characteristics in Isolated Left Ventricular Non-Compaction—Detailed Analysis from the Three-Dimensional Speckle Tracking Echocardiographic MAGYAR-Path Study
Abstract
1. Introduction
2. Methods
- −
- Thickened LV wall showing a two-layered structure with an epicardial compacted layer and a so-called endocardial non-compacted layer including deep intertrabecular recesses and prominent trabeculations.
- −
- A non-compacted to compacted LV myocardial thickness ratio >2 at end-diastole, measured at the moment of maximal thickness.
- −
- Deep intertrabecular recesses having communication with the LV cavity confirmed by color Doppler.
- −
- Absence of concomitant cardiac anomalies.
- −
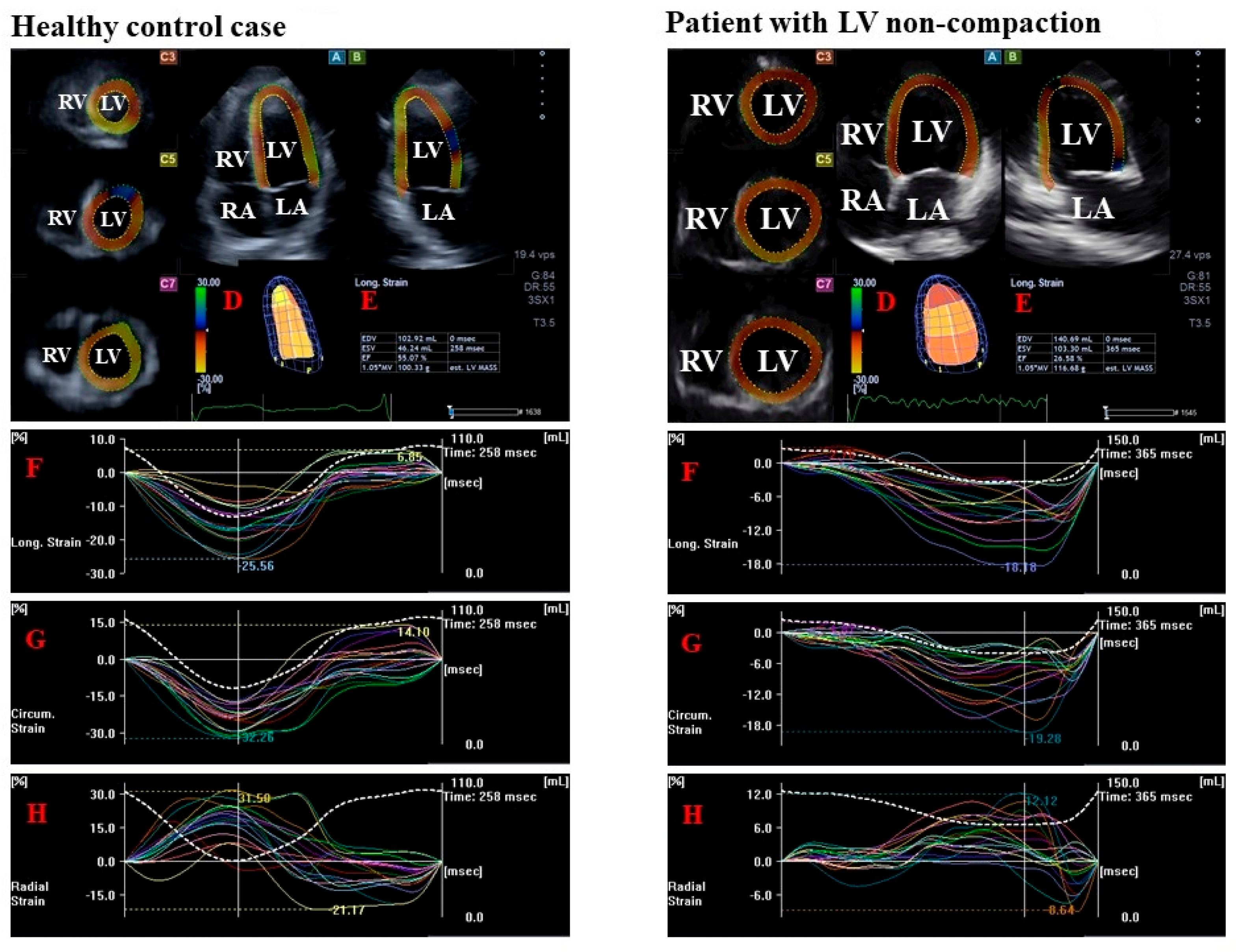
- LV radial strain (RS), which represents thickening/thinning of the LV.
- −
- LV circumferential strain (CS), which represents narrowing/widening of the LV.
- −
- LV longitudinal strain (LS), which represents shortening/lengthening of the LV.
3. Results
4. Discussion
Limitation Section
- −
- Image quality of 2D echocardiography is still better than that of 3DSTE due to several technical reasons like better spatial and temporal resolutions. Moreover, there is a size difference between the transducers for 2D echocardiography and 3DSTE: the larger size of the 3DSTE transducer makes its positioning on the chest more complicated. Moreover, the fact that six subvolumes during six cardiac cycles are required for optimal image quality may lead to stitching and motion artefacts [8,9,10,11,12]. Only acceptable quality images were used during analysis; low-quality images or those with extreme results were excluded. If more than quarter of the LV/AVA could not be visualized, the analysis was considered infeasible.
- −
- It is not clear whether frame rate modifies 3DSTE-derived AAPSE measurements. Moreover, 2D echocardiography-guided M-mode tracings and 3DSTE-derived assessments of the AAPSE were not compared, which could be considered as a technical limitation of the present study but could be a topic for future investigations.
- −
- Only data of a limited number of patients with LVNC were analyzed in this study. However, it should be noted that LVNC is a rare disease. The small LVNC sample size potentially introduced selection bias. Moreover, no power calculation or justification of sample size has been provided, limiting the generalizability and power of the findings.
- −
- Moreover, only Caucasian (Hungarian) subjects from a single center were involved, which can also be considered a limitation.
- −
- The most important preselection bias is that due to low-quality images, 43% of LVNC patients had to be excluded, which could significantly affect the findings and should be considered when interpreting the results.
- −
- According to literature data from the MAGYAR-Healthy Study, similarly to LVNC cases, AVA parameters could not be measured in 40% of healthy subjects due to technical reasons [12]. In the present study, age- and gender-matched healthy subjects served as controls.
- −
- Some cardiovascular risk factors including hypertension and hypercholesterolemia were relatively frequent in patients with LVNC, which could partially explain the findings. In a recent review it has been demonstrated that a lifetime exposure to elevated systolic blood pressure may be associated with an increased risk of (aortic) valvular heart disease [30]. Similarly, hyperlipidemia was found to be associated with aortic valve disease [31].
- −
- Although there are several opportunities, STE-derived or other parameters characterizing AVA dimensions were not intended to be investigated in this study.
- −
- Moreover, grading of valvular regurgitation was mainly performed visually rather than with quantitative and advanced methods, limiting precision.
- −
- The controls and LVNC patients were grouped according to their AVA-A. As can be seen from the data presented, it might have been worthwhile to also group patients according to AVA diameters and perimeter, although this would have significantly exceeded the scope of this scientific work.
- −
- The effect of compaction on the relationship between LV function and AVA was not examined in this study due to the low number of non-compacted basal segments (8%) in LVNC patients. According to literature data, however, similarly reduced LV-LS and LV-CS of compacted and non-compacted segments in LVNC were demonstrated in a recent study. The reduction in LV-RS was more pronounced in the presence of non-compaction [32].
- −
- Moreover, LV rotational mechanics was also not intended to be examined in the present study, which could be a topic of further analysis.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engberding, R.; Bender, F. Identification of a rare congenital anomaly of the myocardium by two-dimensional echocardiography: Persistence of isolated myocardial sinusoids. Am. J. Cardiol. 1984, 53, 1733–1734. [Google Scholar] [CrossRef] [PubMed]
- Filho, D.C.S.; do Rêgo Aquino, P.L.; de Souza Silva, G.; Fabro, C.B. Left Ventricular Noncompaction: New Insights into a Poorly Understood Disease. Curr. Cardiol. Rev. 2021, 17, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Jenni, R.; Oechslin, E.; Schneider, J.; Attenhofer Jost, C.; Kaufmann, J.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular noncompaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Wu, M. Mechanisms of Trabecular Formation and Specification During Cardiogenesis. Pediatr. Cardiol. 2018, 39, 1082–1089. [Google Scholar] [CrossRef]
- Almeida, A.G.; Pinto, F.J. Non-compaction cardiomyopathy. Heart 2013, 99, 1535–1542. [Google Scholar] [CrossRef]
- Llerena-Velastegui, J.; Velastegui-Zurita, S.; Santander-Fuentes, C.; Dominguez-Gavilanes, D.; Roa-Guerra, A.; Jesus, A.C.F.S.; Coelho, P.M.; Carrasco-Perez, P.; Calderon-Lopez, C.; Benitez-Gutierrez, D. Advances and challenges in the diagnosis and management of left ventricular noncompaction in adults: A literature review. Curr. Probl. Cardiol. 2024, 49, 102571. [Google Scholar] [CrossRef]
- Nemes, A. Myocardial Mechanics and Associated Valvular and Vascular Abnormalities in Left Ventricular Noncompaction Cardiomyopathy. J. Clin. Med. 2024, 13, 78. [Google Scholar] [CrossRef]
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, M.F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012, 29, 861–872. [Google Scholar] [CrossRef]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef]
- Nemes, A.; Ambrus, N.; Lengyel, C. Normal reference values of three-dimensional speckle-tracking echocardiography-derived aortic valve annular dimensions in healthy adults—A detailed analysis from the MAGYAR-Healthy Study. Quant. Imaging Med. Surg 2025, 15, 6776–6786. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef]
- Stöllberger, C.; Gerecke, B.; Finsterer, J.; Engberding, R. Refinement of echocardiographic criteria for left ventricular noncom paction. Int. J. Cardiol. 2013, 165, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Naubauer, S. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef]
- Jacquier, A.; Thuny, F.; Jop, B.; Giorgi, R.; Cohen, F.; Gaubert, J.Y.; Vidal, V.; Bartoli, J.M.; Habib, G.; Moulin, G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur. Heart J. 2010, 31, 1098–1104. [Google Scholar] [CrossRef]
- Grothoff, M.; Pachowsky, M.; Hoffmann, J.; Posch, M.; Klaassen, S.; Lehmkuhl, L.; Gutberlet, M. Value of cardiovascular MR in diagnosing left ventricular non-compaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur. Radiol. 2012, 22, 2699–2709. [Google Scholar] [CrossRef]
- Rao, K.; Bhaskaran, A.; Choudhary, P.; Tan, T.C. The role of multimodality imaging in the diagnosis of left ventricular noncom paction. Eur. J. Clin. Investig. 2020, 50, e13254. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 254–275. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar]
- Bennett, C.E.; Freudenberger, R. The Current Approach to Diagnosis and Management of Left Ventricular Noncompaction Cardiomyopathy: Review of the Literature. Cardiol. Res. Pract. 2016, 2016, 5172308. [Google Scholar] [CrossRef]
- Kleijn, S.A.; Aly, M.F.A.; Terwee, C.B.; van Rossum, A.C.; Kamp, O. Comparison between direct volumetric and speckle tracking methodologies for left ventricular and left atrial chamber quantification by three-dimensional echocardiography. Am. J. Cardiol. 2011, 108, 1038–1044. [Google Scholar] [CrossRef]
- Kleijn, S.A.; Aly, M.F.A.; Terwee, C.B.; van Rossum, A.C.; Kamp, O. Reliability of left ventricular volumes and function measurements using three-dimensional speckle tracking echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Kleijn, S.A.; Brouwer, W.P.; Aly, M.F.A.; Russel, I.K.; de Roest, G.J.; Beek, A.M.; van Rossum, A.C.; Kamp, O. Comparison between three-dimensional speckle-tracking echocardiography and cardiac magnetic resonance imaging for quantification of left ventricular volumes and function. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 834–839. [Google Scholar] [CrossRef]
- Nesser, H.J.; Mor-Avi, V.; Gorissen, W.; Weinert, L.; Steringer-Mascherbauer, R.; Niel, J.; Sugeng, L.; Lang, R.M. Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking: Comparison with MRI. Eur. Heart J. 2009, 30, 1565–1573. [Google Scholar] [CrossRef]
- Nemes, A.; Ambrus, N.; Lengyel, C. Differences in left ventricular functional properties in healthy adults with greater and-diastolic versus end-systolic aortic valve annular area—Detailed analysis from the three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. Quant. Imaging Med. Surg. 2025, in press. [Google Scholar]
- Silva, R.C.; Mariani, J., Jr.; Falcão, B.A.A.; Filho, A.E.; Nomura, C.H.; Avila, L.F.R.; Parga, J.R.; Lemos Neto, P.A. Differences between systolic and diastolic dimensions of the aortic valve annulus in computed tomography angiography in patients undergoing percutaneous implantation of aortic valve prosthesis by catheter. Rev. Bras. Cardiol. Invasiva (Engl. Ed.) 2015, 23, 130–133. [Google Scholar] [CrossRef][Green Version]
- Nazarzadeh, M.; Pinho-Gomes, A.C.; Smith Byrne, K.; Canoy, D.; Raimondi, F.; Ayala Solares, J.R.; Otto, C.M.; Rahimi, K. Systolic Blood Pressure and Risk of Valvular Heart Disease: A Mendelian Randomization Study. JAMA Cardiol. 2019, 4, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Rossebø, A.B.; Pedersen, T.R. Hyperlipidaemia and aortic valve disease. Curr. Opin. Lipidol. 2004, 15, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, A.; Domsik, P.; Forster, T.; Nemes, A. Left ventricular strain reduction is not confined to the noncompacted segments in noncompaction cardiomyopathy-insights from the three-dimensional speckle tracking echocardiographic MAGYAR-Path Study. Echocardiography 2014, 31, 638–643. [Google Scholar] [CrossRef] [PubMed]
| Controls (n = 38) | Isolated LVNC Patients (n = 12) | |
|---|---|---|
| Risk factors | ||
| Age (years) | 48.2 ± 8.0 | 54.6 ± 13.6 |
| Male gender (%) | 19 (50) | 7 (60) |
| Hypertension (%) | 0 (0) | 5 (42) * |
| Diabetes mellitus (%) | 0 (0) | 0 (0) |
| Hypercholesterolemia (%) | 0 (0) | 3 (25) * |
| Basal heart rate (1/s) | 78 ± 3 | 85 ± 8 |
| Systolic blood pressure (mm Hg) | 122 ± 5 | 134 ± 12 |
| Diastolic blood pressure (mm Hg) | 80 ± 3 | 85 ± 6 |
| Two-dimensional echocardiography | ||
| LA diameter (mm) | 38.3 ± 4.5 | 46.0 ± 8.8 * |
| LV end-diastolic diameter (mm) | 47.9 ± 3.8 | 62.7 ± 11.7 * |
| LV end-diastolic volume (mL) | 107.4 ± 21.7 | 198.6 ± 79.9 * |
| LV end-systolic diameter (mm) | 32.1 ± 3.4 | 48.0 ± 13.4 * |
| LV end-systolic volume (mL) | 37.3 ± 9.5 | 117.4 ± 67.3 * |
| Interventricular septum (mm) | 9.4 ± 1.2 | 10.2 ± 1.7 |
| LV posterior wall (mm) | 9.5 ± 1.4 | 9.9 ± 1.3 |
| LV ejection fraction (%) | 65.1 ± 3.9 | 39.1 ± 14.3 * |
| All Controls (n = 38) | Controls with Greater End-Diastolic AVA-A (n = 17) | Controls with Greater End-Systolic AVA-A (n = 21) | All Isolated LVNC Patients (n = 12) | Isolated LVNC Patients with Greater End-Diastolic AVA-A (n = 9) | Isolated LVNC Patients with Greater End-Systolic AVA-A (n = 3) | |
|---|---|---|---|---|---|---|
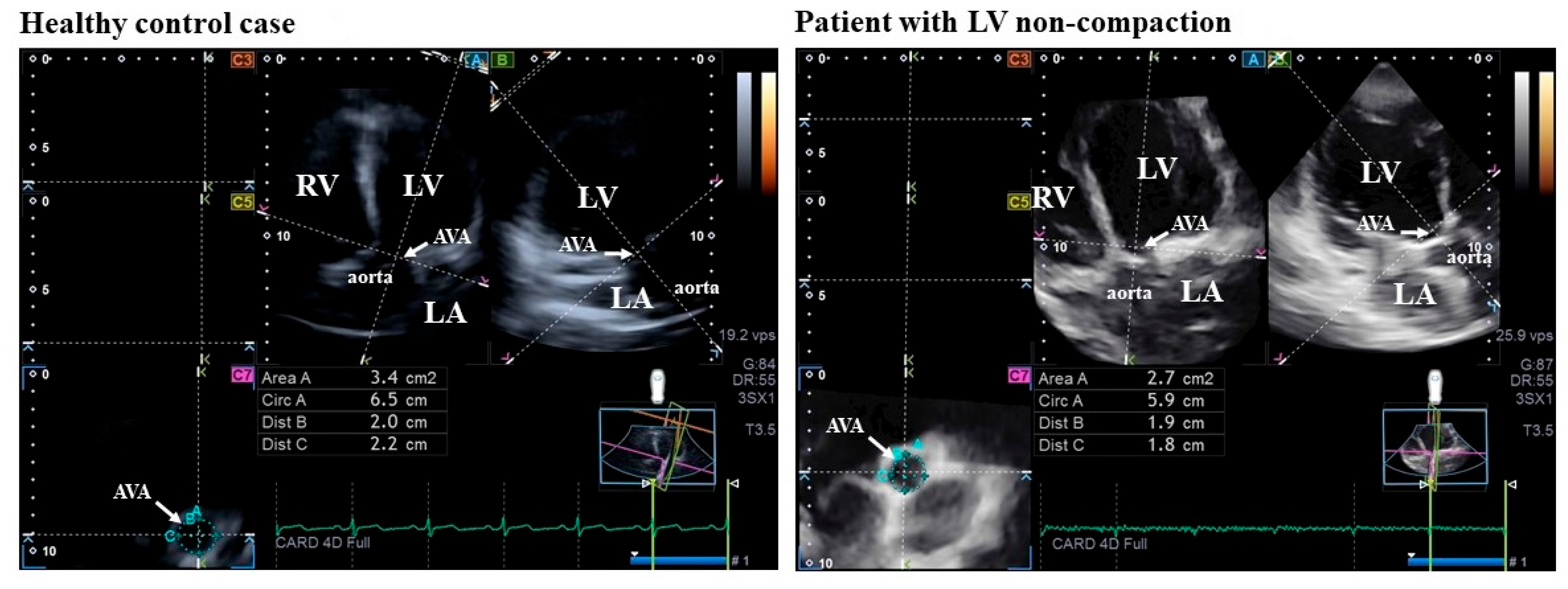
| AVA-Dmax-D (cm) | 2.08 ± 0.26 | 2.18 ± 0.28 * # | 1.99 ± 0.20 * | 2.04 ± 0.48 | 2.08 ± 0.50 * | 1.93 ± 0.37 |
| AVA-Dmin-D (cm) | 1.86 ± 0.26 | 1.94 ± 0.23 ‡ | 1.79 ± 0.27 * | 1.72 ± 0.27 * | 1.70 ± 0.29 * | 1.77 ± 0.21 |
| AVA-A-D (cm2) | 3.30 ± 0.78 | 3.55 ± 0.82 * # | 3.09 ± 0.67 * | 3.19 ± 1.08 | 3.26 ± 1.13 * | 3.00 ± 0.88 |
| AVA-P-D (cm) | 6.48 ± 0.78 | 6.74 ± 0.79 * # | 6.26 ± 0.70 * | 6.33 ± 1.13 | 6.40 ± 1.20 * | 6.13 ± 0.87 |
| AVA-Dmax-S (cm) | 2.02 ± 0.27 | 1.97 ± 0.28 | 2.06 ± 0.26 | 1.92 ± 0.50 | 1.81 ± 0.53 | 2.20 ± 0.22 |
| AVA-Dmin-S (cm) | 1.90 ± 0.29 † | 1.86 ± 0.34 ‡ | 1.93 ± 0.24 | 1.58 ± 0.40 | 1.49 ± 0.39 | 1.83 ± 0.31 |
| AVA-A-S (cm2) | 3.33 ± 0.85 † | 3.07 ± 0.93 | 3.55 ± 0.71 | 2.69 ± 1.04 | 2.37 ± 0.89 | 3.67 ± 0.82 |
| AVA-P-S (cm) | 6.48 ± 0.88 | 6.23 ± 0.94 | 6.69 ± 0.77 | 6.02 ± 1.31 | 5.76 ± 1.38 | 6.80 ± 0.70 |
| AAPSE (cm) | 1.12 ± 0.24 † | 1.11 ± 0.21 ‡ | 1.12 ± 0.27 | 0.78 ± 0.28 | 0.72 ± 0.21 | 0.97 ± 0.37 |
| Basal LV-RS (%) | 31.8 ± 11.8 † | 27.6 ± 8.5 # ‡ | 35.2 ± 12.9 @ | 15.0 ± 8.0 | 15.4 ± 8.4 | 13.4 ± 7.6 |
| Basal LV-CS (%) | −26.1 ± 5.6 † | −27.2 ± 5.6 ‡ | −25.2 ± 5.4 @ | −10.2 ± 3.1 | −10.4 ± 3.3 | −8.6 ± 2.5 |
| Basal LV-LS (%) | −20.4 ± 4.4 † | −18.9 ± 3.5 # ‡ | −21.5 ± 4.6 @ | −9.1 ± 2.2 | −9.0 ± 2.3 | −8.7 ± 2.0 |
| Intraobserver Agreement | Interobserver Agreement | |||
|---|---|---|---|---|
| Mean ± 2SD Difference in Values Obtained by 2 Measurements of the Same Observer | Interclass Correlation Coefficient Between Measurements of the Same Observer | Mean ± 2SD Difference in Values Obtained by 2 Observers | Interclass Correlation Coefficient Between Independent Measurements of 2 Observers | |
| AVA-Dmax-D (cm) | −0.05 ± 0.19 | 0.87 (p < 0.01) | −0.06 ± 0.18 | 0.87 (p < 0.01) |
| AVA-Dmin-D (cm) | −0.01 ± 0.25 | 0.90 (p < 0.01) | −0.03 ± 0.23 | 0.93 (p < 0.01) |
| AVA-A-D (cm2) | −0.13 ± 0.60 | 0.94 (p < 0.01) | −0.11 ± 0.58 | 0.95 (p < 0.01) |
| AVA-P-D (cm) | −0.07 ± 0.65 | 0.90 (p < 0.01) | −0.12 ± 0.70 | 0.95 (p < 0.01) |
| AVA-Dmax-S (cm) | 0.01 ± 0.32 | 0.91 (p < 0.01) | 0.02 ± 0.33 | 0.93 (p < 0.01) |
| AVA-Dmin-S (cm) | 0.07 ± 0.34 | 0.81 (p < 0.01) | 0.04 ± 0.37 | 0.83 (p < 0.01) |
| AVA-A-S (cm2) | 0.12 ± 0.70 | 0.91 (p < 0.01) | 0.13 ± 0.77 | 0.95 (p < 0.01) |
| AVA-P-S (cm) | −0.01 ± 0.58 | 0.91 (p < 0.01) | 0.02 ± 0.53 | 0.92 (p < 0.01) |
| AAPSE (cm) | −0.02 ± 0.20 | 0.90 (p < 0.01) | −0.03 ± 0.20 | 0.90 (p < 0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, A.; Ambrus, N.; Vámos, M.; Gagyi, R.B.; Szili-Török, T.; Ruzsa, Z.; Lengyel, C. Aortic Valve Annular Characteristics in Isolated Left Ventricular Non-Compaction—Detailed Analysis from the Three-Dimensional Speckle Tracking Echocardiographic MAGYAR-Path Study. J. Clin. Med. 2025, 14, 5778. https://doi.org/10.3390/jcm14165778
Nemes A, Ambrus N, Vámos M, Gagyi RB, Szili-Török T, Ruzsa Z, Lengyel C. Aortic Valve Annular Characteristics in Isolated Left Ventricular Non-Compaction—Detailed Analysis from the Three-Dimensional Speckle Tracking Echocardiographic MAGYAR-Path Study. Journal of Clinical Medicine. 2025; 14(16):5778. https://doi.org/10.3390/jcm14165778
Chicago/Turabian StyleNemes, Attila, Nóra Ambrus, Máté Vámos, Rita B. Gagyi, Tamás Szili-Török, Zoltán Ruzsa, and Csaba Lengyel. 2025. "Aortic Valve Annular Characteristics in Isolated Left Ventricular Non-Compaction—Detailed Analysis from the Three-Dimensional Speckle Tracking Echocardiographic MAGYAR-Path Study" Journal of Clinical Medicine 14, no. 16: 5778. https://doi.org/10.3390/jcm14165778
APA StyleNemes, A., Ambrus, N., Vámos, M., Gagyi, R. B., Szili-Török, T., Ruzsa, Z., & Lengyel, C. (2025). Aortic Valve Annular Characteristics in Isolated Left Ventricular Non-Compaction—Detailed Analysis from the Three-Dimensional Speckle Tracking Echocardiographic MAGYAR-Path Study. Journal of Clinical Medicine, 14(16), 5778. https://doi.org/10.3390/jcm14165778







