From the Ocean to the Operating Room: The Role of Fish Skin Grafts in Burn Management—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
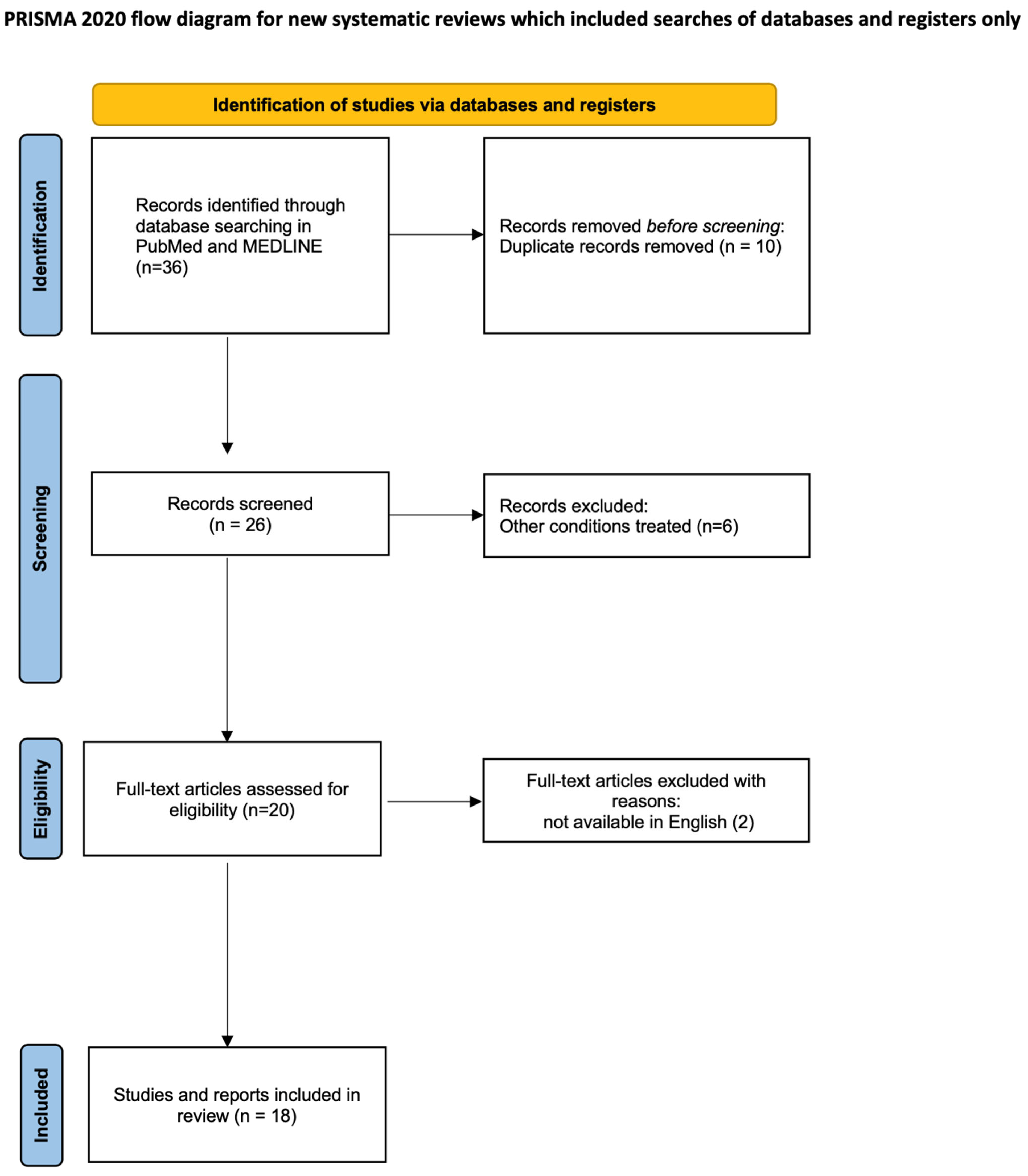
2.2. Study Selection
2.3. Data Extraction
2.4. Data Synthesis
3. Results
3.1. Comparative Preclinical Studies
3.2. Retrospective Preclinical Study
3.3. Clinical Studies
3.4. Case Series and Case Reports
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FSG | Fish skin graft |
| DPTB | Deep partial-thickness burn |
| FTB | Full-thickness burn |
| SPTB | Superficial partial-thickness burn |
| STSG | Split-thickness skin graft |
| TBSA | Total body surface area |
| ADM | Acellular dermal matrix |
| SSD | Silver sulfadiazine |
| PSC | Porcine skin-derived collagen |
| PUFAs | Polyunsaturated fatty acids |
| LCUFAs | Long-chain unsaturated fatty acids |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| SPMs | Specialized pro-resolving mediators |
| VAS | Visual analogue scale |
| CFU | Colony-forming units |
| IHS | Irradiated human skin |
| BTM | Biodegradable temporizing matrix |
| HAM | Hyaluronic acid ester matrix |
| PLA | Polylactic acid |
| POSAS | Patient and observer scar assessment scale |
| NaCMC-Ag | Sodium Carboxymethylcellulose impregnated with Silver |
References
- World Health Organization. Burns. Available online: https://www.who.int/news-room/fact-sheets/detail/burns (accessed on 6 July 2025).
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamolz, L.-P. Recent trends in burn epidemiology worldwide: A systematic review. Burns 2017, 43, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Reda, F.; Kjartansson, H.; Jeffery, S.L.A. Use of Fish Skin Graft in Management of Combat Injuries Following Military Drone Assaults in Field-Like Hospital Conditions. Mil. Med. 2023, 188, e3377–e3381. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Khare, N.A.; Chandramouli, M.V.; Shende, N.; Bharadwaj, S. Comparative Analysis of Early Excision and Grafting vs Delayed Grafting in Burn Patients in a Developing Country. J. Burn Care Res. 2016, 37, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Teplitz, C. Pathogenesis of Pseudomonas vasculitis and septic legions. Arch. Pathol. 1965, 80, 297–307. [Google Scholar]
- Thompson, P.; Herndon, D.N.; Abston, S.; Rutan, T. Effect of early excision on patients with major thermal injury. J. Trauma 1987, 27, 205–207. [Google Scholar] [CrossRef]
- Tam, J.; Wang, Y.; Farinelli, W.A.B.; Jiménez-Lozano, J.; Franco, W.; Sakamoto, F.H.; Cheung, E.J.; Purschke, M.; Doukas, A.G.; Anderson, R.R. Fractional Skin Harvesting: Autologous Skin Grafting without Donor-site Morbidity. Plast. Reconstr. Surg. Glob. Open 2013, 1, e47. [Google Scholar] [CrossRef]
- Pati, F.; Datta, P.; Adhikari, B.; Dhara, S.; Ghosh, K.; Das Mohapatra, P.K. Collagen scaffolds derived from fresh water fish origin and their biocompatibility. J. Biomed. Mater. Res. A 2012, 100, 1068–1079. [Google Scholar] [CrossRef]
- Yamada, S.; Yamamoto, K.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Potency of fish collagen as a scaffold for regenerative medicine. Biomed. Res. Int. 2014, 2014, 302932. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Trautinger, F.; Kokoschka, E.M.; Menzel, E.J. Antibody formation against human collagen and C1q in response to a bovine collagen implant. Arch. Dermatol. Res. 1991, 283, 395–399. [Google Scholar] [CrossRef]
- Yang, C.K.; Polanco, T.O.; Lantis, J.C. A Prospective, Postmarket, Compassionate Clinical Evaluation of a Novel Acellular Fish-skin Graft Which Contains Omega-3 Fatty Acids for the Closure of Hard-to-heal Lower Extremity Chronic Ulcers. Wounds 2016, 28, 112–118. [Google Scholar] [PubMed]
- Magnusson, S.; Baldursson, B.T.; Kjartansson, H.; Rolfsson, O.; Sigurjonsson, G.F. Regenerative and Antibacterial Properties of Acellular Fish Skin Grafts and Human Amnion/Chorion Membrane: Implications for Tissue Preservation in Combat Casualty Care. Mil. Med. 2017, 182, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Dorweiler, B.; Trinh, T.T.; Dünschede, F.; Vahl, C.F.; Debus, E.S.; Storck, M.; Diener, H. The marine Omega3 wound matrix for treatment of complicated wounds: A multicenter experience report. Gefasschirurgie 2018, 23, 46–55. [Google Scholar] [CrossRef]
- Kjartansson, H.; Thorisson, H.; Baldursson, B.T.; Gunnarsson, E.; Jorundsson, E.; Sigurjonsson, G.F. Use of Acellular Fish Skin for Dura Repair in an Ovine Model: A Pilot Study. Open J. Mod. Neurosurg. 2015, 5, 4. [Google Scholar] [CrossRef]
- Rakers, S.; Gebert, M.; Uppalapati, S.; Meyer, W.; Maderson, P.; Sell, A.F.; Kruse, C.; Paus, R. ‘Fish matters’: The relevance of fish skin biology to investigative dermatology. Exp. Dermatol. 2010, 19, 313–324. [Google Scholar] [CrossRef]
- Magnússon, S.; Baldursson, B.T.; Kjartansson, H.; Thorlacius, G.E.; Axelsson, Í.; Rolfsson, Ó.; Petersen, P.H.; Sigurjónsson, G.F. Affrumað roð: Eðliseiginleikar sem styðja vefjaviðgerð [Decellularized fish skin: Characteristics that support tissue repair]. Laeknabladid 2015, 101, 567–573. [Google Scholar] [CrossRef]
- Mil-Homens, D.; Bernardes, N.; Fialho, A.M. The antibacterial properties of docosahexaenoic omega-3 fatty acid against the cystic fibrosis multiresistant pathogen Burkholderia cenocepacia. FEMS Microbiol. Lett. 2012, 328, 61–69. [Google Scholar] [CrossRef]
- Imai, Y. Role of omega-3 PUFA-derived mediators, the protectins, in influenza virus infection. Biochim. Biophys. Acta 2015, 1851, 496–502. [Google Scholar] [CrossRef]
- Piomelli, D.; Hohmann, A.G.; Seybold, V.; Hammock, B.D. A lipid gate for the peripheral control of pain. J. Neurosci. 2014, 34, 15184–15191. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, J.; Guo, Z.; Wu, Z.; Sun, Y.; Wang, K.; Duan, R. Study on the preparation and properties of acellular matrix from the skin of silver carp (Hypophthalmichthys molitrix). J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Varon, D.E.; Carlsson, A.H.; Cooper, L.E.; Chapa, J.; A Valdera, F.; Christy, S.; Christy, R.J.; Chan, R.K.; Nuutila, K.J. Evaluation of Topical Off-The-Shelf Therapies to Improve Prolonged Field Care of Burn-Injured Service Members. Mil. Med. 2023, 188, 3034–3044. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.; Saathoff, E.C.; Larson, D.A.; Wall, J.T.; Wienandt, N.A.; Magnusson, S.; Kjartansson, H.; Natesan, S.; Christy, R.J. Accelerated Wound Closure of Deep Partial Thickness Burns with Acellular Fish Skin Graft. Int. J. Mol. Sci. 2021, 22, 1590. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, H.; Zhang, X.; Chen, Z.; Zhao, D.; Ma, J. A comparative study of two porous sponge scaffolds prepared by collagen derived from porcine skin and fish scales as burn wound dressings in a rabbit model. Regen. Biomater. 2020, 7, 63–70. [Google Scholar] [CrossRef]
- Mauer, E.S.; Maxwell, E.A.; Cocca, C.J.; Ganjei, J.; Spector, D. Acellular fish skin grafts for the management of wounds in dogs and cats: 17 cases (2019–2021). Am. J. Vet. Res. 2022, 83, 188–192. [Google Scholar] [CrossRef]
- Yoon, J.; Yoon, D.; Lee, H.; Lee, J.; Jo, S.; Kym, D.; Yim, H.; Hur, J.; Chun, W.; Kim, G.; et al. Wound healing ability of acellular fish skin and bovine collagen grafts for split-thickness donor sites in burn patients: Characterization of acellular grafts and clinical application. Int. J. Biol. Macromol. 2022, 205, 452–461. [Google Scholar] [CrossRef]
- Lima Júnior, E.M.; de Moraes Filho, M.O.; Costa, B.A.; Fechine, F.V.M.; Vale, M.L.B.; Diógenes, A.K.d.L.B.; Neves, K.R.T.P.; Uchôa, A.M.D.N.B.; Soares, M.F.A.D.N.B.; de Moraes, M.E.A.M. Nile Tilapia Fish Skin-Based Wound Dressing Improves Pain and Treatment-Related Costs of Superficial Partial-Thickness Burns: A Phase III Randomized Controlled Trial. Plast. Reconstr. Surg. 2021, 147, 1189–1198. [Google Scholar] [CrossRef]
- Lima Júnior, E.M.; de Moraes Filho, M.O.; Costa, B.A.; Fechine, F.V.; Rocha, M.B.S.; Vale, M.L.; Diógenes, A.K.d.L.; Uchôa, A.M.D.N.; Júnior, F.R.S.; Martins, C.B.; et al. A Randomized Comparison Study of Lyophilized Nile Tilapia Skin and Silver-Impregnated Sodium Carboxymethylcellulose for the Treatment of Superficial Partial-Thickness Burns. J. Burn Care Res. 2021, 42, 41–48. [Google Scholar] [CrossRef]
- Lima Júnior, E.M.; De Moraes Filho, M.O.; Costa, B.A.; Rohleder, A.V.P.; Rocha, M.B.S.; Fechine, F.V.; Forte, A.J.; Alves, A.P.N.N.; Júnior, F.R.S.; Martins, C.B.; et al. Innovative Burn Treatment Using Tilapia Skin as a Xenograft: A Phase II Randomized Controlled Trial. J. Burn Care Res. 2020, 41, 585–592. [Google Scholar] [CrossRef]
- Lima Júnior, E.M.; Moraes Filho, M.O.; Forte, A.J.; Costa, B.A.; Fechine, F.V.; Alves, A.P.N.N.; de Moraes, M.E.A.; Rocha, M.B.S.; Júnior, F.R.S.; Soares, M.F.A.D.N.; et al. Pediatric Burn Treatment Using Tilapia Skin as a Xenograft for Superficial Partial-Thickness Wounds: A Pilot Study. J. Burn Care Res. 2020, 41, 241–247. [Google Scholar] [CrossRef]
- Badois, N.; Bauër, P.; Cheron, M.; Hoffmann, C.; Nicodeme, M.; Choussy, O.; Lesnik, M.; Poitrine, F.C.; Fromantin, I. Acellular fish skin matrix on thin-skin graft donor sites: A preliminary study. J. Wound Care 2019, 28, 624–628. [Google Scholar] [CrossRef]
- Dawson, K.A.; Mickelson, M.A.; Blong, A.E.; Walton, R.A. Management of severe burn injuries with novel treatment techniques including maggot debridement and applications of acellular fish skin grafts and autologous skin cell suspension in a dog. J. Am. Vet. Med. Assoc. 2022, 260, 428–435. [Google Scholar] [CrossRef]
- Wallner, C.; Holtermann, J.; Drysch, M.; Schmidt, S.; Reinkemeier, F.; Wagner, J.M.; Dadras, M.; Sogorski, A.; Houschyar, K.S.; Becerikli, M.; et al. The Use of Intact Fish Skin as a Novel Treatment Method for Deep Dermal Burns Following Enzymatic Debridement: A Retrospective Case-Control Study. Eur. Burn J. 2022, 3, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Lima Júnior, E.M.; de Moraes Filho, M.O.; Costa, B.A.; Alves, A.P.N.N.; de Moraes, M.E.A.; Uchôa, A.M.D.N.; Martins, C.B.; Bandeira, T.d.J.P.G.; Rodrigues, F.A.R.; Paier, C.R.K.; et al. Lyophilised tilapia skin as a xenograft for superficial partial thickness burns: A novel preparation and storage technique. J. Wound Care 2020, 29, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.A.; Lima Júnior, E.M.; de Moraes Filho, M.O.; Fechine, F.V.; de Moraes, M.E.A.; Júnior, F.R.S.; Soares, M.F.A.D.N.; Rocha, M.B.S. Use of Tilapia Skin as a Xenograft for Pediatric Burn Treatment: A Case Report. J. Burn Care Res. 2019, 40, 714–717. [Google Scholar] [CrossRef]
- Sandness, B.; Struble, A.-M. Use of an Acellular Fish Skin Graft Rich in Omega-3 (Kerecis Omega3 BURN) in a Canine Burn Wound. Michigan State University College of Veterinary Medicine. Available online: https://cvm.msu.edu/vetschool-tails/kerecis-graft-canine-burn-wound (accessed on 29 July 2019).
- Alam, K.; Jeffery, S.L.A. Acellular Fish Skin Grafts for Management of Split Thickness Donor Sites and Partial Thickness Burns: A Case Series. Mil. Med. 2019, 184, 16–20. [Google Scholar] [CrossRef]
- Lima-Junior, E.M.; de Moraes Filho, M.O.; Costa, B.A.; Fechine, F.V.; de Moraes, M.E.A.; Silva-Junior, F.R.; Soares, M.F.A.D.N.; Rocha, M.B.S.; Leontsinis, C.M.P. Innovative treatment using tilapia skin as a xenograft for partial thickness burns after a gunpowder explosion. J. Surg. Case Rep. 2019, 2019, rjz181. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, R.; Tian, Y.; Konno, K. Characterisation of acid-soluble collagen from skin of silver carp (Hypophthalmichthys molitrix). Food Chem. 2009, 116, 318–322. [Google Scholar] [CrossRef]
- Sun, L.; Li, B.; Song, W.; Si, L.; Hou, H. Characterization of Pacific cod (Gadus macrocephalus) skin collagen and fabrication of collagen sponge as a good biocompatible biomedical material. Process Biochem. 2017, 63, 229–235. [Google Scholar] [CrossRef]
- Horst, M.; Milleret, V.; Nötzli, S.; Madduri, S.; Sulser, T.; Gobet, R.; Eberli, D. Increased porosity of electrospun hybrid scaffolds improved bladder tissue regeneration. J. Biomed. Mater. Res. A 2014, 102, 2116–2124. [Google Scholar] [CrossRef]
- Farhat, W.; Chen, J.; Erdeljan, P.; Shemtov, O.; Courtman, D.; Khoury, A.; Yeger, H. Porosity of porcine bladder acellular matrix: Impact of ACM thickness. J. Biomed. Mater. Res. A 2003, 67, 970–974. [Google Scholar] [CrossRef]
- Li, D.; Sun, W.Q.; Wang, T.; Gao, Y.; Wu, J.; Xie, Z.; Zhao, J.; He, C.; Zhu, M.; Zhang, S.; et al. Evaluation of a novel tilapia-skin acellular dermis matrix rationally processed for enhanced wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112202. [Google Scholar] [CrossRef]
- Freyman, T.; Yannas, I.; Gibson, L. Cellular materials as porous scaffolds for tissue engineering. Prog. Mater. Sci. 2001, 46, 273–282. [Google Scholar] [CrossRef]
- Patel, M.; Lantis, J.C., II. Fish skin acellular dermal matrix: Potential in the treatment of chronic wounds. Chronic Wound Care Manag. Res. 2019, 6, 59–70. [Google Scholar] [CrossRef]
- Kotronoulas, A.; de Lomana, A.L.G.; Karvelsson, S.T.; Heijink, M.; Ii, R.S.; Giera, M.; Rolfsson, O. Lipid mediator profiles of burn wound healing: Acellular cod fish skin grafts promote the formation of EPA and DHA derived lipid mediators following seven days of treatment. Prostaglandins Leukot. Essent. Fat. Acids 2021, 175, 102358. [Google Scholar] [CrossRef] [PubMed]
- Fiakos, G.; Kuang, Z.; Lo, E. Improved skin regeneration with acellular fish skin grafts. Eng. Regen. 2020, 1, 95–101. [Google Scholar] [CrossRef]
- Kotronoulas, A.; Jónasdóttir, H.S.; Sigurðardóttir, R.S.; Halldórsson, S.; Haraldsson, G.G.; Rolfsson, Ó. Wound healing grafts: Omega-3 fatty acid lipid content differentiates the lipid profiles of acellular Atlantic cod skin from traditional dermal substitutes. J. Tissue Eng. Regen. Med. 2020, 14, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Bush, K.; Gertzman, A.A. Process development and manufacturing of human and animal acellular dermal matrices. In Skin Tissue Engineering and Regenerative Medicine; Albanna, M.Z., Holmes, J.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 83–103. [Google Scholar]
- Wang, Y.; Zhang, C.L.; Zhang, Q.; Li, P. Composite electrospun nanomembranes of fish scale collagen peptides/chito-oligosaccharides: Antibacterial properties and potential for wound dressing. Int. J. Nanomed. 2011, 6, 667–676. [Google Scholar] [CrossRef]
- Nie, F.; Su, D.; Shi, Y.; Chen, J.; Wang, H.; Qin, W.; Wang, S.; Chen, Y. Early high volume lung lavage for acute severe smoke inhalation injury in dogs. Mol. Med. Rep. 2014, 9, 863–871. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Maskrey, B.H.; Megson, I.L.; Rossi, A.G.; Whitfield, P.D. Emerging importance of omega-3 fatty acids in the innate immune response: Molecular mechanisms and lipidomic strategies for their analysis. Mol. Nutr. Food Res. 2013, 57, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Escudero, G.E.; Romañuk, C.B.; Toledo, M.E.; Olivera, M.E.; Manzo, R.H.; Laino, C.H. Analgesia enhancement and prevention of tolerance to morphine: Beneficial effects of combined therapy with omega-3 fatty acids. J. Pharm. Pharmacol. 2015, 67, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Strandvik, B. Fatty acid metabolism in cystic fibrosis. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 121–129. [Google Scholar] [CrossRef]
- Mimoun, M.; Coste, T.C.; Lebacq, J.; Lebecque, P.; Wallemacq, P.; Leal, T.; Armand, M. Increased tissue arachidonic acid and reduced linoleic acid in a mouse model of cystic fibrosis are reversed by supplemental glycerophospholipids enriched in docosahexaenoic acid. J. Nutr. 2009, 139, 2358–2364. [Google Scholar] [CrossRef]
- Olveira, G.; Olveira, C.; Acosta, E.; Espíldora, F.; Garrido-Sánchez, L.; García-Escobar, E.; Rojo-Martínez, G.; Gonzalo, M.; Soriguer, F. Fatty acid supplements improve respiratory, inflammatory and nutritional parameters in adults with cystic fibrosis. Arch. Bronconeumol. 2010, 46, 70–77. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef]
- Menon, R.; Krzyszczyk, P.; Berthiaume, F. Pro-resolution potency of resolvins D1, D2 and E1 on neutrophil migration and in dermal wound healing. Nano Life 2017, 7, 1750002. [Google Scholar] [CrossRef]


| Study (Author, Year) | Study Type | Burn Etiology and Depth | Fish Skin Type | Comparison Product | Cohort/Animal Model | Treatment Period | Endpoint(s) | Main Results |
|---|---|---|---|---|---|---|---|---|
| Wei et al., 2023 [22] | Comparative Animal Study | Flame, DPTB | Silver carp (Hypophthalmichthys molitrix) | Commercial product | 3 Kunming mice (3 groups: no treatment, commercial product, fish skin) | 20 days | Wound healing rate | Superior healing in the fish skin group (93.9%) compared to no treatment (+5.5%) and commercial (+7.3%). |
| Reda et al., 2023 [3] | Case Series | Combat injury, SPTB, DPTB | North Atlantic cod (Gadus morhua) | None | 3 patients with burns and blast injuries | 7 days | Granulation tissue formation | Rapid granulation tissue formation. |
| Dawson et al., 2022 [33] | Case Report | Flame, SPTB, DPTB | North Atlantic cod (Gadus morhua) | None | 1 dog with SPTB and DPTB burns | 35 days | Wound healing | More rapid healing. |
| Varon et al., 2023 [23] | Randomized Trial | Flame, DPTB | North Atlantic cod (Gadus morhua) | Human allograft, BTM, PLA, HAM | 5 anesthetized pigs | 28 days | Contraction, revascularization, re-epithelialization, scar index, colony-forming units (CFU) | 100% re-epithelialization at day 28. |
| Yoon J. et al., 2022 [27] | Prospective Comparative Study | Flame, SPTB, DPTB | North Atlantic cod (Gadus morhua) | Bovine collagen (ProHeAL) | 52 patients (STSG donor sites) | Up to 17 days | Healing time | Faster healing by ~2 days with FSG compared to no treatment and bovine collagen. |
| Mauer et al., 2022 [26] | Retrospective Animal Study | Flame, SPTB, DPTB | North Atlantic cod (Gadus morhua) | None | 17 animals (13 dogs, 4 cats), 3 with burns | - | Wound closure | Time to wound closure: 26–145 days (median: 71). |
| Wallner et al., 2022 [34] | Retrospective Case–Control Study | Flame, DPTB | North Atlantic cod (Gadus morhua) | Synthetic skin (Suprathel®), STSG | 12 patients | 28 days | Re-epithelialization time, scar quality | Shorter re-epithelialization time with FSG (22 days) compared to STSG (12 days) and Suprathel (23 days). |
| Stone et al., 2021 [24] | Pre-clinical Trial | Flame, DPTB | North Atlantic cod (Gadus morhua) | Fetal bovine dermis (FBD) | 6 Yorkshire pigs | 28 days | Closure rate, transepidermal water loss, hydration, blood flow | Significantly faster re-epithelialization at day 14 compared to FBD (50.2% vs. 23.5%). |
| Lima Júnior et al., 2021 [28] | Phase III RCT | Flame, SPTB, DPTB | Nile tilapia (Oreochromis niloticus) | 1% SSD | 115 patients (57 FSG, 58 SSD) | 11 days | Re-epithelialization time, n. of dressings, costs, pain | Reduced healing time (−0.5 days), n. of dressings, pain and costs (−42.1%) with FSG. |
| Lima Júnior et al., 2021 [29] | Randomized Pilot Clinical Study | Flame, SPTB | Nile tilapia (Oreochromis niloticus) | Sodium carboxymethylcellulose with silver (NaCMC-Ag) | 24 patients | 11 days | N. of dressings, pain (VAS) | Fewer dressings (1 vs. 2) and significantly lower pain (p = 0.0142) in the FSG group. |
| Shi et al., 2020 [25] | Comparative Animal Study | Flame, SPTB, DPTB | Grass carp (Ctenopharyngodon idellus) | Porcine collagen (PSC), gauze, Vaseline gauze | 2 New Zealand rabbits | 28 days | Water uptake, water vapor transmission rate | Complete healing at 28 days for collagen groups (fish and porcine). Superior water uptake for fish collagen. |
| Lima Júnior et al., 2020 [35] | Case Report | Flame, SPTB | Nile tilapia (Oreochromis niloticus) | None | 1 patient (10% TBSA, SPTB) | 10 days | Re-epithelialization time | Complete re-epithelialization in 10 days. |
| Lima Júnior et al., 2020 [30] | Phase II RCT | Flame, SPTB, DPTB | Nile tilapia (Oreochromis niloticus) | 1% SSD | 62 patients (3 study arms by depth/TBSA) | 23 days | Re-epithelialization time, pain, n. of dressings | Reduced re-epithelialization time by 1.1 to 3.2 days in favor of tilapia over SSD. |
| Lima Júnior et al., 2020 [31] | Phase II Pilot Study | Flame, SPTB | Nile tilapia (Oreochromis niloticus) | 1% SSD | 30 pediatric patients | 11 days | Re-epithelialization time, n. of dressings | Complete re-epithelialization at day 10: 86.7% (tilapia) vs. 53.3% (SSD). Significantly fewer dressings required. |
| Costa et al., 2019 [36] | Case Report | Flame, SPTB, DPTB | Nile tilapia (Oreochromis niloticus) | None | 1 pediatric patient (18% TBSA, SPTB) | 10 days | Re-epithelialization time | Complete re-epithelialization in 10 days. |
| Sandness B et al., 2019 [37] | Case Report | Flame, SPTB, DPTB | North Atlantic cod (Gadus morhua) | None | 1 dog (10% TBSA, FTB) | 19 days | Wound dimensions (length, width) | 95% reduction in wound size after 56 days. |
| Lima Junior et al., 2019 [39] | Case Report | Combat injury, SPTB, DPTB | Nile tilapia (Oreochromis niloticus) | None | 1 patient (16% TBSA, SPTB) | 17 days | Re-epithelialization time | Complete re-epithelialization in 12 and 17 days for the two upper limbs. |
| Alam et al., 2019 [38] | Case Series | Flame, SPTB, DPTB | North Atlantic cod (Gadus morhua) | None | 10 patients (STSG donor sites) | 16 days | Re-epithelialization time, pain, infection | Complete re-epithelialization in an average of 11.5 days. Low pain scores. No infections. |
| Study | Burn Depth | Animal/Patient | Fish Skin Graft | Wound Healing Time |
|---|---|---|---|---|
| Yoon et al., 2022 [27] | SPTB and DPTB | Humans | Nile tilapia (Oreochromis niloticus) | 9.1 ± 1.0 days for group 1 treated with FSG, 10.7 ± 1.5 days for group 2 treated with FSG. |
| Lima et al., 2021 [28] | SPTB and DPTB | Humans | Nile tilapia (Oreochromis niloticus) | 9.7 ± 0.6 days for complete re-epithelialization. |
| Lima et al., 2020 [30] | SPTB and DPTB | Humans | Nile tilapia (Oreochromis niloticus) | 9.77 ± 0.83 days for SPTB group A, 10.56 ± 1.13 days for SPTB group B, 18.10 ± 0.99 days for DPTB group C. |
| Lima et al., 2019 [39] | SPTB and DPTB | Humans | Nile tilapia (Oreochromis niloticus) | 12 days for SPTB, 17 days for DPTB. |
| Lima et al., 2020 [35] | SPTB | Humans | Nile tilapia (Oreochromis niloticus) | 10 days for complete re-epithelialization. |
| Lima et al., 2020 [31] | SPTB | Humans | Nile tilapia (Oreochromis niloticus) | 10.07 ± 0.46 days. |
| Wei et al., 2023 [22] | DPTB | Kumming mice | Silver carp (Hypophthalmichthys molitrix) | 14 days for a wound healing rate of 93.89% ± 3.15%. |
| Wallner et al., 2022 [34] | DPTB | Humans | North Atlantic cod (Gadus morhua) | 22 ± 6.3 days. |
| Varon et al., 2023 [23] | DPTB | Pigs | North Atlantic cod (Gadus morhua) | 28 days for 100% re-epithelialization rate. |
| Stone et al., 2021 [24] | DPTB | Yorkshire pigs | North Atlantic cod (Gadus morhua) | 28 days for a re-epithelialization of >90%. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Araby, M.M.; Marcaccini, G.; Susini, P.; Giardino, F.R.; Pozzi, M.; Pizzo, V.; Grimaldi, L.; Innocenti, A.; Cuomo, R.; Nisi, G.; et al. From the Ocean to the Operating Room: The Role of Fish Skin Grafts in Burn Management—A Systematic Review. J. Clin. Med. 2025, 14, 5750. https://doi.org/10.3390/jcm14165750
El Araby MM, Marcaccini G, Susini P, Giardino FR, Pozzi M, Pizzo V, Grimaldi L, Innocenti A, Cuomo R, Nisi G, et al. From the Ocean to the Operating Room: The Role of Fish Skin Grafts in Burn Management—A Systematic Review. Journal of Clinical Medicine. 2025; 14(16):5750. https://doi.org/10.3390/jcm14165750
Chicago/Turabian StyleEl Araby, Mohamed Marzouk, Gianluca Marcaccini, Pietro Susini, Francesco Ruben Giardino, Mirco Pozzi, Vera Pizzo, Luca Grimaldi, Alessandro Innocenti, Roberto Cuomo, Giuseppe Nisi, and et al. 2025. "From the Ocean to the Operating Room: The Role of Fish Skin Grafts in Burn Management—A Systematic Review" Journal of Clinical Medicine 14, no. 16: 5750. https://doi.org/10.3390/jcm14165750
APA StyleEl Araby, M. M., Marcaccini, G., Susini, P., Giardino, F. R., Pozzi, M., Pizzo, V., Grimaldi, L., Innocenti, A., Cuomo, R., Nisi, G., Pascone, C., & Di Lonardo, A. (2025). From the Ocean to the Operating Room: The Role of Fish Skin Grafts in Burn Management—A Systematic Review. Journal of Clinical Medicine, 14(16), 5750. https://doi.org/10.3390/jcm14165750








