Bilateral–Contralateral Endoscopic Decompression as a Fusion-Deferral Strategy in Upper Lumbar Stenosis: A Structural Rationale and Conditional Framework—A Technical Note with Cases Review
Abstract
1. Introduction
Surgical Technique
2. Materials and Methods
2.1. Case 1: Bilateral–Contralateral Decompression in Upper Lumbar Stenosis
2.2. Case 2: Bilateral–Contralateral Decompression in Adjacent Segmental Stenosis (ASS) After Lumbar Fusion
2.3. Case 3: Bilateral–Contralateral Decompression in Spondylolisthesis
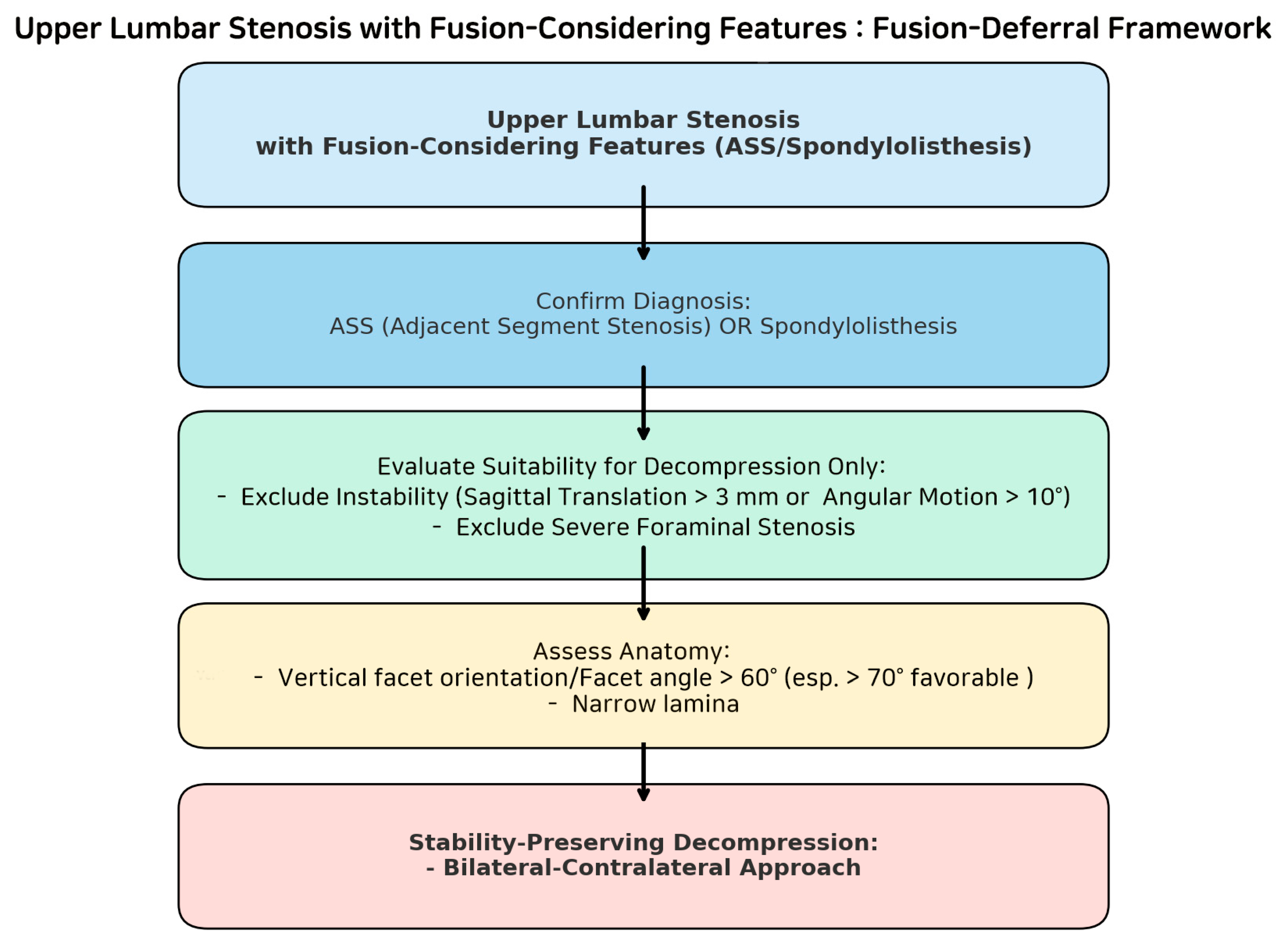
3. Discussion
4. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASS | Adjacent segment stenosis |
| ASD | Adjacent segment disease |
| MRI | Magnetic resonance imaging |
| RCT | Randomized controlled trial |
| UBE | Unilateral biportal endoscopy |
| ULBD | Unilateral laminotomy for bilateral decompression |
References
- Albert, T.J.; Balderston, R.A.; Heller, J.G.; Herkowitz, H.N.; Garfin, S.R.; Tomany, K.; An, H.S.; Simeone, F.A. Upper lumbar disc herniations. J. Spinal Disord. 1993, 6, 351–359. [Google Scholar] [CrossRef]
- Sanderson, S.P.; Houten, J.; Errico, T.; Forshaw, D.; Bauman, J.; Cooper, P.R. The unique characteristics of “Upper” lumbar disc herniations. Neurosurgery 2004, 55, 385–389, discussion 389. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, J.K.; Moon, K.S.; Hur, H.; Kim, Y.S.; Kim, S.H. Transdural approach for calcified central disc herniations of the upper lumbar spine. Technical note. J. Neurosurg. Spine 2007, 7, 370–374. [Google Scholar] [CrossRef]
- Nishikawa, H.; Fujimoto, M.; Tanioka, S.; Ikezawa, M.; Nakatsuka, Y.; Araki, T.; Suzuki, H.; Mizuno, M. Novel transdural Epiarachnoid approach for Large Central disk herniation in upper lumbar spine. Oper. Neurosurg. 2022, 22, e58–e61. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, S.H.; Moon, K.H.; Lee, H.Y. Surgical results of the Oblique paraspinal Approach in Upper Lumbar Disc Herniation and thoracolumbar Junction. Neurosurgery 2009, 65, 95–99, discussion 99. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, S.H.; Lee, J.H.; Kim, J.U.; Liu, W.C. Transforaminal percutaneous endoscopic lumbar discectomy for upper lumbar disc herniation: Clinical outcome, prognostic factors, and technical consideration. Acta Neurochir. 2009, 151, 199–206. [Google Scholar] [CrossRef]
- Hur, J.W.; Kim, J.S.; Cho, D.Y.; Shin, J.M.; Lee, J.H.; Lee, S.H. Video-assisted thoracoscopic surgery under O-arm navigation system guidance for the treatment of thoracic disk herniations: Surgical techniques and early clinical results. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2014, 75, 415–421. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, C.K.; Kim, J.S.; Hwang, J.S.; Lee, J.Y.; Lee, D.G.; Jang, J.W.; Kim, J.Y.; Cho, Y.E.; Lee, D.C. O-arm navigation-based transforaminal unilateral biportal endoscopic discectomy for upper lumbar disc herniation: An innovative preliminary study. Asian Spine J. 2025, 19, 194–204. [Google Scholar] [CrossRef]
- Hwang, J.S.; Lee, S.H.; Jeong, D.; Jang, J.W.; Cho, Y.E.; Lee, D.G.; Park, C.K.; Chough, C.K. Far-lateral transforaminal unilateral biportal endoscopic lumbar discectomy for upper lumbar disc herniations. Neurospine 2025, 22, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Masharawi, Y.M.; Alperovitch-Najenson, D.; Steinberg, N.; Dar, G.; Peleg, S.; Rothschild, B.; Salame, K.; Hershkovitz, I. Lumbar facet orientation in spondylolysis: A skeletal study. Spine 2007, 32, E176–E180. [Google Scholar] [CrossRef] [PubMed]
- Jentzsch, T.; Geiger, J.; Zimmermann, S.M.; Slankamenac, K.; Nguyen-Kim, T.D.L.; Werner, C.M.L. Lumbar facet joint arthritis is associated with more coronal orientation of the facet joints at the upper lumbar spine. Radiol. Res. Pract. 2013, 2013, 693971. [Google Scholar] [CrossRef]
- Njoku, I.U.; Park, J.Y.K.; Munim, M.A.; Clarke, A.; Cheng, C.W. An anatomic study examining lumbar pars interarticularis distance and spinal canal width in relation to lumbar decompressive surgery. Int. J. Spine Surg. 2022, 16, 646–650. [Google Scholar] [CrossRef]
- Van Schaik, J.P.; Verbiest, H.; Van Schaik, F.D. The orientation of laminae and facet joints in the lower lumbar spine. Spine 1985, 10, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Veerapen, R.; O’Laoire, S.A. Relief of lumbar canal stenosis using multilevel subarticular fenestrations as an alternative to wide laminectomy: Preliminary report. Neurosurgery 1988, 23, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Jang, H.J.; Moon, B.J.; Kim, K.H.; Chin, D.K.; Kim, K.S.; Park, J.Y. Comparative outcomes of biportal endoscopic decompression, conventional subtotal laminectomy, and minimally invasive transforaminal lumbar interbody fusion for lumbar central stenosis. Neurospine 2024, 21, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- So, J.Y.; Park, J.Y. Comparison of postoperative bone healing in patients with unilateral biportal endoscopic lumbar discectomy and microscopic lumbar discectomy. J. Minim. Invasive Spine Surg. Tech. 2023, 8 (Suppl. S1), S29–S38. [Google Scholar] [CrossRef]
- Park, M.K.; Park, J.Y.; Son, S.K. Complications of endoscopic thoracic spine surgery: Overview and complication avoidance. World Neurosurg. 2023, 179, 127–132. [Google Scholar] [CrossRef]
- Park, D.Y.; Olson, T.E.; Upfill-Brown, A.; Adejuyigbe, B.; Shah, A.A.; Sheppard, W.L.; Park, C.W.; Heo, D.H. Biportal endoscopic approach for lumbar degenerative disease in the ambulatory outpatient vs inpatient setting: A comparative study. Int. J. Spine Surg. 2023, 17, 858–865. [Google Scholar] [CrossRef]
- Park, S.M.; Song, K.S.; Ham, D.W.; Kang, M.S.; You, K.H.; Park, C.K.; Kim, J.S.; Park, H.J. Comparing the efficacy and safety of biportal endoscopic discectomy with microscopic discectomy for lumbar herniated intervertebral disc: A multicentre, prospective, assessor-blinded, randomized controlled trial. Bone Joint J. 2025, 107B, 529–539. [Google Scholar] [CrossRef]
- Kwon, H.; Park, J.Y. The role and future of endoscopic spine surgery: A narrative review. Neurospine 2023, 20, 43–55. [Google Scholar] [CrossRef]
- Kim, H.S.; Patel, R.; Paudel, B.; Jang, J.S.; Jang, I.T.; Oh, S.H.; Park, J.E.; Lee, S. Early outcomes of endoscopic contralateral foraminal and lateral recess decompression via an interlaminar approach in patients with unilateral radiculopathy from unilateral foraminal stenosis. World Neurosurg. 2017, 108, 763–773. [Google Scholar] [CrossRef]
- Heo, D.H.; Kim, J.S.; Park, C.W.; Quillo-Olvera, J.; Park, C.K. Contralateral sublaminar endoscopic approach for removal of lumbar Juxtafacet cysts using percutaneous biportal endoscopic surgery: Technical report and preliminary results. World Neurosurg. 2019, 122, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jang, J.W.; Park, W.M.; Park, C.W. Contralateral keyhole biportal endoscopic surgery for ruptured lumbar herniated disc: A technical feasibility and early clinical outcomes. Neurospine 2020, 17 (Suppl. S1), S110–S119. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Lee, S.H.; Jang, J.W.; Lee, D.G.; Cho, Y.E.; Park, C.K.; Kim, I.S. Comparison of complications of biportal endoscopic discectomy: Ipsilateral versus contralateral approach. J. Clin. Neurosci. 2025, 137, 111282. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, C.K.; Jang, J.W.; Lee, D.G. Safety and utility of bilateral-contralateral decompression for adjacent segment stenosis after lumbar interbody fusion using unilateral biportal endoscopy. Clin. Spine Surg. 2025. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, D.G.; Park, C.K.; Jang, J.W.; Hwang, J.S.; Kim, J.Y.; Cho, Y.E.; Lee, S.W.; Lee, D.C.; Han, B.S.; et al. Saving stabilizing structure treatment with bilateral-contralateral decompression for spinal stenosis in degenerative spondylolisthesis using unilateral biportal endoscopy. Neurospine 2023, 20, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Pestonji, M.D.; Langaliya, M.K.; Banka, P. A novel transforaminal approach for Upmigrated lumbar disc herniations in the hidden zone of MacNab: A surgical technical note. J. Minim. Invasive Spine Surg. Tech. 2024, 9 (Suppl. S2), S185–S193. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, J.S.; Jang, J.W.; Cho, Y.E.; Lee, D.G.; Park, C.K. Far-lateral transforaminal approach for biportal endoscopic upper lumbar discectomy: 2-dimensional operative video. Oper. Neurosurg. 2025, 9900. [Google Scholar] [CrossRef]
- Liang, Y.H.; Kavishwar, R.A.; Pedraza, M.; Setiawan, D.R.; Kim, J.H.; Kim, J.S. Hybrid endoscopic thoracic discectomy using robotic arm and navigation for highly migrated calcified disc herniation. Neurospine 2024, 21, 1126–1130. [Google Scholar] [CrossRef]
- Haher, T.R.; O’Brien, M.; Dryer, J.W.; Nucci, R.; Zipnick, R.; Leone, D.J. The role of the lumbar facet joints in spinal stability. Identification of alternative paths of loading. Spine 1994, 19, 2667–2670, discussion 2671. [Google Scholar] [CrossRef]
- McDonald, C.L.; Alsoof, D.; Glueck, J.; Osorio, C.; Stone, B.; McCluskey, L.; Diebo, B.G.; Daniels, A.H.; Basques, B.A. Adjacent segment disease after spinal fusion. JBJS Rev. 2023, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Louie, P.K.; Harada, G.K.; Sayari, A.J.; Mayo, B.C.; Khan, J.M.; Varthi, A.G.; Yacob, A.; Samartzis, D.; An, H.S. Etiology-based classification of adjacent segment disease following lumbar spine fusion. HSS J. 2020, 16, 130–136. [Google Scholar] [CrossRef]
- Tobert, D.G.; Antoci, V.; Patel, S.P.; Saadat, E.; Bono, C.M. Adjacent segment disease in the cervical and lumbar spine. Clin. Spine Surg. 2017, 30, 94–101. [Google Scholar] [CrossRef]
- Telfeian, A.E. Transforaminal endoscopic surgery for adjacent segment disease after lumbar fusion. World Neurosurg. 2017, 97, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Drysch, A.; Ajiboye, R.M.; Sharma, A.; Li, J.; Reza, T.; Harley, D.; Park, D.Y.; Pourtaheri, S. Effectiveness of reoperations for adjacent segment disease following lumbar spinal fusion. Orthopedics 2018, 41, e161–e167. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Dmitriev, A.E.; Helgeson, M.; Lehman, R.A.; Kuklo, T.R.; Rosner, M.K. Does superior-segment facet violation or laminectomy destabilize the adjacent level in lumbar transpedicular fixation? An in vitro human cadaveric assessment. Spine 2008, 33, 2868–2873. [Google Scholar] [CrossRef]
- Eissa, M.A.; Mohamed, E.S.; Ahmed, A.E. Adjacent segment degeneration following laminectomy versus degeneration without laminectomy in levels adjacent to those operated upon by lumbar posterolateral fixation: A comparative study. Med. J. Cairo Univ. 2023, 91, 1213–1218. [Google Scholar] [CrossRef]
- Hamasaki, T.; Tanaka, N.; Kim, J.; Okada, M.; Ochi, M.; Hutton, W.C. Biomechanical assessment of minimally invasive decompression for lumbar spinal canal stenosis: A cadaver study. J. Spinal Disord. Tech. 2009, 22, 486–491. [Google Scholar] [CrossRef]
- Wang, M.Y.; Green, B.A.; Shah, S.; Vanni, S.; Levi, A.D.O. Complications associated with lumbar stenosis surgery in patients older than 75 years of age. Neurosurg. Focus. 2003, 14, e7. [Google Scholar] [CrossRef]
- Ghogawala, Z.; Dziura, J.; Butler, W.E.; Dai, F.; Terrin, N.; Magge, S.N.; Coumans, J.V.C.E.; Harrington, J.F.; Amin-Hanjani, S.; Schwartz, J.S.; et al. Laminectomy plus Fusion versus laminectomy Alone for Lumbar spondylolisthesis. N. Engl. J. Med. 2016, 374, 1424–1434. [Google Scholar] [CrossRef]
- Schroeder, G.D.; Kepler, C.K.; Kurd, M.F.; Vaccaro, A.R.; Hsu, W.K.; Patel, A.A.; Savage, J.W. Rationale for the surgical treatment of lumbar degenerative spondylolisthesis. Spine 2015, 40, E1161–E1166. [Google Scholar] [CrossRef]
- Eismont, F.J.; Norton, R.P.; Hirsch, B.P. Surgical management of lumbar degenerative spondylolisthesis. J. Am. Acad. Orthop. Surg. 2014, 22, 203–213. [Google Scholar] [CrossRef]
- Urakawa, H.; Jones, T.; Samuel, A.; Vaishnav, A.S.; Othman, Y.; Virk, S.; Katsuura, Y.; Iyer, S.; McAnany, S.; Albert, T.; et al. The necessity and risk factors of subsequent fusion after decompression alone for lumbar spinal stenosis with lumbar spondylolisthesis: 5 years follow-up in two different large populations. Spine J. 2020, 20, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.R.; Gallo, M.C.; Kebaish, K.; Hang, N.; Ton, A.; Hernandez, F.; Abdou, M.; Karakash, W.J.; Wang, J.C.; Hah, R.J.; et al. National trends in lumbar degenerative spondylolisthesis with stenosis treated with fusion versus decompression. Neurospine 2024, 21, 1068–1077. [Google Scholar] [CrossRef]
- Sriphirom, P.; Siramanakul, C.; Sumritsopak, M.; Chokviriyaprasert, P.; Uttamo, N.; Songchou, K. Clinical outcomes of interlaminar percutaneous endoscopic uniportal pars decompression for lumbar spondylolysis. Int. J. Spine Surg. 2023, 17, 335–342. [Google Scholar] [CrossRef]
- White, A.A.; Panjabi, M.M. Clinical Biomechanics of the Spine; Lippincott: New York, NY, USA, 1978. [Google Scholar]
- Levy, H.A.; Astudillo Potes, M.D.; Nassr, A.N.; Freedman, B.A.; Sebastian, A.S. Biomechanical analysis of lumbar decompression technique and the effect on spinal instability: A narrative review. AME Med. J. 2024, 9, 12. [Google Scholar] [CrossRef]
- Blumenthal, C.; Curran, J.; Benzel, E.C.; Potter, R.; Magge, S.N.; Harrington, J.F., Jr.; Coumans, J.V.; Ghogawala, Z. Radiographic predictors of delayed instability following decompression without fusion for degenerative Grade I lumbar spondylolisthesis. J. Neurosurg. Spine 2013, 18, 340–346. [Google Scholar] [CrossRef]
- Shin, M.H.; Kim, J.S.; Ryu, K.S.; Hur, J.W. Bilateral decompression via microscopic TubularCrossing laminotomy (MTCL) for lumbar spinal stenosis: Technique and early surgical result. Neurol. Med. Chir. 2015, 55, 570–577. [Google Scholar] [CrossRef][Green Version]
- Ahn, J.S.; Lee, H.J.; Park, E.J.; Kim, S.B.; Choi, D.J.; Kwon, Y.S.; Chung, H.J. Multifidus muscle changes after biportal endoscopic spinal surgery: Magnetic resonance imaging evaluation. World Neurosurg. 2019, 130, e525–e534. [Google Scholar] [CrossRef] [PubMed]
- Solheim, O. Randomized controlled trials in surgery and the glass ceiling effect. Acta Neurochir. 2019, 161, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K.U.; León, J.F.R.; Dowling, Á.; Garcia, M.R.; Rugeles, J.G.; Ramirez, C.; Garcia, A.; Valerio, J.; Carvalho, P.S.T.; Duchén Rodríguez, L.M.; et al. Breaking through the glass ceiling effect of high-grade clinical evidence creation in orthopaedics & trauma. Rev. Colomb. Ortop. Traumatol. 2022, 36, 215–228. [Google Scholar] [CrossRef]
- Woolf, S.H.; George, J.N. Evidence-based medicine. Interpreting studies and setting policy. Hematol. Oncol. Clin. N. Am. 2000, 14, 761–784. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K.U.; Yeung, A. Meaningful outcome research to validate endoscopic treatment of common lumbar pain generators with durability analysis. J. Spine Surg. 2020, 6 (Suppl. S1), S6–S13. [Google Scholar] [CrossRef] [PubMed]
- Eap, S.; Nagayama Hall, G.C. Relevance of RCTs to diverse groups. In Evidence-Based Outcome Research; Nezu, A.M., Nezu, C.M., Eds.; Oxford University Press: New York, NY, USA, 2007; pp. 425–444. [Google Scholar] [CrossRef]

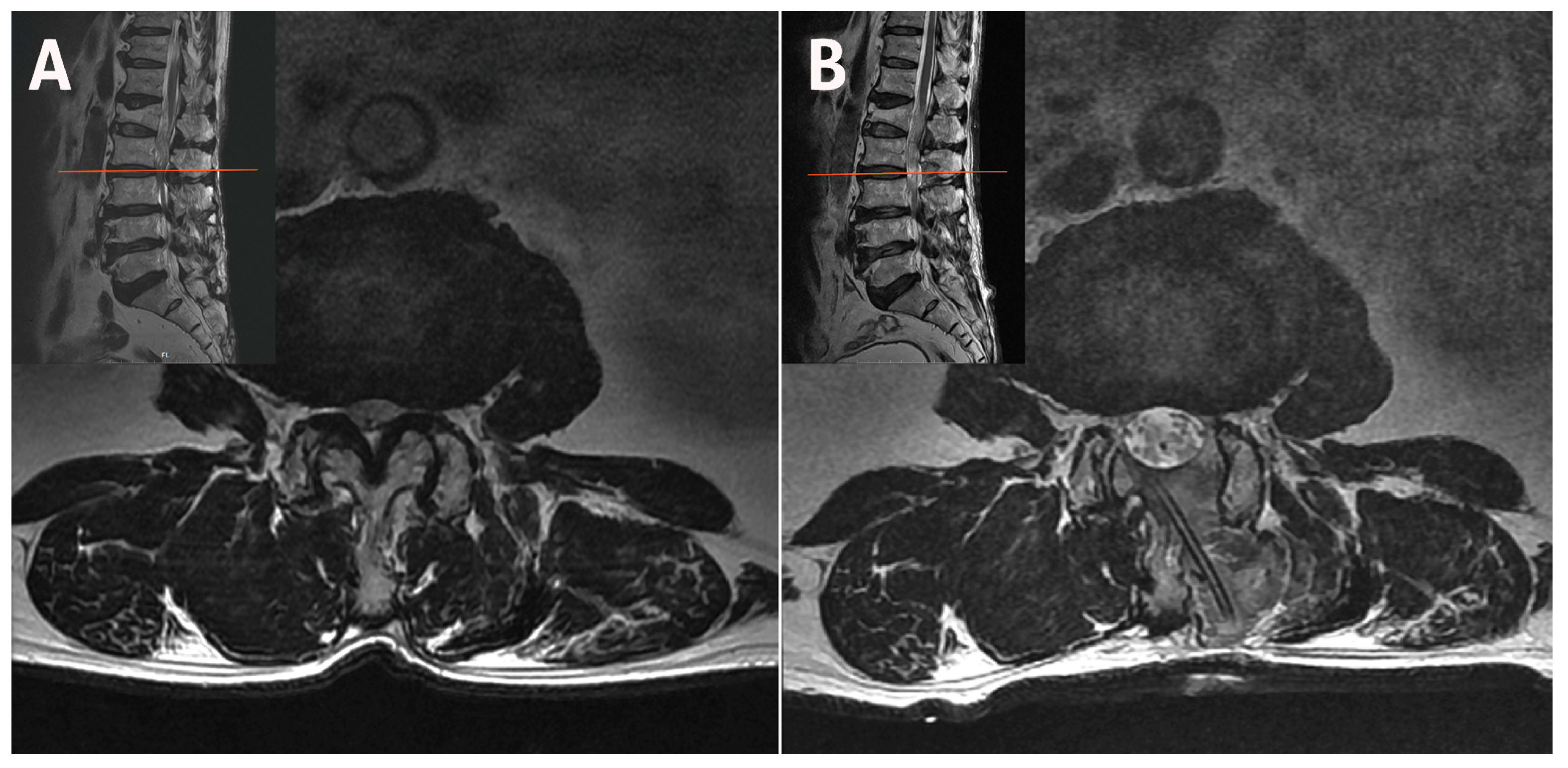

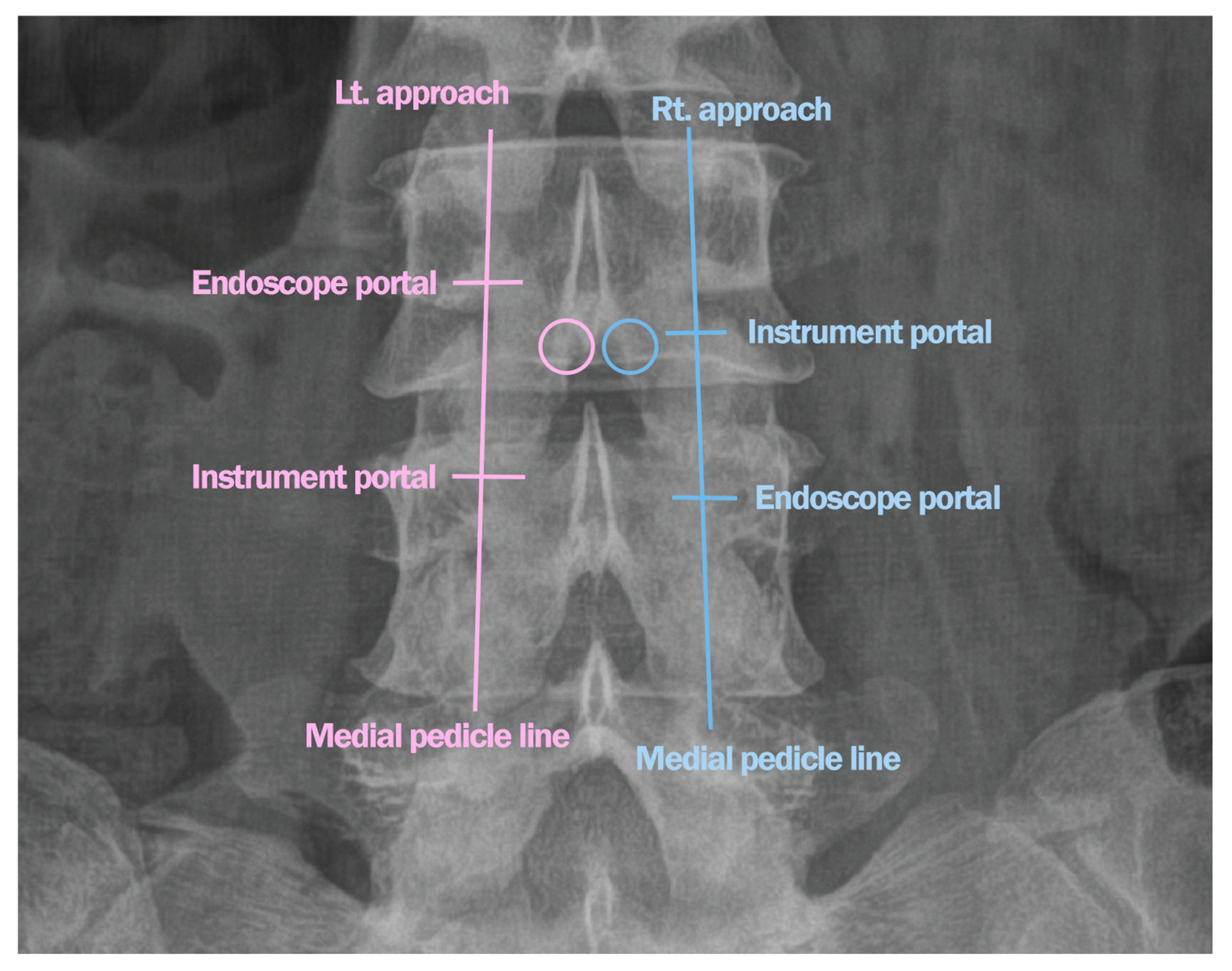
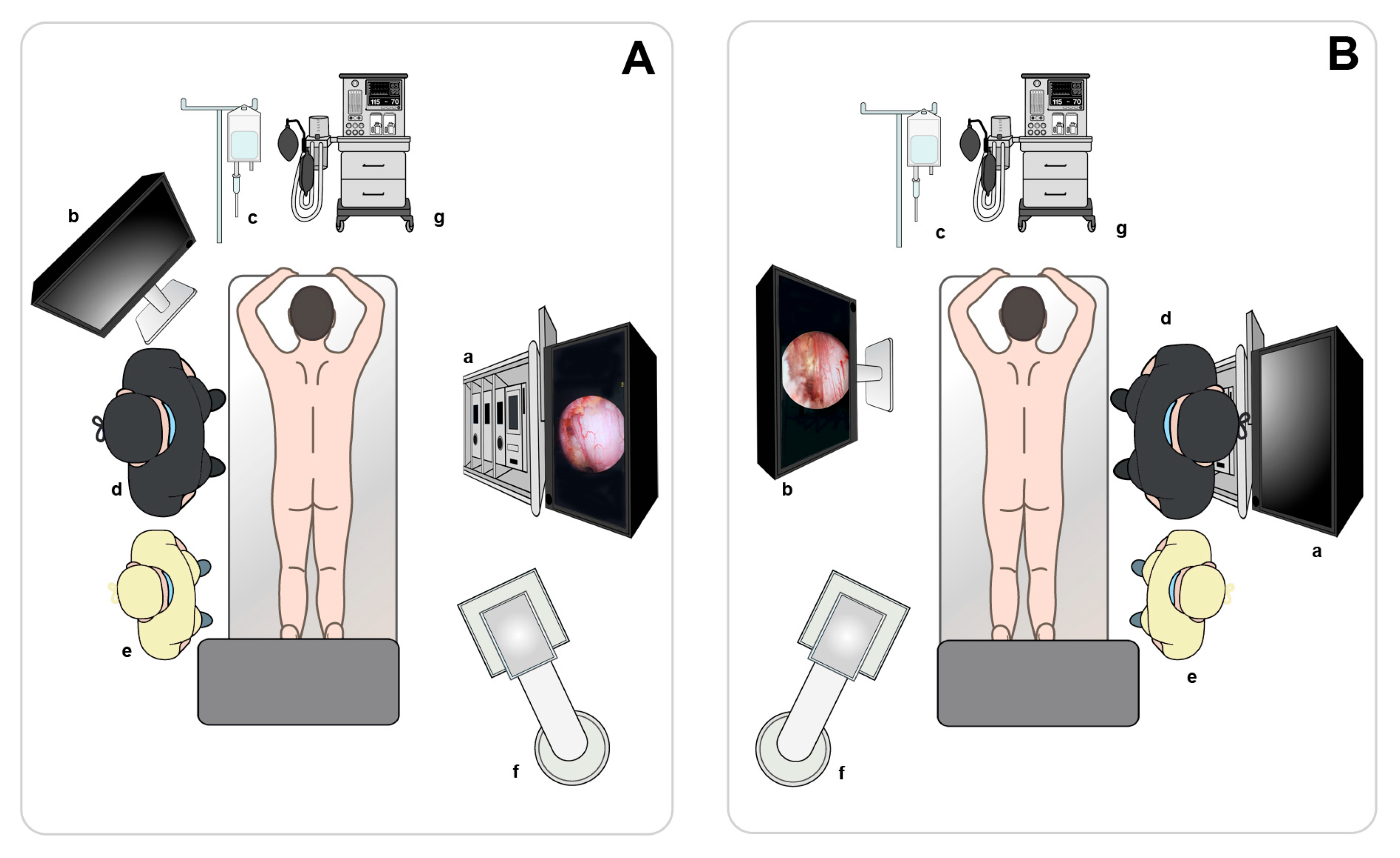
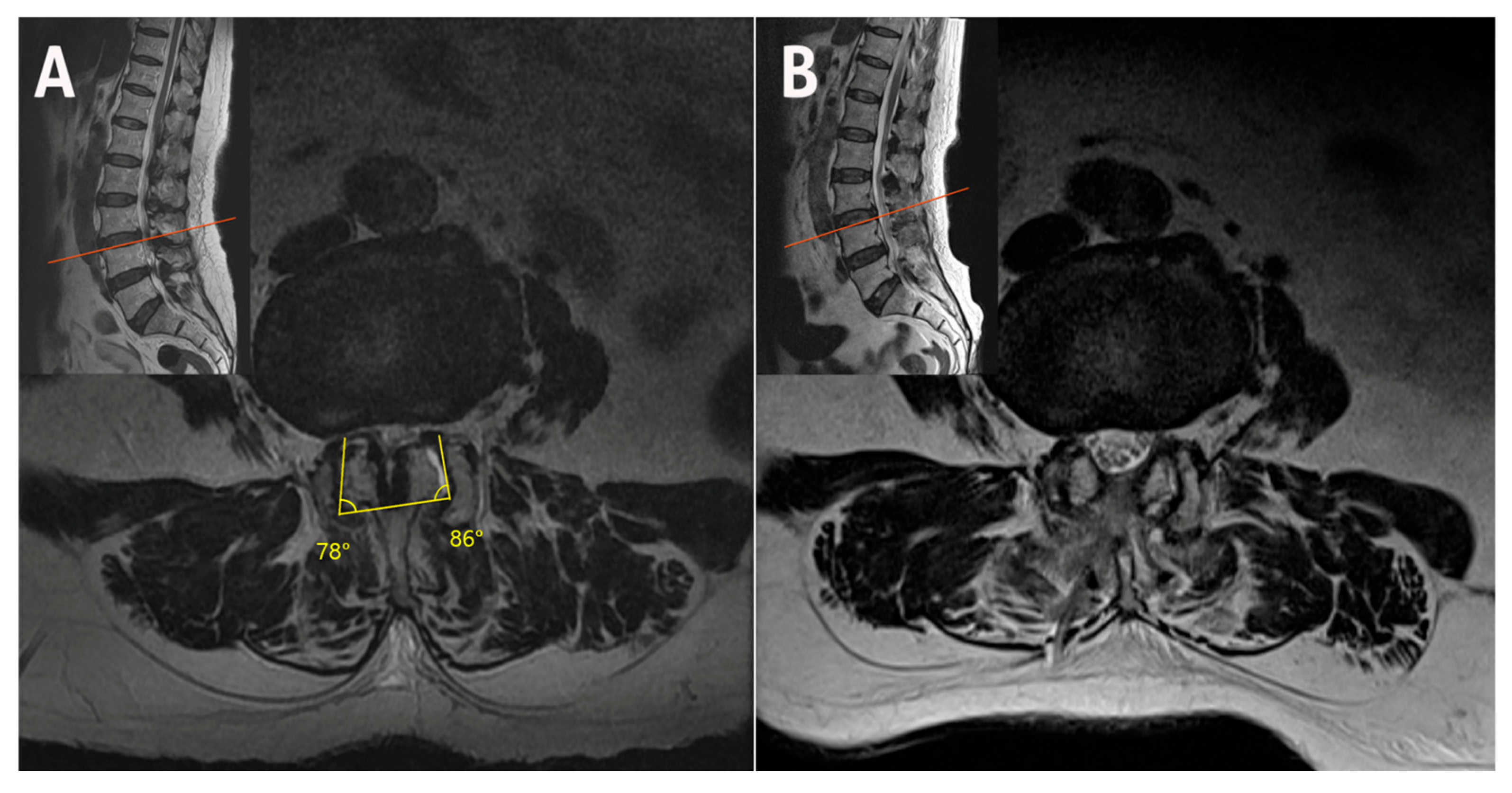
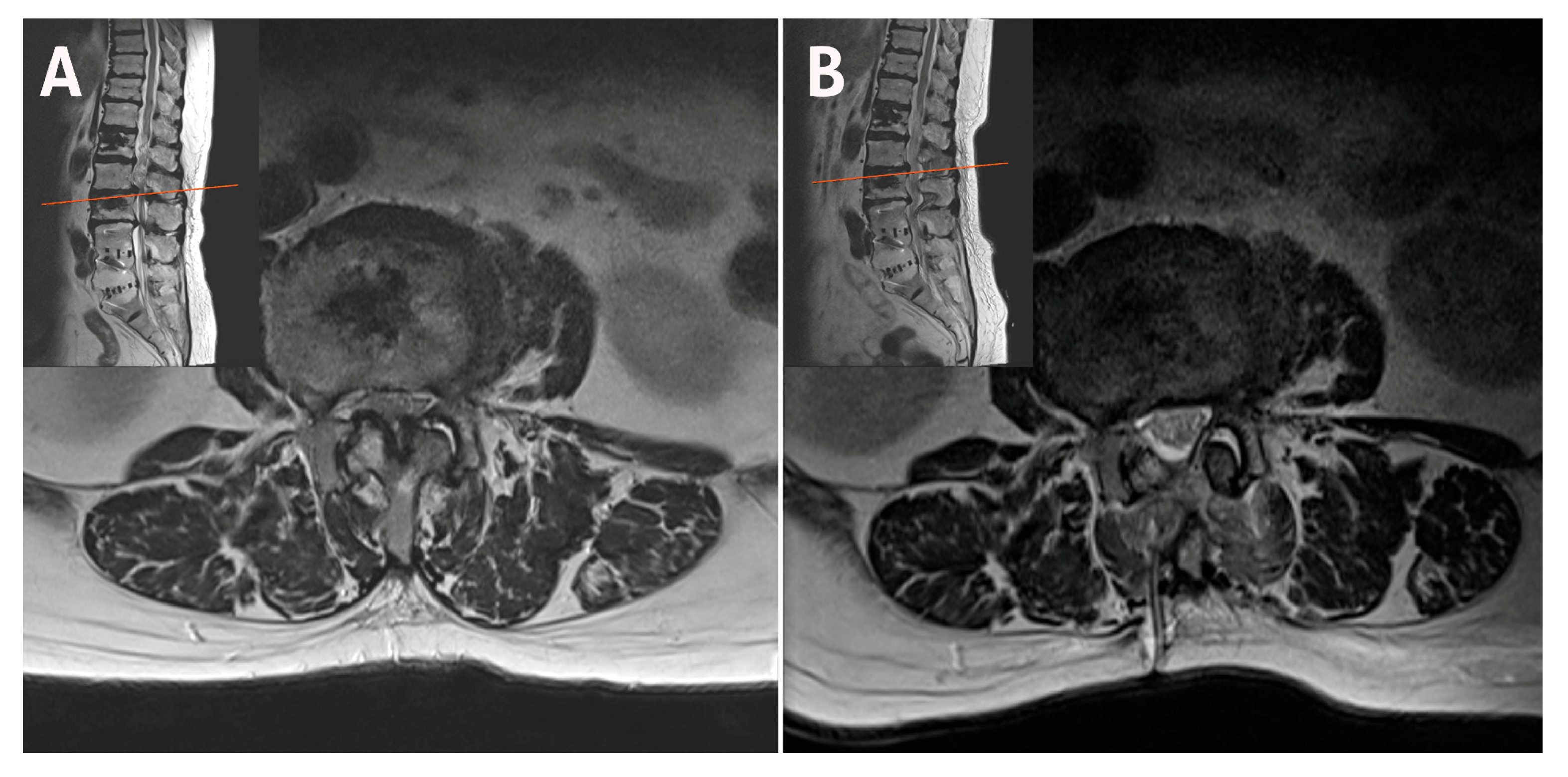

| Case | Age (years) | Sex | Diagnosis | Level | Operative Time (min) | Pre-op Back VAS | Post-op Back VAS | Pre-op Leg VAS | Post-op Leg VAS | Pre-op ODI | Post-op ODI | Complications | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | M | Upper Lumbar Stenosis | L3–L4 | 55 | 6 | 2 | 7 | 1 | 48 | 16 | None | 6 |
| 2 | 82 | F | Adjacent Segment Stenosis | L2–L3 | 68 | 7 | 3 | 8 | 3 | 58 | 26 | None | 6 |
| 3 | 78 | F | Stenosis with Spondylolisthesis | L3–L4 | 64 | 6 | 2 | 8 | 2 | 50 | 20 | None | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.H.; Han, S.Y.; Jeong, S.Y.; Jang, I.-T. Bilateral–Contralateral Endoscopic Decompression as a Fusion-Deferral Strategy in Upper Lumbar Stenosis: A Structural Rationale and Conditional Framework—A Technical Note with Cases Review. J. Clin. Med. 2025, 14, 5726. https://doi.org/10.3390/jcm14165726
Lee DH, Han SY, Jeong SY, Jang I-T. Bilateral–Contralateral Endoscopic Decompression as a Fusion-Deferral Strategy in Upper Lumbar Stenosis: A Structural Rationale and Conditional Framework—A Technical Note with Cases Review. Journal of Clinical Medicine. 2025; 14(16):5726. https://doi.org/10.3390/jcm14165726
Chicago/Turabian StyleLee, Dong Hyun, Sang Yeop Han, Seung Young Jeong, and Il-Tae Jang. 2025. "Bilateral–Contralateral Endoscopic Decompression as a Fusion-Deferral Strategy in Upper Lumbar Stenosis: A Structural Rationale and Conditional Framework—A Technical Note with Cases Review" Journal of Clinical Medicine 14, no. 16: 5726. https://doi.org/10.3390/jcm14165726
APA StyleLee, D. H., Han, S. Y., Jeong, S. Y., & Jang, I.-T. (2025). Bilateral–Contralateral Endoscopic Decompression as a Fusion-Deferral Strategy in Upper Lumbar Stenosis: A Structural Rationale and Conditional Framework—A Technical Note with Cases Review. Journal of Clinical Medicine, 14(16), 5726. https://doi.org/10.3390/jcm14165726






