A Digital Twin Strategy to Predict Thrombotic Recurrence in Antiphospholipid Syndrome Patients Treated with Direct Oral Anticoagulants vs. Vitamin K Antagonists Using Data from Real-World Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Setting
2.2. Baseline Variables
2.3. Outcome
2.4. Digital Twin Creation
2.4.1. Generative Modeling
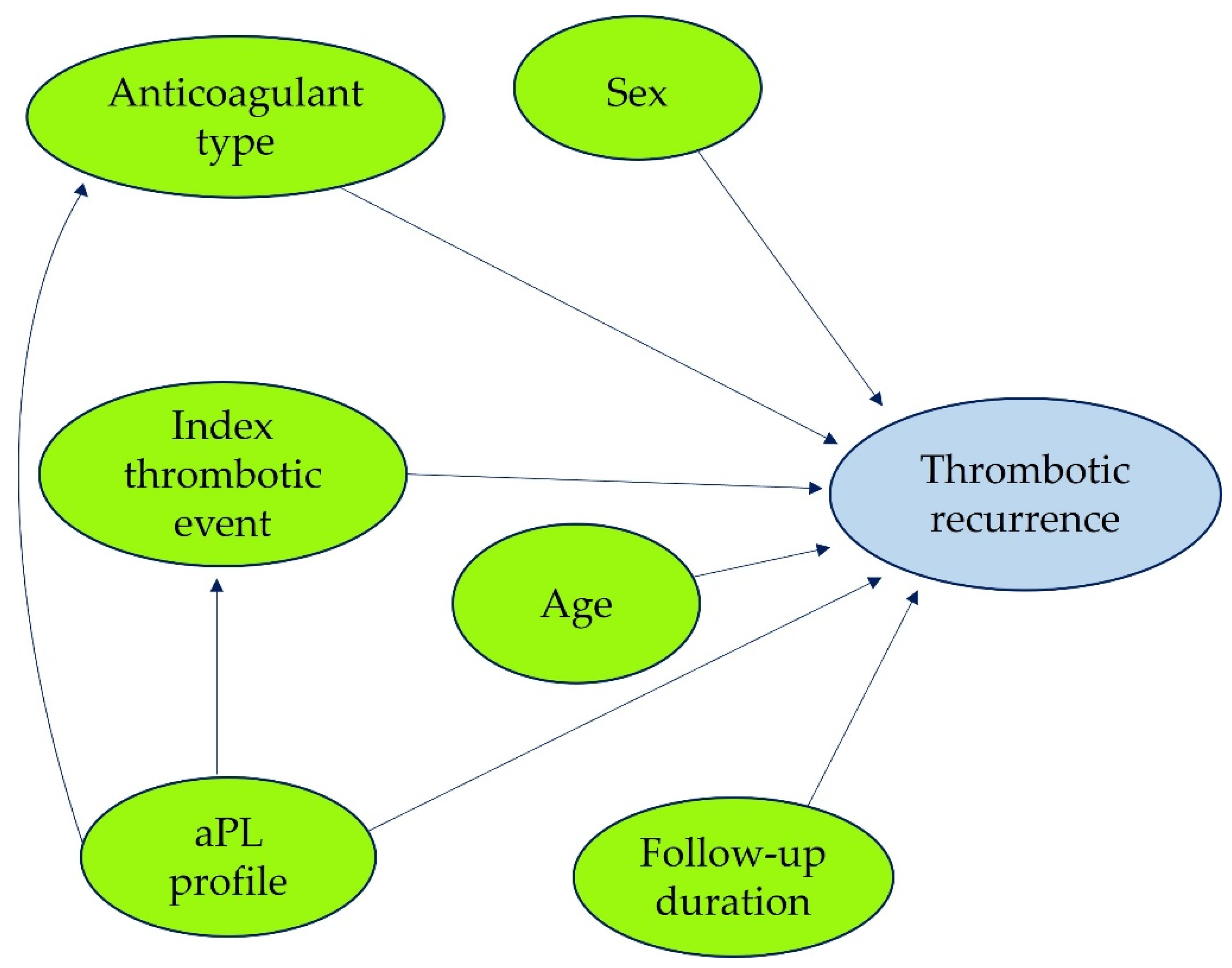
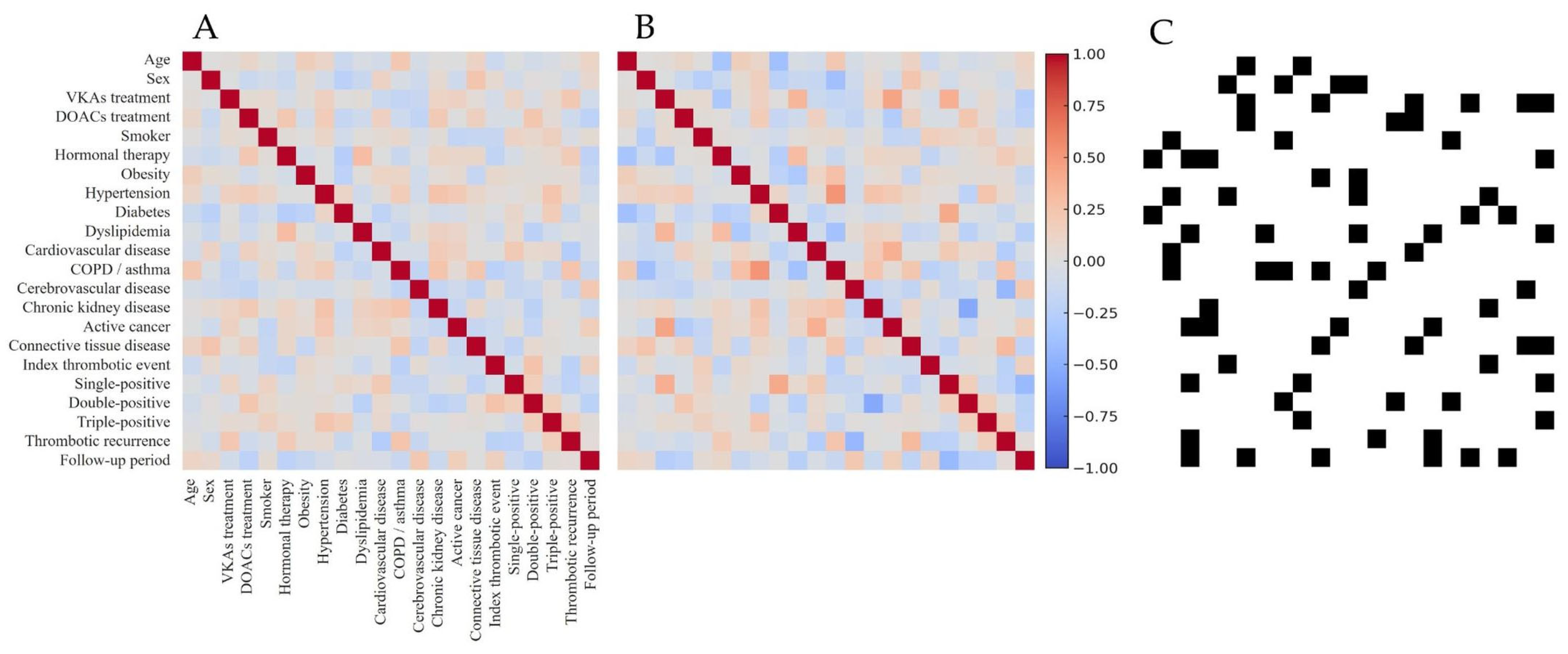
2.4.2. Variable Relationships and DAG Construction
2.5. Model Evaluation (Digital Twins)
2.6. Sensitivity Analyses
2.7. Statistical Analyses in the Original Cohort
3. Results
3.1. Characteristics and Outcomes in Real-World Patients
3.2. Digital Twin
3.2.1. Non-Conditioned Digital Twin Cohort and Internal Structure Validation
3.2.2. Conditioned Twins and Treatment Effect Estimation
3.2.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DOACs | Direct oral anticoagulants |
| VKAs | Vitamin K antagonists |
| APS | Antiphospholipid syndrome |
| DT | Digital twin |
| CGANs | Generative adversarial networks |
| DAG | Directed acyclic graph |
| ASMD | Absolute standardized mean differences |
| MASMD | Mean absolute standardized mean differences |
| aPL | Antiphospholipid antibodies |
| LA | Lupus anticoagulant |
| aCL | Anti-cardiolipin antibodies |
| anti-β2GPI | Anti-beta-2-glycoprotein I antibodies |
| ACR | American College of Rheumatology |
| EULAR | European Alliance of Associations for Rheumatology |
| VTE | Venous thromboembolism |
| RCTs | Randomized controlled trials |
| OR | Odds ratio |
| INR | International normalized ratio |
| SD | Standard deviation |
| IQR | Interquartile range |
| TTR | Time therapeutic range |
References
- Barbhaiya, M.; Zuily, S.; Naden, R.; Hendry, A.; Manneville, F.; Amigo, M.C.; Amoura, Z.; Andrade, D.; Andreoli, L.; Artim-Esen, B.; et al. The 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria. Arthritis Rheumatol. 2023, 75, 1687–1702. [Google Scholar] [CrossRef]
- Salet, D.M.; Bekkering, S.; Middeldorp, S.; van den Hoogen, L.L. Targeting thromboinflammation in antiphospholipid syndrome. J. Thromb. Haemost. 2023, 21, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Serrano, R.; Pons-Estel, G.J.; Ceberio-Hualde, L.; Shoenfeld, Y.; de Ramón, E.; Buonaiuto, V.; Jacobsen, S.; Zeher, M.M.; Tarr, T.; et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann. Rheum. Dis. 2015, 74, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Krnic-Barrie, S.; O’Connor, C.R.; Looney, S.W.; Pierangeli, S.S.; Harris, E.N. A retrospective review of 61 patients with antiphospholipid syndrome. Analysis of factors influencing recurrent thrombosis. Arch. Intern. Med. 1997, 157, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Parpia, S.; Spencer, F.A.; Baglin, T.; Stevens, S.M.; Bauer, K.A.; Lentz, S.R.; Kessler, C.M.; Douketis, J.D.; Moll, S.; et al. Antiphospholipid antibodies and recurrent thrombosis after a first unprovoked venous thromboembolism. Blood 2018, 131, 2151–2160. [Google Scholar] [CrossRef]
- Cohen, H.; Hunt, B.J.; Efthymiou, M.; Arachchillage, D.R.; Mackie, I.J.; Clawson, S.; Sylvestre, Y.; Machin, S.J.; Bertolaccini, M.L.; Ruiz-Castellano, M.; et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): A randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016, 3, e426–e436. [Google Scholar] [CrossRef]
- Pengo, V.; Denas, G.; Zoppellaro, G.; Jose, S.P.; Bison, E.; Bracco, A.; Pegoraro, C.; Iliceto, S.; Prisco, D.; Ruffatti, A. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018, 132, 1365–1371. [Google Scholar] [CrossRef]
- Ordi-Ros, J.; Sáez-Comet, L.; Pérez-Conesa, M.; Vidal, X.; Riera-Mestre, A.; Castro-Salomó, A.; Cuquet-Pedragosa, J.; Ortiz-Santamaria, V.; Mauri-Plana, M.; Solé, C.; et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: A randomized noninferiority trial. Ann. Intern. Med. 2019, 171, 685–694. [Google Scholar] [CrossRef]
- Woller, S.C.; Stevens, S.M.; Kaplan, D.; Wang, T.F.; Branch, D.W.; Groat, D.; Wilson, E.L.; Armbruster, B.; Aston, V.T.; Lloyd, J.F.; et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: A randomized trial. Blood Adv. 2022, 6, 1661–1670. [Google Scholar] [CrossRef]
- Khairani, C.D.; Bejjani, A.; Piazza, G.; Jimenez, D.; Monreal, M.; Chatterjee, S.; Pengo, V.; Woller, S.C.; Cortes-Hernandez, J.; Connors, J.M.; et al. Direct Oral Anticoagulants vs Vitamin K Antagonists in Patients With Antiphospholipid Syndromes: Meta-Analysis of Randomized Trials. J. Am. Coll. Cardiol. 2023, 81, 16–30. [Google Scholar] [CrossRef]
- Bejjani, A.; Khairani, C.D.; Assi, A.; Piazza, G.; Sadeghipour, P.; Talasaz, A.H.; Fanikos, J.; Connors, J.M.; Siegal, D.M.; Barnes, G.D.; et al. When Direct Oral Anticoagulants Should Not Be Standard Treatment: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2024, 83, 444–465, https://doi.org/10.1016/j.jacc.2023.10.038; Erratum in J. Am. Coll. Cardiol. 2024, 83, 1351. [Google Scholar] [CrossRef] [PubMed]
- Riahi, V.; Diouf, I.; Khanna, S.; Boyle, J.; Hassanzadeh, H. Digital Twins for Clinical and Operational Decision-Making: Scoping Review. J. Med. Internet Res. 2025, 27, e55015. [Google Scholar] [CrossRef] [PubMed]
- Katsoulakis, E.; Wang, Q.; Wu, H.; Shahriyari, L.; Fletcher, R.; Liu, J.; Achenie, L.; Liu, H.; Jackson, P.; Xiao, Y.; et al. Digital twins for health: A scoping review. NPJ Digit. Med. 2024, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A. Envisioning the Future of Personalized Medicine: Role and Realities of Digital Twins. J. Med. Internet Res. 2024, 26, e50204. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; DEGroot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liskiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef]
- NASA. Digital Twins and Living Models at NASA. NASA Technical Reports Server. 2021. Available online: https://ntrs.nasa.gov/citations/20210023699 (accessed on 15 May 2025).
- Ferrari, A.; Willcox, K. Digital twins in mechanical and aerospace engineering. Nat. Comput. Sci. 2024, 4, 178–183. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, X.; Lu, J.; Yang, P.; Sun, J. Construction of Data-Driven Performance Digital Twin for a Real-World Gas Turbine Anomaly Detection Considering Uncertainty. Sensors 2023, 23, 6660. [Google Scholar] [CrossRef]
- Abd Wahab, N.H.; Hasikin, K.; Wee Lai, K.; Xia, K.; Bei, L.; Huang, K.; Wu, X. Systematic review of predictive maintenance and digital twin technologies challenges, opportunities, and best practices. PeerJ Comput. Sci. 2024, 10, e1943. [Google Scholar] [CrossRef] [PubMed]
- Moiceanu, G.; Paraschiv, G. Digital Twin and Smart Manufacturing in Industries: A Bibliometric Analysis with a Focus on Industry 4.0. Sensors 2022, 22, 1388. [Google Scholar] [CrossRef]
- John, A.; Alhajj, R.; Rokne, J. A systematic review of AI as a digital twin for prostate cancer care. Comput. Methods Programs Biomed. 2025, 268, 108804. [Google Scholar] [CrossRef]
- Lu, M.; Saeys, W.; Maryam, M.; Gjeleshi, I.; Nazarahari, H.; Truijen, S.; Scataglini, S. Using 3D and 4D digital human modeling in extended reality-based rehabilitation: A systematic review. Front. Bioeng. Biotechnol. 2025, 13, 1496168. [Google Scholar] [CrossRef]
- Faiella, E.; Pileri, M.; Ragone, R.; Grasso, R.F.; Zobel, B.B.; Santucci, D. Digital twins in radiology: A systematic review of applications, challenges, and future perspectives. Eur. J. Radiol. 2025, 189, 112166. [Google Scholar] [CrossRef]
- Franco-Moreno, A.; Izquierdo-Martínez, A.; Ancos-Aracil, C. Rethinking the use of direct oral anticoagulants for secondary thromboprophylaxis in patients with thrombotic antiphospholipid syndrome. Drug Discov. Ther. 2024, 18, 213–219. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Fava, A.; Petri, M.A.; Bikdeli, B.; Galli, M. Direct oral anticoagulants versus Vitamin K antagonists in antiphospholipid syndrome: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2025, 73, 152741. [Google Scholar] [CrossRef]

| RAPS [6] | TRAPS [7] | Ordi-Ros et al. [8] | ASTRO-APS [9] | |
|---|---|---|---|---|
| Year | 2016 | 2018 | 2019 | 2022 |
| Country | United Kingdom | Italy | Spain | United States |
| Index thrombotic event | VTE | Arterial and venous | Arterial and venous | Arterial and venous |
| Median follow-up in months | 7 | 20.4 | 36 | 12 |
| DOAC/comparison | Rivaroxaban 20 mg day vs. warfarin (INR 2.5) | Rivaroxaban 20 or 15 mg day vs. warfarin (INR 2.5) | Rivaroxaban 20 or 15 mg day vs. VKA (INR 2–3) | Apixaban 5 or 2.5 mg twice daily vs. warfarin (INR 2–3) |
| Sample size | 57/59 | 59/61 | 95/95 | 23/25 |
| Mean age | 47/50 | 46.5/46.1 | 47/51 * | 46/48.5 |
| Female sex, % | 74/71 | 66/62 | 64/63 | 83/84 |
| aPL profile, % | ||||
| Simple | 60/48 | 0/0 | 34/32 | 22/30 |
| Double | 28/32 | 0/0 | 5/8 | 17/8 |
| Triple | 12/20 | 100/100 | 61/60 | 30/28 |
| Recurrent thrombosis | ||||
| Total | None for both groups | 7 (12%) vs. 0; p = 0.005 | 11 (11.6%) vs. 3 (6.3%); p = 0.21 | 6 (26%) vs. 0; p = 0.008 |
| Arterial | ― | 7 (12%) † vs. 0 | 10 (10.5%) § vs. 3 (3.2%); p = 0.06 | 6 (26%) € vs. 0; p = 0.008 |
| Venous | ― | 0 vs. 0 | 2 (2.1%) vs. 3 (3.2%); p = 0.65 | 0 vs. 0 |
| Major bleeding | None for both groups | 4 (7%) vs. 2 (3%); p = 0.30 | 6 (6.3%) vs. 7 (7.4%); p = 0.77 | 0 vs. 1 (4%), p = 1.0 |
| Baseline Characteristics | Total (n = 89) | VKA Group (n = 70) | DOAC Group (n = 19) | p-Value |
|---|---|---|---|---|
| Demographic data | ||||
| Age (mean ± SD) | 56.2 (15.6) | 55.2 (16.1) | 60.1 (12.9) | 0.328 |
| Female sex, n (%) | 41 (46.1) | 37 (52.9) | 4 (21.1) | 0.014 |
| Previous conditions, n (%) | ||||
| Hypertension | 44 (49.4) | 32 (45.7) | 12 (63.1) | 0.177 |
| Diabetes | 19 (21.3) | 12 (17.1) | 7 (36.8) | 0.063 |
| Dyslipidemia | 38 (42.7) | 26 (37.1) | 12 (63.2) | 0.042 |
| Obesity (BMI ≥ 30 kg/m2) | 32 (36) | 24 (34.3) | 8 (42.1) | 0.529 |
| Smoking | 37 (41.6) | 27 (38.6) | 10 (52.6) | 0.270 |
| Chronic heart failure | 13 (14.6) | 9 (12.8) | 4 (21.1) | 0.370 |
| COPD/asthma | 29 (32.6) | 25 (35.7) | 4 (21.1) | 0.227 |
| Cerebrovascular disease | 13 (14.6) | 13 (18.6) | 0 | 0.042 |
| Chronic kidney disease | 11 (12.4) | 10 (14.3) | 1 (5.3) | 0.289 |
| Connective tissue disease * | 12 (13.5) | 11 (15.7) | 1 (5.3) | 0.237 |
| Systemic lupus erythematosus | 5 (5.6) | 5 | 0 | 0.230 |
| Active cancer | 5 (5.6) | 4 (5.7) | 1 (5.3) | 0.940 |
| Concomitant antiplatelet therapy | 7 (7.9) | 5 (7.1) | 2 (10.5) | 0.638 |
| Index thrombotic event, n (%) | ||||
| Arterial | 26 (29.2) | 18 (25.7) | 8 (42.1) | 0.267 |
| Venous | 63 (70.8) | 52 (74.3) | 11 (57.9) | 0.267 |
| Type of index arterial or venous thrombotic event, n (%) | ||||
| Stroke | 17 (19.1) | 14 (20.0) | 3 (15.8) | 0.628 |
| Myocardial infarction | 5 (5.6) | 2 (2.8) | 3 (15.8) | 0.063 |
| Peripheral artery disease | 2 (2.2) | 1 (1.4) | 1 (5.3) | 0.383 |
| Mesenteric artery thrombosis | 1 (1.1) | 1 (1.4) | 0 | 0.802 |
| Thrombotic endocarditis | 1 (1.1) | 0 | 1 (5.3) | 0.213 |
| VTE | 63 (70.8) | 52 (74.3) | 11 (57.9) | 0.267 |
| Serological profile, n (%) | ||||
| Single-positive | 34 (38.2) | 26 (37.1) | 8 (42.1) | 0.418 |
| Double-positive | 39 (43.8) | 34 (48.6) | 5 (26.3) | 0.032 |
| Triple-positive | 16 (18.0) | 10 (14.3) | 6 (31.6) | 0.082 |
| Type of serological profile, n (%) | ||||
| Lupus anticoagulant (LA) | 54 (60.7) | 42 (60.0) | 12 (63.1) | 0.803 |
| Anticardiolipin antibodies (aCL) | 56 (62.9) | 42 (60.0) | 14 (73.7) | 0.273 |
| Anti-beta 2 glycoprotein I antibodies (anti-β2GPI) | 52 (58.4) | 39 (55.7) | 13 (68.4) | 0.319 |
| Another thrombophilia, n (%) | 4 (4.5) | 3 (4.3) | 1 (5.3) | 0.855 |
| Thrombotic recurrence during follow-up, n (%) | ||||
| Total | 22 (24.7) | 17 (24.3) | 5 (26.3) | 0.856 |
| Arterial | 7 (7.9) | 6 (8.5) | 1 (5.3) | 0.635 |
| Venous | 15 (16.8) | 11 (15.7) | 4 (21.1) | 0.730 |
| Type of arterial or venous thrombosis during follow-up, n (%) | ||||
| Stroke | 4 (4.5) | 4 (5.7) | 0 | 0.574 |
| Myocardial infarction | 2 (2.2) | 1 (1.4) | 1 (5.3) | 0.383 |
| Placental thrombosis | 1 (1.1) | 1 (1.4) | 0 | 0.802 |
| VTE | 15 (16.8) | 11 (15.7) | 4 (21.1) | 0.730 |
| Mean time to thrombotic recurrence (months) | 21.8 | 23.2 | 16.6 | 0.195 |
| Hemoglobin (median, IQR) † | 13.5 (12.6–14.8) | 13.4 (12.2–14.7) | 13.8 (12.8–14.9) | 0.350 |
| Platelets (median, IQR) † | 203 (160–239) | 198 (155–237) | 222 (179–246) | 0.280 |
| Major bleeding during follow-up, n (%) | 3 (3.4) | 3 (4.3) | 0 | 0.359 |
| Follow-up period (median, IQR) | 46 (36.1–58.7) | 48 (38.2–60.5) | 43 (30.1–52.0) | 0.138 |
| Mortality, n (%) | 7 (7.9) | 6 (8.5) | 1 (5.2) | 0.635 |
| aPL Profile | Treatment Comparison | Outcome | OR (95% CI) | p-Value |
|---|---|---|---|---|
| All patients | DOACs vs. VKAs | Total thrombosis | 0.90 (0.28–2.86) | 0.859 |
| Arterial thrombosis | 1.69 (0.19–14.94) | 0.637 | ||
| Venous thrombosis | 0.70 (0.19–2.51) | 0.588 | ||
| Triple-positive | DOACs vs. VKAs | Total thrombosis | 0.67 (0.10–4.35) | 0.677 |
| Arterial thrombosis | 1.75 (0.13–23.70) | 0.673 | ||
| Venous thrombosis | 0.42 (0.05–3.43) | 0.421 | ||
| Non-triple-positive | DOACs vs. VKAs | Total thrombosis | 2.77 (0.32–23.64) | 0.353 |
| Arterial thrombosis | 1.57 (0.08–31.89) | 0.768 | ||
| Venous thrombosis | 1.76 (0.20–15.52) | 0.611 |
| Type of Index Thrombosis | VKAs—Predicted Recurrence | DOACs—Predicted Recurrence |
|---|---|---|
| Arterial | 25.1% | 46.8% |
| Venous | 18.3% | 17.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado-Suela, M.Á.; Torres-Macho, J.; Izquierdo-Martínez, A.; Ancos-Aracil, C.L.; Ferreira-Burguillos, L.; Madroñal-Cerezo, E.; Talaván-Zañón, T.; Castañeda-Mata, A.; Escobar-Curbelo, L.; de la Casa-Muñoz, A.M.; et al. A Digital Twin Strategy to Predict Thrombotic Recurrence in Antiphospholipid Syndrome Patients Treated with Direct Oral Anticoagulants vs. Vitamin K Antagonists Using Data from Real-World Populations. J. Clin. Med. 2025, 14, 5716. https://doi.org/10.3390/jcm14165716
Casado-Suela MÁ, Torres-Macho J, Izquierdo-Martínez A, Ancos-Aracil CL, Ferreira-Burguillos L, Madroñal-Cerezo E, Talaván-Zañón T, Castañeda-Mata A, Escobar-Curbelo L, de la Casa-Muñoz AM, et al. A Digital Twin Strategy to Predict Thrombotic Recurrence in Antiphospholipid Syndrome Patients Treated with Direct Oral Anticoagulants vs. Vitamin K Antagonists Using Data from Real-World Populations. Journal of Clinical Medicine. 2025; 14(16):5716. https://doi.org/10.3390/jcm14165716
Chicago/Turabian StyleCasado-Suela, Miguel Ángel, Juan Torres-Macho, Aida Izquierdo-Martínez, Cristina Lucía Ancos-Aracil, Luis Ferreira-Burguillos, Elena Madroñal-Cerezo, Tamar Talaván-Zañón, Adela Castañeda-Mata, Luis Escobar-Curbelo, Ana Martínez de la Casa-Muñoz, and et al. 2025. "A Digital Twin Strategy to Predict Thrombotic Recurrence in Antiphospholipid Syndrome Patients Treated with Direct Oral Anticoagulants vs. Vitamin K Antagonists Using Data from Real-World Populations" Journal of Clinical Medicine 14, no. 16: 5716. https://doi.org/10.3390/jcm14165716
APA StyleCasado-Suela, M. Á., Torres-Macho, J., Izquierdo-Martínez, A., Ancos-Aracil, C. L., Ferreira-Burguillos, L., Madroñal-Cerezo, E., Talaván-Zañón, T., Castañeda-Mata, A., Escobar-Curbelo, L., de la Casa-Muñoz, A. M., Ruiz-Navío, E., Bustamante-Fermosel, A., & Franco-Moreno, A. (2025). A Digital Twin Strategy to Predict Thrombotic Recurrence in Antiphospholipid Syndrome Patients Treated with Direct Oral Anticoagulants vs. Vitamin K Antagonists Using Data from Real-World Populations. Journal of Clinical Medicine, 14(16), 5716. https://doi.org/10.3390/jcm14165716






