The Prognostic Value of the Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score in Lung Cancer: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strings
2.2. Inclusion and Exclusion Criteria
2.3. Study Quality Score Evaluation
2.4. Data Extraction
2.5. Statistical Methods
3. Results
3.1. Study Characteristics
3.2. HLAP Score and OS
3.3. HLAP Score and PFS/DFS
3.4. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HALP | Hemoglobin, Albumin, Lymphocyte, and Platelet |
| NSCLC | Non-Small Cell Lung Cancer |
| SCLC | Small Cell Lung Cancer |
| TNM | Tumor, Node, Metastasis (staging system) |
| OS | Overall Survival |
| DFS | Disease-Free Survival |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| CNKI | China National Knowledge Infrastructure |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| PFS | Progression-Free Survival |
| NOS | Newcastle–Ottawa Scale |
References
- Sharma, R. Mapping of global, regional and national incidence, mortality and mortality-to-incidence ratio of lung cancer in 2020 and 2050. Int. J. Clin. Oncol. 2022, 27, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Akerley, W.; Bepler, G.; Blum, M.G.; Chang, A.; Cheney, R.T.; Chirieac, L.R.; D’Amico, T.A.; Demmy, T.L.; Ganti, A.K.P.; et al. Non-small cell lung cancer. J. Natl. Compr. Cancer Netw. 2010, 8, 740–801. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R.; Nishimura, K.K.; Giroux, D.J.; Detterbeck, F.; Cardillo, G.; Edwards, J.G.; Fong, K.M.; Giuliani, M.; Huang, J.; Kernstine, K.H.; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groups in the Forthcoming (Ninth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2024, 19, 1007–1027. [Google Scholar] [CrossRef]
- Liu, C.A.; Liu, T.; Li, H.C.; Song, M.M.; Ge, Y.Z.; Ruan, G.T.; Deng, L.; Zhang, Q.; Xie, H.-L.; Lin, S.-Q.; et al. Nutrition impact symptoms: Noteworthy prognostic indicators for lung cancer. Clin. Nutr. 2023, 42, 550–558. [Google Scholar] [CrossRef]
- Xie, H.; Ruan, G.; Ge, Y.; Zhang, Q.; Zhang, H.; Lin, S.; Song, M.; Zhang, X.; Liu, X.; Li, X.; et al. Inflammatory burden as a prognostic biomarker for cancer. Clin. Nutr. 2022, 41, 1236–1243. [Google Scholar] [CrossRef]
- Chen, X.L.; Xue, L.; Wang, W.; Chen, H.N.; Zhang, W.H.; Liu, K.; Chen, X.-Z.; Yang, K.; Zhang, B.; Chen, Z.-X.; et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: A retrospective cohort study. Oncotarget 2015, 6, 41370–41382. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.F.; Wang, L.; Yang, X. The preoperative hemoglobin, albumin, lymphocyte and platelet (HALP) score is a useful predictor in patients with resectable esophageal squamous cell carcinoma. Bosn. J. Basic Med. Sci. 2021, 21, 773–781. [Google Scholar] [CrossRef]
- Yalav, O.; Topal, U.; Unal, A.G.; Eray, I.C. Prognostic significance of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients undergoing curative resection for colorectal cancer. Ann. Ital. Chir. 2021, 92, 283–292. [Google Scholar]
- Liu, Q.; Xie, H.; Cheng, W.; Liu, T.; Liu, C.; Zhang, H.; Lin, S.; Liu, X.; Tian, H.; Li, X.; et al. The preoperative hemoglobin, albumin, lymphocyte, and platelet score (HALP) as a prognostic indicator in patients with non-small cell lung cancer. Front. Nutr. 2024, 11, 1428950. [Google Scholar] [CrossRef]
- Wei, S.; Shao, J.; Wang, J.; Wang, G. The preoperative hemoglobin, albumin, lymphocyte, and platelet score is a prognostic factor for non-small cell lung cancer patients undergoing adjuvant chemotherapy: A retrospective study. Ann. Transl. Med. 2022, 10, 457. [Google Scholar] [CrossRef]
- Taylor, M.; Evison, M.; Michael, S.; Obale, E.; Fritsch, N.C.; Abah, U.; Smith, M.; Martin, G.P.; Shackcloth, M.; Granato, F.; et al. Pre-Operative Measures of Systemic Inflammation Predict Survival After Surgery for Primary Lung Cancer. Clin. Lung Cancer 2024, 25, 460–467.e7. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.; Rothstein, H. Meta-Analysis: Fixed Effect vs. Random Effects. Meta-analysis.com: Englewood, NJ, USA, 2007; Volume 1, p. 30. [Google Scholar]
- Zhai, B.; Chen, J.; Wu, J.; Yang, L.; Guo, X.; Shao, J.; Xu, H.; Shen, A. Predictive value of the hemoglobin, albumin, lymphocyte, and platelet (HALP) score and lymphocyte-to-monocyte ratio (LMR) in patients with non-small cell lung cancer after radical lung cancer surgery. Ann. Transl. Med. 2021, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Cavdar, E.; Karaboyun, K.; Kara, K. Comprehensive analysis of the prognostic role of laboratory indices in advanced lung cancer patients. Asia-Pac. J. Clin. Oncol. 2024, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mazzella, A.; Maiolino, E.; Maisonneuve, P.; Loi, M.; Alifano, M. Systemic Inflammation and Lung Cancer: Is It a Real Paradigm? Prognostic Value of Inflammatory Indexes in Patients with Resected Non-Small-Cell Lung Cancer. Cancers 2023, 15, 1854. [Google Scholar] [CrossRef]
- Fang, Q.; Yu, J.; Li, W.; Luo, J.; Deng, Q.; Chen, B.; He, Y.; Zhang, J.; Zhou, C. Prognostic value of inflammatory and nutritional indexes among advanced NSCLC patients receiving PD-1 inhibitor therapy. Clin. Exp. Pharmacol. Physiol. 2023, 50, 178–190. [Google Scholar] [CrossRef]
- Gao, S.; Huang, Q.; Wei, S.; Lv, Y.; Xie, Y.; Hao, Y. Prognostic nomogram based on pre-treatment HALP score for patients with advanced non-small cell lung cancer. Clinics 2024, 79, 100371. [Google Scholar] [CrossRef]
- Güç, Z.G.; Alacacıoğlu, A.; Kalender, M.E.; Oflazoğlu, U.; Ünal, S.; Yıldız, Y.; Salman, T.; Küçükzeybek, Y.; Tarhan, M.O. HALP score and GNRI: Simple and easily accessible indexes for predicting prognosis in advanced stage NSCLC patients. The İzmir oncology group (IZOG) study. Front. Nutr. 2022, 9, 905292. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Xu, C. Correlation analysis of hemoglobin, albumin, lymphocyte, platelet score and platelet to albumin ratio and prognosis in patients with lung adenosquamous carcinoma. Front. Oncol. 2023, 13, 1166802. [Google Scholar] [CrossRef]
- Huo, J.C.; Wang, Y.; Su, J.W.; Liu, S.; Osoegawa, A.; Jia, Z.F.; Wang, Y.-X.; Yang, J. Correlation of hemoglobin, albumin, lymphocyte, and platelet score with prognosis in patients with stage III squamous lung cancer. J. Thorac. Dis. 2024, 16, 7016–7028. [Google Scholar] [CrossRef]
- Shen, X.B.; Zhang, Y.X.; Wang, W.; Pan, Y.Y. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score in Patients with Small Cell Lung Cancer Before First-Line Treatment with Etoposide and Progression-Free Survival. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 5630–5639. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, P.; Meng, R.; Yang, J.; Zhou, C.; Jiao, Z. Predictive value of preoperative hemoglobin, albumin, lymphocyte and platelet (HALP) score for prognosis in elderly patients with non-small cell lung cancer undergoing surgery. Pract. Geriatr. 2022, 36, 1259–1263. (In Chinese) [Google Scholar]
- Mao, J.; Ji, S.; Zhang, Z.; Zhang, H. Prognostic value of a hemoglobin-albumin-lymphocyte-platelet nomogram in non-small cell lung cancer. Chin. Clin. Dr. 2023, 51, 545–551. (In Chinese) [Google Scholar]
- Garner, H.; de Visser, K.E. Immune crosstalk in cancer progression and metastatic spread: A complex conversation. Nat. Rev. Immunol. 2020, 20, 483–497. [Google Scholar] [CrossRef]
- Wiseman, M.J. Nutrition and cancer: Prevention and survival. Br. J. Nutr. 2019, 122, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.-G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef] [PubMed]
- Sarvaiya, P.J.; Guo, D.; Ulasov, I.; Gabikian, P.; Lesniak, M.S. Chemokines in tumor progression and metastasis. Oncotarget 2013, 4, 2171–2485. [Google Scholar] [CrossRef] [PubMed]
- Gonda, T.A.; Tu, S.; Wang, T.C. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle 2009, 8, 2005–2013. [Google Scholar] [CrossRef]
- Varlotto, J.; Stevenson, M.A. Anemia, tumor hypoxemia, and the cancer patient. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 25–36. [Google Scholar] [CrossRef]
- Tas, F.; Eralp, Y.; Basaran, M.; Sakar, B.; Alici, S.; Argon, A.; Gulistan, B.; Hakan, C.; Adnan, A.; Erkan, T. Anemia in oncology practice: Relation to diseases and their therapies. Am. J. Clin. Oncol. 2002, 25, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, T.J. The impact of hemoglobin levels on treatment outcomes in patients with cancer. Semin. Oncol. 2001, 28 (Suppl. S8), 49–53. [Google Scholar] [CrossRef]
- Nazha, B.; Moussaly, E.; Zaarour, M.; Weerasinghe, C.; Azab, B. Hypoalbuminemia in colorectal cancer prognosis: Nutritional marker or inflammatory surrogate? World J. Gastrointest. Surg. 2015, 7, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, A.S.; Dolan, R.D.; Edwards, C.A.; McMillan, D.C. Hypoalbuminemia Reflects Nutritional Risk, Body Composition and Systemic Inflammation and Is Independently Associated with Survival in Patients with Colorectal Cancer. Cancers 2020, 12, 1986. [Google Scholar] [CrossRef]
- Ikeda, M.; Furukawa, H.; Imamura, H.; Shimizu, J.; Ishida, H.; Masutani, S.; Tatsuta, M.; Satomi, T. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann. Surg. Oncol. 2002, 9, 287–291. [Google Scholar] [CrossRef]
- Feng, F.; Zheng, G.; Wang, Q.; Liu, S.; Liu, Z.; Xu, G.; Wang, F.; Guo, M.; Lian, X.; Zhang, H. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. 2018, 18, 148. [Google Scholar] [CrossRef]
- Sargin, Z.G.; Dusunceli, I. The Effect of HALP Score on the Prognosis of Gastric Adenocarcinoma. J. Coll. Physicians Surg.—Pak. 2022, 32, 1154–1159. [Google Scholar]
- Okazaki, K.; Furukawa, K.; Haruki, K.; Onda, S.; Shirai, Y.; Tsunematsu, M.; Taniai, T.; Matsumoto, M.; Hamura, R.; Akaoka, M.; et al. Prognostic significance of the hemoglobin, albumin, lymphocyte, platelet (HALP) score after hepatectomy for colorectal liver metastases. Surg. Today 2025. [Google Scholar] [CrossRef]
- Farag, C.M.; Antar, R.; Akosman, S.; Ng, M.; Whalen, M.J. What is hemoglobin, albumin, lymphocyte, platelet (HALP) score? A comprehensive literature review of HALP’s prognostic ability in different cancer types. Oncotarget 2023, 14, 153–172. [Google Scholar] [CrossRef]
- Portale, G.; Cavallin, F.; Cipollari, C.; Spolverato, Y.; Di Miceli, D.; Zuin, M.; Mazzeo, A.; Morabito, A.; Sava, T.; Fiscon, V. Preoperative Prognostic Nutritional Index was not predictive of short-term complications after laparoscopic resection for rectal cancer. Langenbeck’s Arch. Surg. 2023, 408, 263. [Google Scholar] [CrossRef] [PubMed]
- Aro, R.; Meriläinen, S.; Sirniö, P.; Väyrynen, J.P.; Pohjanen, V.M.; Herzig, K.H.; Rautio, T.T.; Mäkäräinen, E.; Häivälä, R.; Klintrup, K.; et al. Sarcopenia and Myosteatosis Are Associated with Neutrophil to Lymphocyte Ratio but Not Glasgow Prognostic Score in Colorectal Cancer Patients. J. Clin. Med. 2022, 11, 2656. [Google Scholar] [CrossRef] [PubMed]

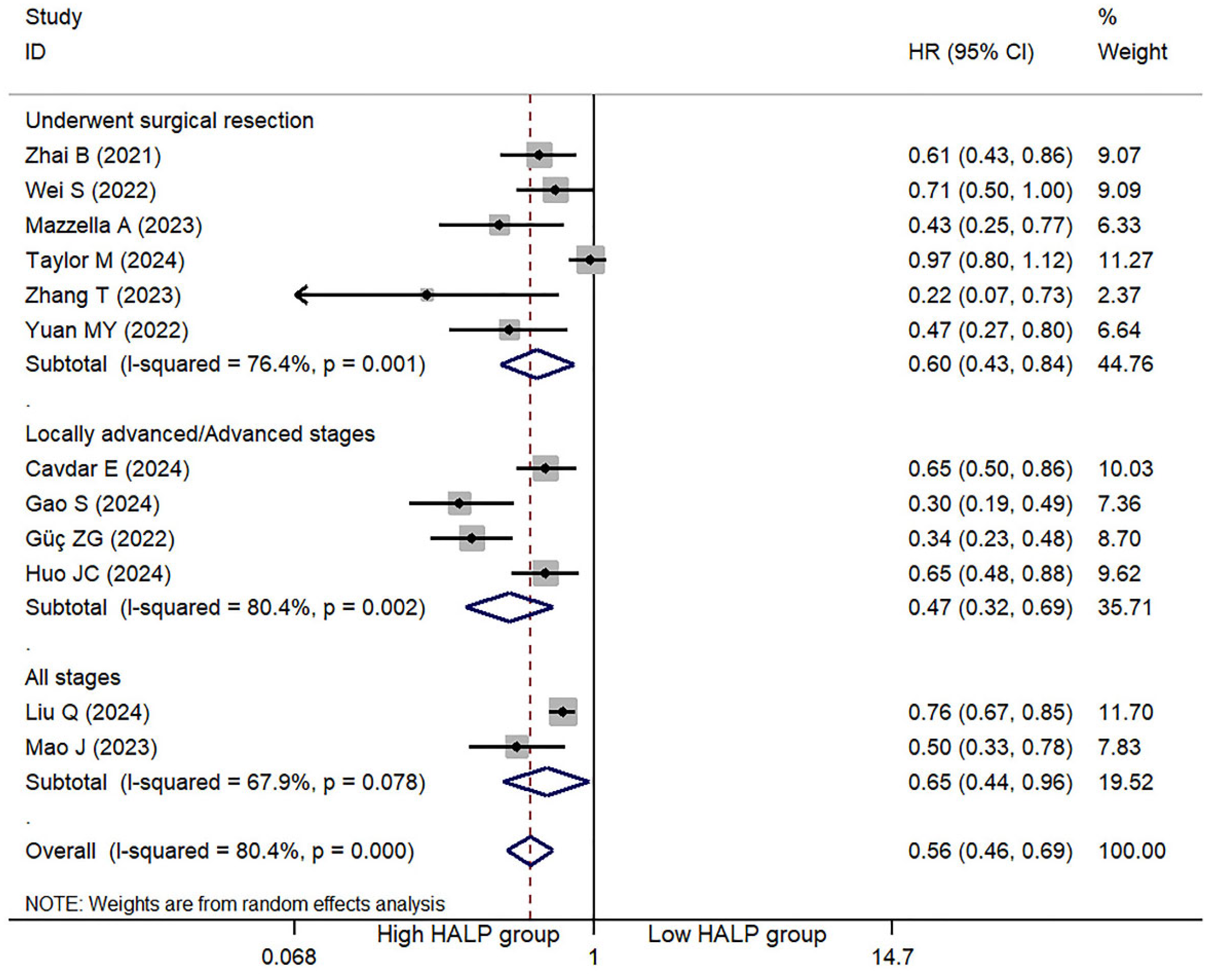
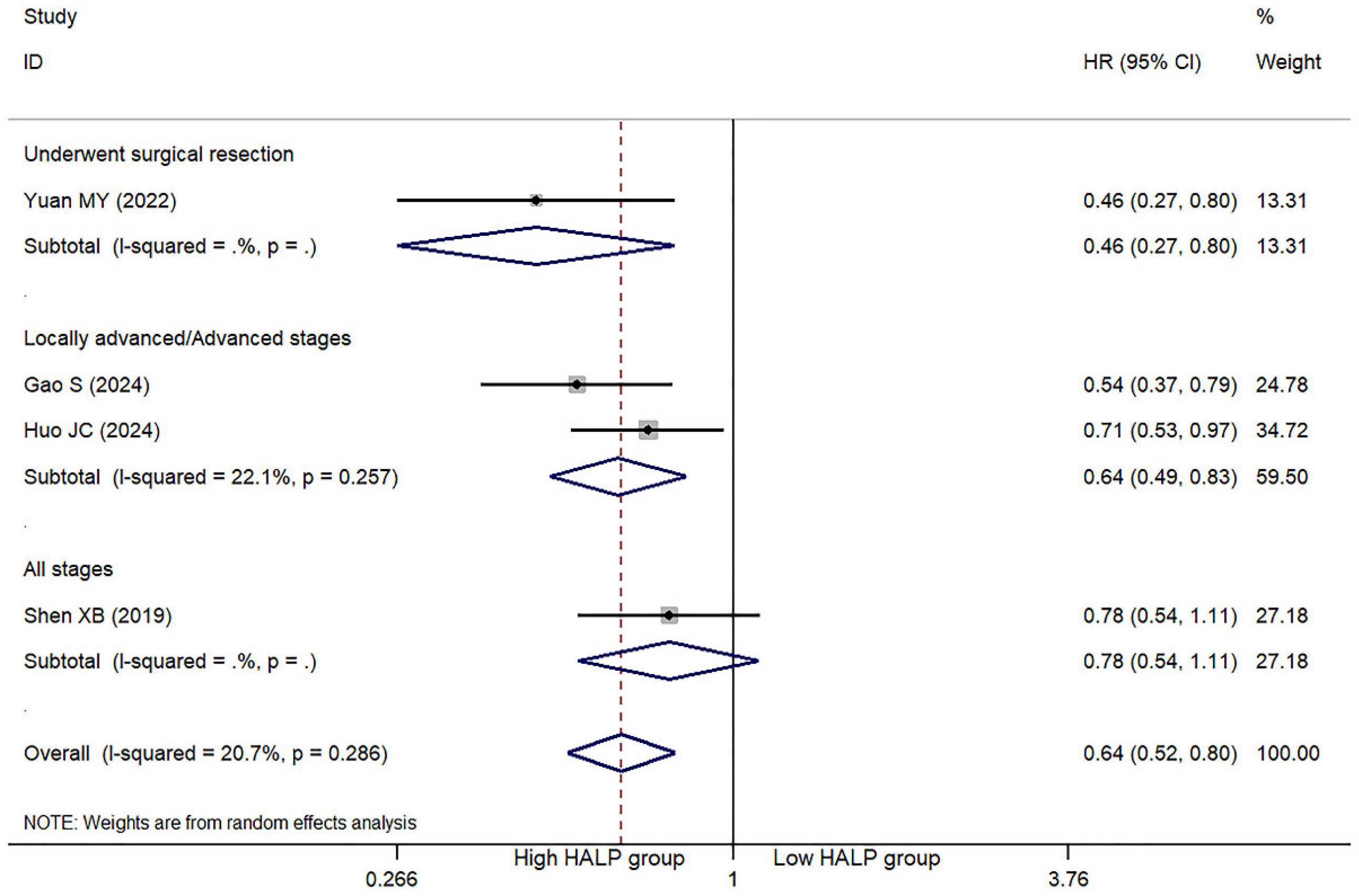
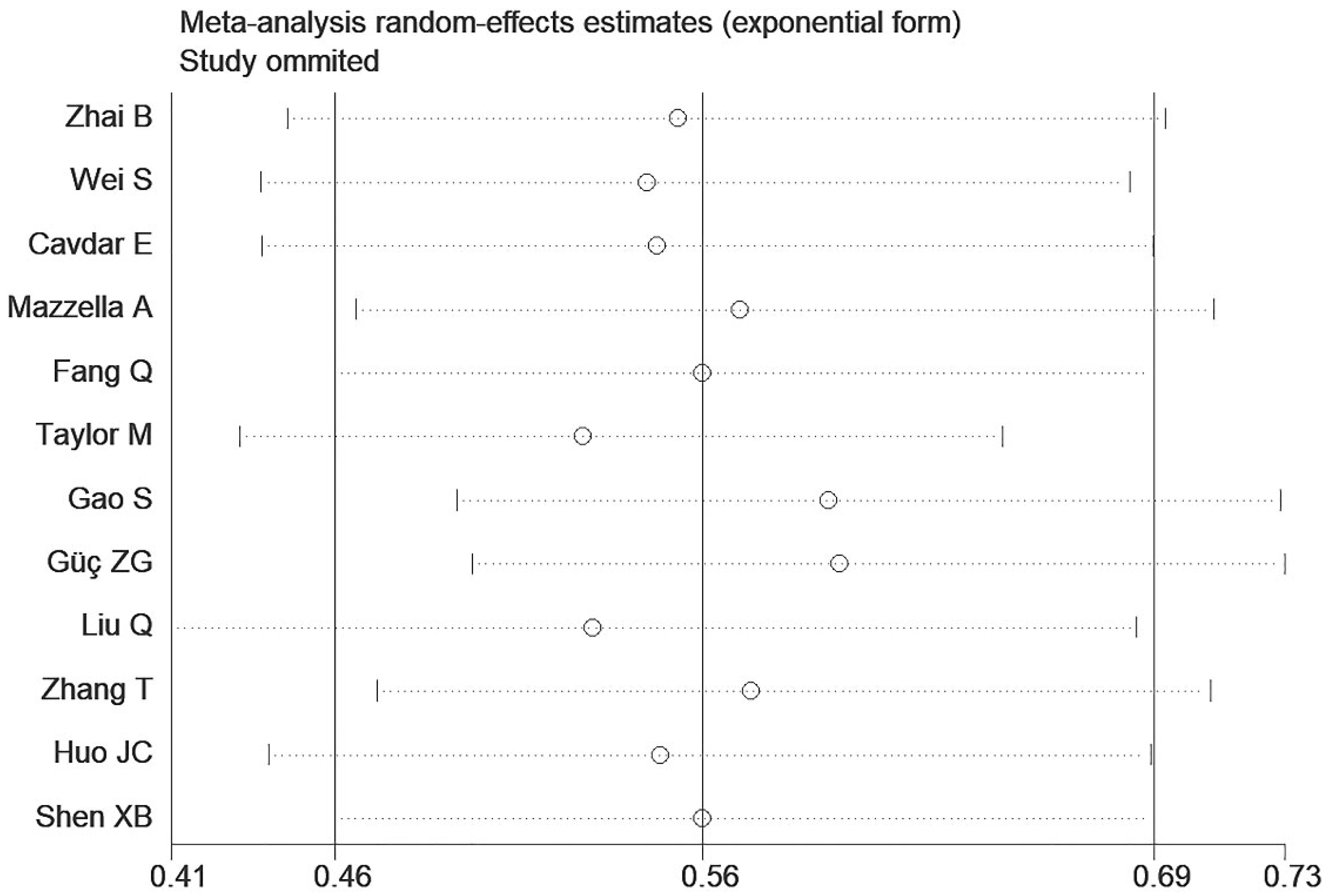
| First Author | Year | Country | N | M/F | Age | Type | Classification | Cut-off | High HALP | Low HALP | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhai B [15] | 2021 | China | 238 | 150/80 | 62.3 ± 8.4 | NSCLC | Underwent surgical resection | 48 | 139 | 99 | OS |
| Wei S [11] | 2022 | China | 362 | 217/145 | NA | NSCLC | Underwent surgical resection | 48.2 | 127 | 77 | OS, DFS |
| Cavdar E [16] | 2024 | Turkey | 278 | 260/18 | 40-82 | NSCLC | Locally advanced/Advanced stages | 26 | 139 | 139 | OS |
| Mazzella A [17] | 2023 | France | 257 | 149/108 | 65 ± 10.2 | NSCLC | Underwent surgical resection | 32.2 | 91 | 66 | OS |
| Fang Q [18] | 2023 | China | 223 | 189/34 | 60.4 | NSCLC | Locally advanced/Advanced stages | 39.33 | 111 | 112 | OS, PFS |
| Taylor M [12] | 2024 | UK | 5029 | 2444/2585 | 68.6 ± 9.1 | NSCLC | Underwent surgical resection | 36.87 | OS | ||
| Gao S [19] | 2024 | China | 203 | 140/63 | 59.6 ± 9.7 | NSCLC | Locally advanced/Advanced stages | 28.02 | 71 | 132 | OS, PFS |
| Güç ZG [20] | 2022 | Turkey | 401 | 317/84 | 63.47 ± 9.75 | NSCLC | Locally advanced/Advanced stages | 23.24 | 171 | 230 | OS |
| Liu Q [10] | 2024 | China | 2053 | 1346/707 | 60.73 ± 9.8 | NSCLC | All stages | 29.17 | 1090 | 963 | OS |
| Zhang T [21] | 2023 | China | 52 | 35/17 | 43-79 | NSCLC | Underwent surgical resection | 24.3 | 46 | 6 | OS, DFS |
| Huo JC [22] | 2024 | China | 206 | 193/13 | 28-83 | NSCLC | Locally advanced/Advanced stages | 24.3 | 127 | 79 | OS, PFS |
| Shen XB [23] | 2019 | China | 178 | 142/36 | 61.24 ± 9.27 | SCLC | All stages | 25.8 | 130 | 48 | PFS |
| Yuan MY [24] | 2022 | China | 270 | 166/104 | 68 ± 6 | NSCLC | Underwent surgical resection | 39 | 104 | 166 | OS, PFS |
| Mao J [25] | 2023 | China | 432 | 256/176 | NA | NSCLC | All stages | 42.2 | 185 | 247 | OS |
| Author | Selection | Comparability | Exposure | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Representativeness of Exposed Cohorts | 2. Selection of Non-Exposed Cohorts | 3. Ascertainment of Exposure | 4. Demonstration that Outcome of Interest was not Present at Start of Study | 1. Study Controls for the Most Important Factor | 2. Comparability for Any Additional Factor | 1. Assessment of Outcome | 2. Was Follow-Up Long Enough for Outcomes to Occur | 3. Adequacy of Follow-Up | ||
| Zhai B [15] | * | * | * | * | - | * | * | * | * | 8 |
| Wei S [11] | * | * | * | * | * | - | * | * | * | 8 |
| Cavdar E [16] | * | * | * | * | - | * | * | * | - | 7 |
| Mazzella A [17] | * | * | * | * | * | * | * | * | - | 8 |
| Fang Q [18] | * | * | * | * | * | * | * | * | - | 8 |
| Taylor M [12] | * | * | * | * | - | - | * | * | - | 6 |
| Gao S [19] | * | * | * | * | * | - | * | * | * | 8 |
| Güç ZG [20] | * | * | * | * | - | - | * | * | * | 7 |
| Liu Q [10] | * | * | * | * | * | - | * | * | * | 8 |
| Zhang T [21] | * | * | * | * | * | - | * | * | * | 8 |
| Huo JC [22] | * | * | * | * | - | * | * | * | * | 8 |
| Shen XB [23] | * | * | * | * | * | * | * | * | - | 8 |
| Yuan MY [24] | * | * | * | * | - | - | * | * | * | 7 |
| Mao J [25] | * | * | * | * | * | - | * | * | * | 8 |
| First Author | OS Univariate | OS Multivariate | DFS Univariate | DFS Multivariate | PFS Univariate | PFS Multivariate | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | LCI | UCI | HR | LCI | UCI | HR | LCI | UCI | HR | LCI | UCI | HR | LCI | UCI | HR | LCI | UCI | |
| Zhai B [15] | 0.531 | 0.379 | 0.744 | 0.610 | 0.433 | 0.860 | / | / | / | / | / | / | / | / | / | / | / | / |
| Wei S [11] | 0.672 | 0.479 | 0.942 | 0.707 | 0.503 | 0.995 | 0.648 | 0.475 | 0.882 | 0.671 | 0.491 | 0.916 | / | / | / | / | / | / |
| Cavdar E [16] | 0.630 | 0.480 | 0.820 | 0.650 | 0.500 | 0.860 | / | / | / | / | / | / | / | / | / | / | / | / |
| Mazzella A [17] | 0.360 | 0.210 | 0.610 | 0.430 | 0.250 | 0.770 | / | / | / | / | / | / | / | / | / | / | / | / |
| Fang Q [18] | 0.996 | 0.709 | 1.401 | / | / | / | / | / | / | / | / | / | 0.771 | 0.569 | 1.045 | / | / | / |
| Taylor M [12] | / | / | / | 0.975 | 0.800 | 1.118 | / | / | / | / | / | / | / | / | / | / | / | / |
| Gao S [19] | 0.380 | 0.240 | 0.600 | 0.300 | 0.190 | 0.490 | / | / | / | / | / | / | 0.600 | 0.420 | 0.850 | 0.540 | 0.370 | 0.790 |
| Güç ZG [20] | 0.333 | 0.230 | 0.482 | 0.335 | 0.231 | 0.484 | / | / | / | / | / | / | / | / | / | / | / | / |
| Liu Q [10] | 0.613 | 0.546 | 0.690 | 0.756 | 0.671 | 0.853 | / | / | / | / | / | / | / | / | / | / | / | / |
| Zhang T [21] | 0.172 | 0.066 | 0.443 | 0.224 | 0.068 | 0.733 | 0.126 | 0.046 | 0.347 | 0.268 | 0.085 | 0.847 | / | / | / | / | / | / |
| Huo JC [22] | 0.589 | 0.437 | 0.793 | 0.650 | 0.481 | 0.880 | / | / | / | / | / | / | 0.631 | 0.469 | 0.850 | 0.715 | 0.528 | 0.968 |
| Shen XB [23] | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 0.777 | 0.544 | 1.112 |
| Yuan MY [24] | / | / | / | 0.466 | 0.273 | 0.795 | / | / | / | / | / | / | / | / | / | 0.460 | 0.266 | 0.796 |
| Mao J [25] | 0.343 | 0.233 | 0.423 | 0.503 | 0.325 | 0.778 | / | / | / | / | / | / | / | / | / | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Xie, C.; Liu, S.; Fan, H.; Li, Z.; Tong, X. The Prognostic Value of the Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score in Lung Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 5701. https://doi.org/10.3390/jcm14165701
Zhang M, Xie C, Liu S, Fan H, Li Z, Tong X. The Prognostic Value of the Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score in Lung Cancer: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(16):5701. https://doi.org/10.3390/jcm14165701
Chicago/Turabian StyleZhang, Min, Chuangying Xie, Sitong Liu, Hong Fan, Zhenzhen Li, and Xiang Tong. 2025. "The Prognostic Value of the Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score in Lung Cancer: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 16: 5701. https://doi.org/10.3390/jcm14165701
APA StyleZhang, M., Xie, C., Liu, S., Fan, H., Li, Z., & Tong, X. (2025). The Prognostic Value of the Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score in Lung Cancer: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(16), 5701. https://doi.org/10.3390/jcm14165701








