Comparative Performance of the CamPROBE Local Anaesthetic Transperineal Biopsy Device Versus an In-Line Device for Detection of Significant Prostate Cancer
Abstract
1. Introduction
2. Patients and Methods
2.1. Cohort Assembly
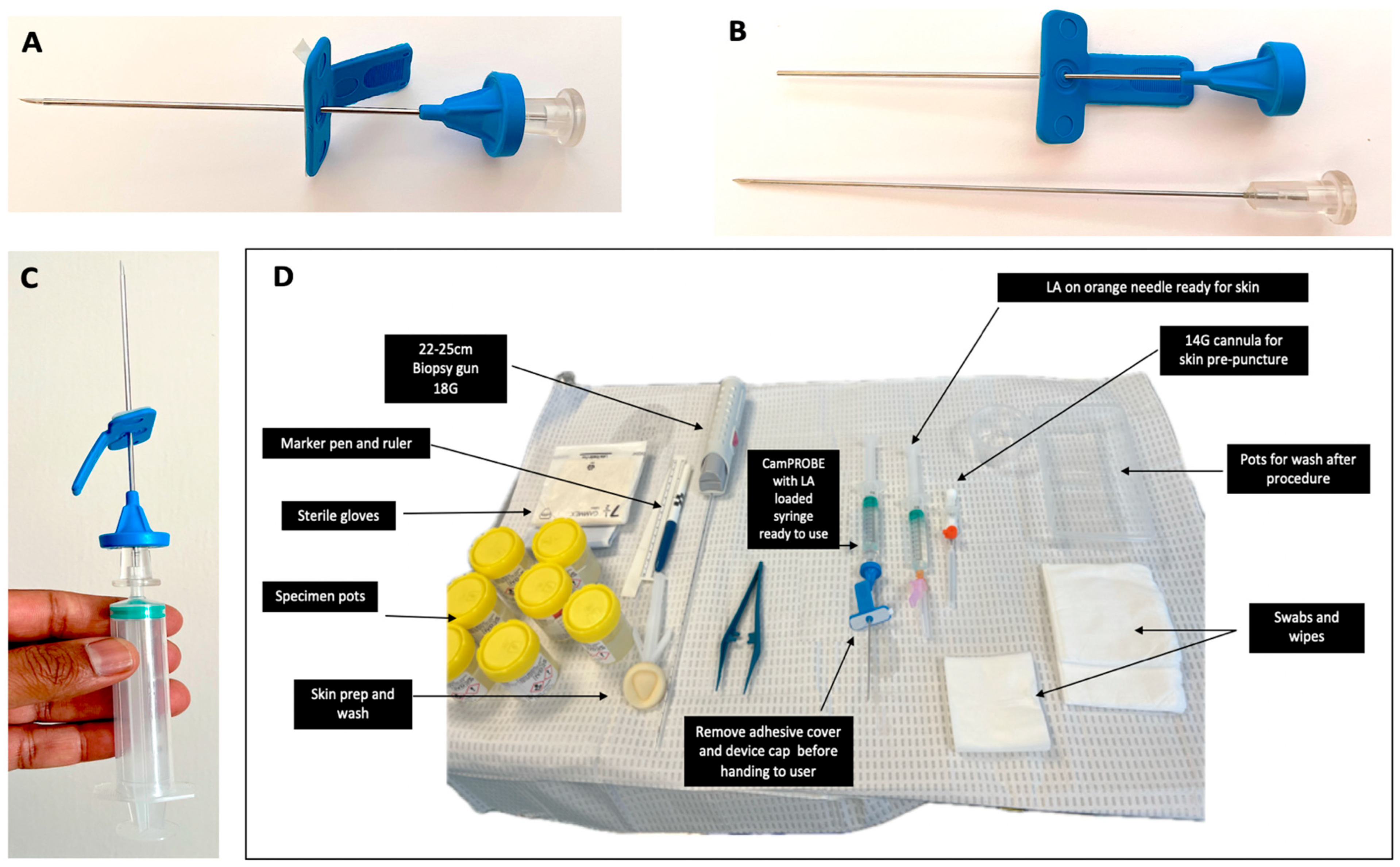
2.2. Biopsy Procedure
2.3. Variables Collected and Data Analysis
3. Results
3.1. Primary Cohort Characteristics and Cancer Diagnosis Rates
3.2. Validation Cohort Characteristics and Cancer Diagnosis Rates
3.3. Device Accuracy Analysis
3.4. Comparison with Other Biopsy Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honore, A.; Moen, C.A.; Juliebo-Jones, P.; Reisaeter, L.A.R.; Gravdal, K.; Chaudhry, A.A.; Rawal, R.; Sandoy, A.; Beisland, C. Transitioning from transrectal to transperineal prostate biopsy using a freehand cognitive approach. BJU Int. 2024, 133, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-H.; Wu, R.; Xu, H.-X.; Xu, J.-M.; Wu, J.; Wang, S.; Bo, X.-W.; Liu, B.-J. Comparison between Ultrasound Guided Transperineal and Transrectal Prostate Biopsy: A Prospective, Randomized and Controlled Trial. Sci. Rep. 2015, 5, 16089. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.J.; Marian, I.R.; Williams, R.; Lopez, J.F.; Mercader, C.; Raslan, M.; Berridge, C.; Whitburn, J.; Campbell, T.; Tuck, S.; et al. Local anaesthetic transperineal biopsy versus transrectal prostate biopsy in prostate cancer detection (TRANSLATE): A multicentre, randomised, controlled trial. Lancet Oncol. 2025, 26, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Xu, Y.; Cheng, M.J.; Jain, A.; Woo, H.H. Tolerability of Transperineal Prostate Biopsy Under Local Anaesthetic Using Pre-Emptive Over-the-Counter Analgesia: An Interventional Study in Patients with Abnormal Clinical Prostate Findings. Société Int. D’urologie J. 2024, 5, 852–864. [Google Scholar] [CrossRef]
- Noureldin, M.E.; Connor, M.J.; Boxall, N.; Miah, S.; Shah, T.; Walz, J. Current techniques of prostate biopsy: An update from past to present. Transl. Androl. Urol. 2020, 9, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Maxim, L.Ș.; Rotaru, R.M.; Scârneciu, C.C.; Moga, M.A.; Sabou, F.L.P.; Hogea, A.C.; Gherasim, R.D.; Ghicavîi, A.; Mulțescu, R.-D.; Badea, M.-A.; et al. Single-Center Comparative Evaluation of Freehand Transperineal and Transrectal Prostate Biopsy Techniques Performed Under Local Anesthesia. Diagnostics 2025, 15, 1929. [Google Scholar] [CrossRef]
- Gnanapragasam, V.J.; Leonard, K.; Sut, M.; Ilie, C.; Ord, J.; Roux, J.; Prieto, M.C.H.; Warren, A.; Tamer, P. Multicentre clinical evaluation of the safety and performance of a simple transperineal access system for prostate biopsies for suspected prostate cancer: The CAMbridge PROstate Biopsy DevicE (CamPROBE) study. J. Clin. Urol. 2020, 13, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.; Omer, A.; Lopez, F.; Bryant, R.J.; Lamb, A.D. Perspectives on technology—Prostate cancer: Is local anaesthetic transperineal prostate biopsy really better than transrectal biopsy? BJU Int. 2024, 134, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Schmeusser, B.; Levin, B.; Lama, D.; Sidana, A. Hundred years of transperineal prostate biopsy. Ther. Adv. Urol. 2022, 14, 17562872221100590. [Google Scholar] [CrossRef] [PubMed]
- Kum, F.; Elhage, O.; Maliyil, J.; Wong, K.; Faure Walker, N.; Kulkarni, M.; Namdarian, B.; Challacombe, B.; Cathcart, P.; Popert, R. Initial outcomes of local anaesthetic freehand transperineal prostate biopsies in the outpatient setting. BJU Int. 2020, 125, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Orecchia, L.; Germani, S.; Colalillo, G.; Fasano, A.; Ricci, M.; Rosato, E.; Asimakopoulos, A.D.; Albisinni, S.; Finazzi Agro, E.; Manenti, G.; et al. Prospective per-target analysis of the added value of the PrecisionPoint Transperineal Access System in cognitive prostate biopsy of MRI targets. BJUI Compass 2024, 5, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Slough, R.; Sushentsev, N.; Shaida, N.; Koo, B.C.; Caglic, I.; Kozlov, V.; Warren, A.Y.; Thankappannair, V.; Pinnock, C.; et al. Three-year experience of a dedicated prostate mpMRI pre-biopsy programme and effect on timed cancer diagnostic pathways. Clin. Radiol. 2019, 74, 894.e1–894.e9. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Prostate Cancer: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng131 (accessed on 13 May 2025).
- Gnanapragasam, V.J.; Bratt, O.; Muir, K.; Lee, L.S.; Huang, H.H.; Stattin, P.; Lophatananon, A. The Cambridge Prognostic Groups for improved prediction of disease mortality at diagnosis in primary non-metastatic prostate cancer: A validation study. BMC Med. 2018, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.F.; Campbell, A.; Omer, A.; Stroman, L.; Bondad, J.; Austin, T.; Reeves, T.; Phelan, C.; Leiblich, A.; Philippou, Y.; et al. Local anaesthetic transperineal (LATP) prostate biopsy using a probe-mounted transperineal access system: A multicentre prospective outcome analysis. BJU Int. 2021, 127, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.L.; Barrett, T.; Kesch, C.; Pepdjonovic, L.; Bonekamp, D.; O’Sullivan, R.; Distler, F.; Warren, A.; Samel, C.; Hadaschik, B.; et al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy-naive men with suspicion of prostate cancer. BJU Int. 2018, 122, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Albnhawy, A.T.; Mostafa, A.; Hussain, E.; Hanna, G.; Das, S.; Hossain, A.; Abdalla, M.; Durrant, J.; Papikinos, P. Patient-reported outcome measures for pain and tolerability of transperineal prostate biopsy under local anaesthesia using the PrecisionPoint™ transperineal access system: A prospective study for a real-world patient experience. Investig. Clin. Urol. 2025, 66, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.R.; Joice, G.A.; Schwen, Z.R.; Partin, A.W.; Allaf, M.E.; Gorin, M.A. Initial Experience Performing In-office Ultrasound-guided Transperineal Prostate Biopsy Under Local Anesthesia Using the PrecisionPoint Transperineal Access System. Urology 2018, 115, 8–13. [Google Scholar] [CrossRef] [PubMed]
- National Health Service. NHS Supply Chain. Available online: https://www.supplychain.nhs.uk/ (accessed on 31 May 2025).
- Wilson, J. What makes a health system good? From cost-effectiveness analysis to ethical improvement in health systems. Med. Health Care Philos. 2023, 26, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Thurtle, D.; Starling, L.; Leonard, K.; Stone, T.; Gnanapragasam, V.J. Improving the safety and tolerability of local anaesthetic outpatient transperineal prostate biopsies: A pilot study of the CAMbridge PROstate Biopsy (CAMPROBE) method. J. Clin. Urol. 2018, 11, 192–199. [Google Scholar] [CrossRef] [PubMed]
| CamPROBE n = 100 | In-Line Device n = 97 | ||
|---|---|---|---|
| Age (y) | p = 0.01 | ||
| Mean | 66 | 69 | |
| Median | 65 | 69 | |
| Interquartile range | 61–73 | 63–75 | |
| PSA (ng/mL) | p = 0.72 | ||
| Mean | 25.8 | 21.5 | |
| Median | 6.1 | 7.4 | |
| Interquartile range | 4.1–12.4 | 4.9–11.6 | |
| Prostate volume (mls) | p = 0.02 | ||
| Mean | 44.9 | 52.7 | |
| Median | 39.5 | 46.7 | |
| Interquartile range | 30–53.8 | 33–63 | |
| No data | 6 | 0 | |
| MRI stage (pre–biopsy) | p = 0.92 | ||
| T1–T2 | 78 | 81 | |
| T3–T4 | 14 | 14 | |
| Metastatic | n = 8 | n = 2 | |
| MRI Likert score | p = 0.78 | ||
| 1–2 | 10 | 9 | |
| 3 | 8 | 7 | |
| 4–5 | 75 | 78 | |
| No data available | n = 7 | n = 3 | |
| Target positivity (%) | p = 0.59 | ||
| All MRI lesions | 56/83 (67.4%) | 54/85 (63.5%) | |
| Likert 3 | 2/8 (25.0%) | 2/7 (28.6%) | |
| Likert 4 | 28/45 (62.2%) | 22/42 (52.3%) | |
| Likert 5 | 26/30 (86.7%) | 30/36 (83.3%) | |
| Cancer detection (%) | |||
| Any cancer | 78% | 79% | p = 0.81 |
≥Grade Group 2 | 60% | 56.7% | p = 0.64 |
| ≥Grade Group 3 | 31% | 20.6% | p = 0.09 |
≥Cambridge Prognostic Group 2 | 62% | 60.8% | p = 0.86 |
| ≥Cambridge Prognostic Group 3 | 37% | 32.9% | p = 0.55 |
| CamPROBE n = 38 | In-Line Device n = 44 | ||
|---|---|---|---|
| Age (y) | p = 0.15 | ||
| Mean | 68.1 | 70.6 | |
| Median | 67.5 | 71.5 | |
| Interquartile range | 63–76 | 66–76 | |
| PSA (ng/mL) | p = 0.95 | ||
| Mean | 11.3 | 11.1 | |
| Median | 7.9 | 8.3 | |
| Interquartile range | 5.9–14.9 | 7–12.5 | |
| Prostate volume (mls) | p = 0.14 | ||
| Mean | 44.3 | 51.3 | |
| Median | 41.5 | 47 | |
| Interquartile range | 32.7–58.7 | 34.9–67.7 | |
| MRI stage (pre-biopsy) | p = 0.95 | ||
| T1–T2 | 28 | 34 | |
| T3–T4 | 8 | 10 | |
| MRI Likert score | p = 0.76 | ||
| 1–2 | 2 | 3 | |
| 3 | 6 | 8 | |
| 4–5 | 30 | 33 | |
| Target positivity (%) | p = 0.08 | ||
| All MRI lesions | 27/33 (81.8%) | 26/41 (63.4%) | |
| Likert 3 | 2/4 (50.0%) | 2/8 (25.0%) | |
| Likert 4 | 9/11 (81.8%) | 7/12 (58.3%) | |
| Likert 5 | 16/18 (88.8%) | 17/21 (80.9%) | |
| Cancer detection (%) | |||
| Any cancer | 84.2% | 84.0% | p = 0.98 |
≥Grade Group 2 | 73.6% | 68.1% | p = 0.58 |
| ≥Grade Group 3 | 50% | 27.2% | p = 0.03 |
≥Cambridge Prognostic Group 2 | 76.3% | 75.0% | p = 0.96 |
| ≥Cambridge Prognostic Group 3 | 55.2% | 34.0% | p = 0.05 |
| Number with MRI targets | CamPROBE | In line device | |
|---|---|---|---|
| Target positivity by laterality RIGHT LEFT BOTH/DIFFUSE | n = 83 27/42 (64.3%) 18/24 (75.0%) 11/17(64.7%) Within group comparison p = 0.36 | n = 83* 26/44 (59.0%) 19/31 (61.2%) 8/8 (100%) Within group comparison p = 0.84 | p = 0.62 p = 0.28 p = 0.12 |
| Target positivity by lesion location # Anterior Posterior Lateral Diffuse/extensive | n = 83 7/11 (63.6%) 14/23 (60.9%) 29/43 (67.4%) 6/6 (100%) Within group comparison p = 0.86 | n = 83 * 8/13 (61.5%) 20/33 (60.6%) 20/33 (60.6%) 4/4 (100%) Within group comparison p = 0.99 | p = 0.91 p = 0.98 p = 0.54 NA |
| Target positivity by prostate size (mls) Prostate ≤50 with target Prostate >50 with target | n = 83 41/59 (69.5%) 15/24 (62.5%) Within group comparison p = 0.53 | n = 83 * 39/49 (79.6%) 13/34 (38.2%) Within group comparison p = 0.0003 | p = 0.23 p = 0.06 |
| Target positivity by MRI lesion size (mm2) # Lesion size ≤100 Lesion size >100 | n = 76 ** 26/44 (59.0%) 24/32 (75.0%) Within group comparison p = 0.14 | n = 76 ** 21/43 (48.8%) 24/33 (72.7%) Within group comparison p = 0.017 | p = 0.33 p = 0.94 |
| Biopsy core length from positive target (mm) # Mean Median Interquartile range | n = 56 7.41 7 4–11 | n = 52 7.0 6.5 3–10 | p = 0.68 |
| Device for First Biopsy | Number | Overall Cancer Detection Rate (%) | ≥Grade Group 2 Detection Rate (%) |
|---|---|---|---|
| CamPROBE (this study) Primary cohort Validation cohort | 100 38 | 78 (78.0) 32 (84.2) | 60 (60.0) 28 (73.7) |
| In-line LATP device Kum et al. 2020 [10] a Lopez et al. 2021 [15] a (multi-centre) Bryant et al. [3] b (multi-centre RCT-TP arm) | 115 674 562 | 81 (70.4) 487 (72.0) 360 (69.4) | 57 (49.6) 406 (60.2) 329 (58.5) |
| GA grid-based TP biopsies Hansen et al. [16] c (multi-centre) | 807 | 546 (67.6) | 392 (49.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandhu, K.; Shah, S.; Thorman, H.; Saeb-Parsy, K.; Gnanapragasam, V.J.; Miah, S.; Ahmed, A. Comparative Performance of the CamPROBE Local Anaesthetic Transperineal Biopsy Device Versus an In-Line Device for Detection of Significant Prostate Cancer. J. Clin. Med. 2025, 14, 5702. https://doi.org/10.3390/jcm14165702
Sandhu K, Shah S, Thorman H, Saeb-Parsy K, Gnanapragasam VJ, Miah S, Ahmed A. Comparative Performance of the CamPROBE Local Anaesthetic Transperineal Biopsy Device Versus an In-Line Device for Detection of Significant Prostate Cancer. Journal of Clinical Medicine. 2025; 14(16):5702. https://doi.org/10.3390/jcm14165702
Chicago/Turabian StyleSandhu, Kieran, Syed Shah, Hannah Thorman, Kasra Saeb-Parsy, Vincent J. Gnanapragasam, Saiful Miah, and Adham Ahmed. 2025. "Comparative Performance of the CamPROBE Local Anaesthetic Transperineal Biopsy Device Versus an In-Line Device for Detection of Significant Prostate Cancer" Journal of Clinical Medicine 14, no. 16: 5702. https://doi.org/10.3390/jcm14165702
APA StyleSandhu, K., Shah, S., Thorman, H., Saeb-Parsy, K., Gnanapragasam, V. J., Miah, S., & Ahmed, A. (2025). Comparative Performance of the CamPROBE Local Anaesthetic Transperineal Biopsy Device Versus an In-Line Device for Detection of Significant Prostate Cancer. Journal of Clinical Medicine, 14(16), 5702. https://doi.org/10.3390/jcm14165702








