Treatment Outcomes in Patients Receiving Carbon-Ion Radiotherapy Versus Hepatectomy for Hepatocellular Carcinoma (≥4 cm): A Retrospective Study in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Carbon-Ion Radiotherapy
2.3. Clinical Outcome Evaluation
2.4. Statistical Analysis
2.5. Ethics Approval
3. Results
3.1. Characteristics of Patients Who Underwent CIRT
3.2. Recurrence/Nonrecurrence After CIRT
3.3. Characteristics of Patients Who Underwent Hepatectomy
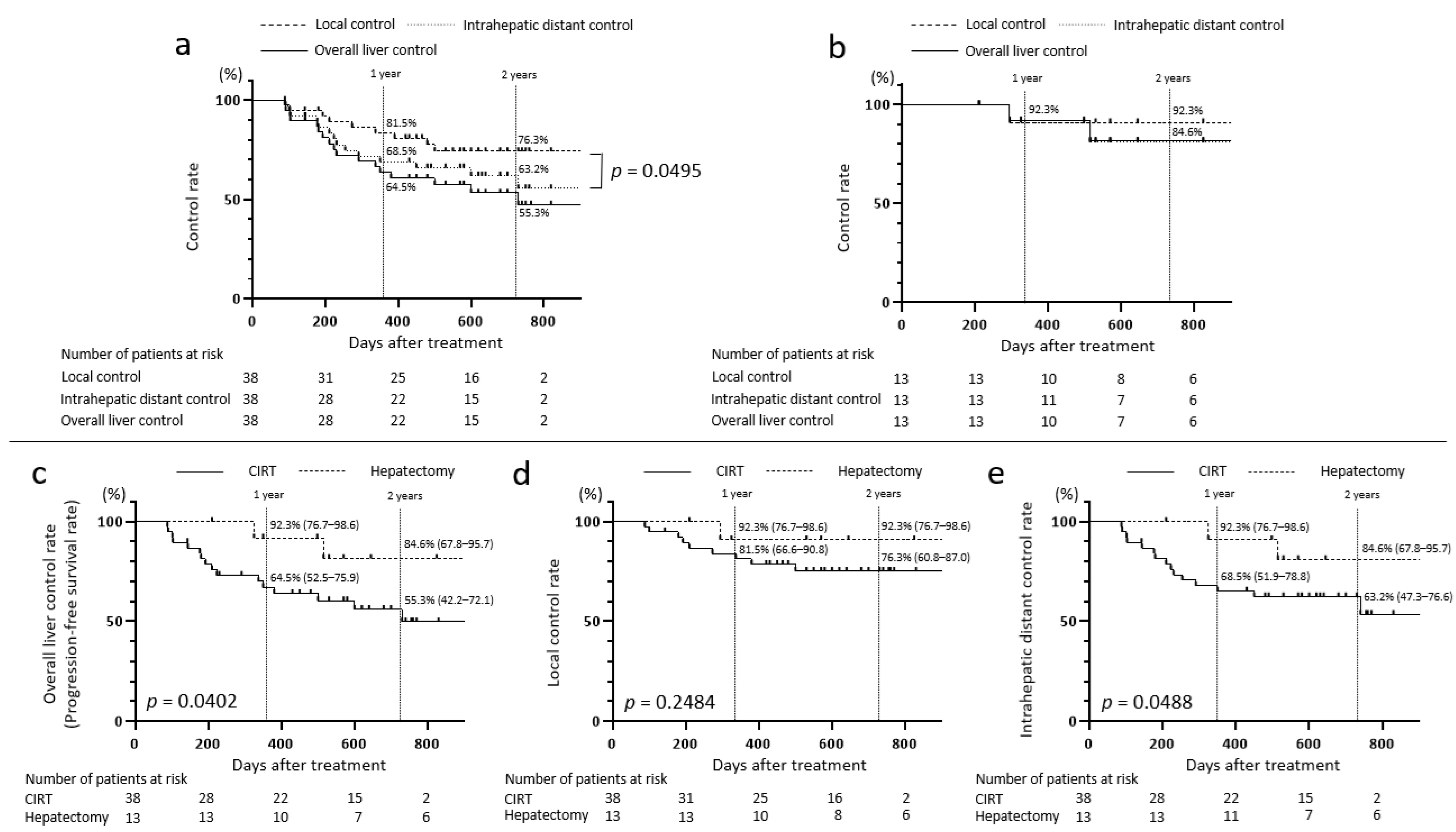
3.4. Disease Control Rates and OS Rates in the CIRT and Hepatectomy Groups
3.5. Comparison Between the CIRT and Hepatectomy Groups in Patients Aged Below 80 Years
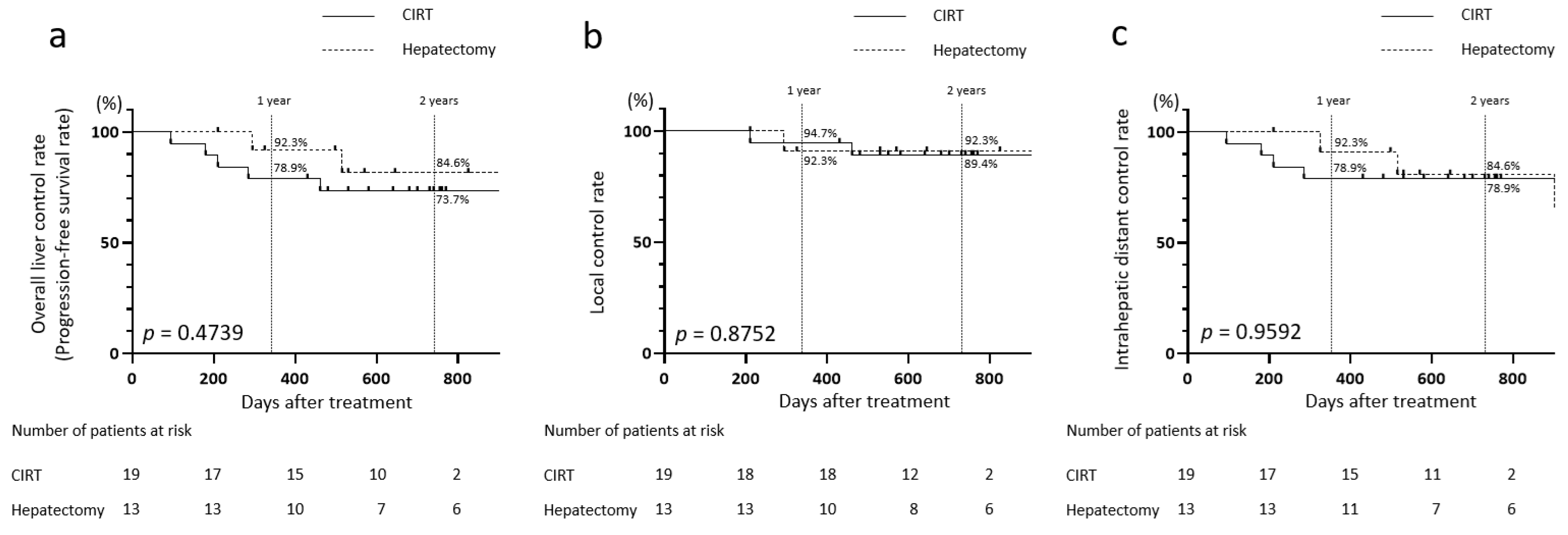
3.6. Comparison Between the CIRT and Hepatectomy Groups Using Matching
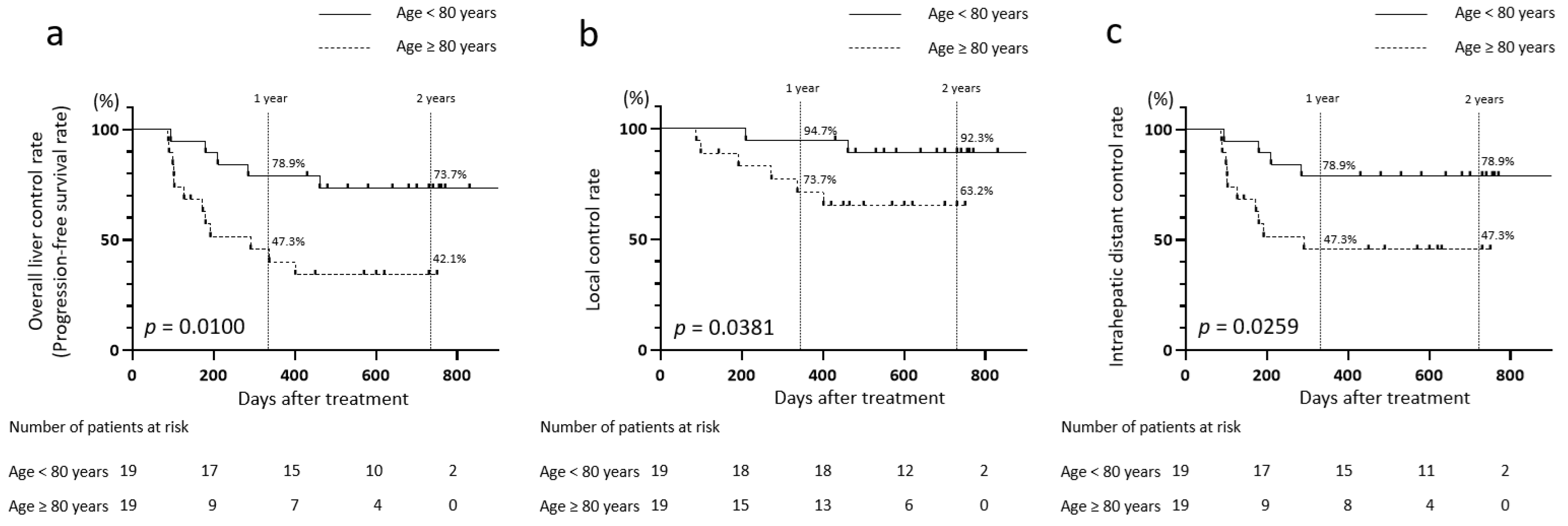
3.7. Comparison of Disease Control Rates in Patients with CIRT Aged Below 80 Years and 80 Years and Older
3.8. AEs After CIRT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALBI score | albumin–bilirubin score |
| BCLC | Barcelona Clinic Liver Cancer |
| CIRT | carbon-ion radiotherapy |
| CR | complete response |
| CT | computed tomography |
| CTV | clinical target volume |
| GTV | gross tumor volume |
| HCC | hepatocellular carcinoma |
| ITV | internal target volume |
| LEN | lenvatinib |
| mALBI grade | modified albumin–bilirubin grade |
| MASH | metabolic dysfunction-associated steatohepatitis |
| MRI | magnetic resonance imaging |
| OAR | organs at risk |
| PBC | primary biliary cholangitis |
| PD | progressive disease |
| PFS | progression-free survival |
| PR | partial response |
| RFA | radiofrequency ablation |
| SD | stable disease |
| TACE | transarterial chemoembolization |
References
- Omiya, S.; Komatsu, S.; Terashima, K.; Yamasaki, N.; Matsuo, Y.; Toyama, H.; Tokumaru, S.; Okimoto, T.; Fukumoto, T. Hepatic resection vs particle therapy as an initial treatment for single hepatocellular carcinoma: Bi-institutional propensity score-matched analysis. J. Am. Coll. Surg. 2023, 236, 972–981. [Google Scholar] [CrossRef]
- Tsujii, H.; Mizoe, J.; Kamada, T.; Baba, M.; Tsuji, H.; Kato, H.; Kato, S.; Yamada, S.; Yasuda, S.; Ohno, T.; et al. Clinical results of carbon ion radiotherapy at NIRS. J. Radiat. Res. 2007, 48, A1–A13. [Google Scholar] [CrossRef]
- Byun, H.K.; Kim, C.; Seong, J. Carbon ion radiotherapy in the treatment of hepatocellular carcinoma. Clin. Mol. Hepatol. 2023, 29, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, G.; Kato, H.; Yasuda, S.; Tsuji, H.; Yamada, S.; Haruyama, Y.; Kobashi, G.; Ebner, D.K.; Okada, N.N.; Makishima, H. Progressive hypofractionated carbon-ion radiotherapy for hepatocellular carcinoma: Combined analyses of 2 prospective trials. Cancer 2017, 123, 3955–3965. [Google Scholar] [CrossRef] [PubMed]
- Shiba, S.; Abe, T.; Shibuya, K.; Katoh, H.; Koyama, Y.; Shimada, H.; Kakizaki, S.; Shirabe, K.; Kuwano, H.; Ohno, T. Carbon ion radiotherapy for 80 years or older patients with hepatocellular carcinoma. BMC Cancer 2017, 17, 721. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Ohno, T.; Terashima, K.; Toyama, S.; Yasuda, S.; Tsuji, H.; Okimoto, T.; Shioyama, Y.; Nemoto, K.; Kamada, T. Short-course carbon-ion radiotherapy for hepatocellular carcinoma: A multi-institutional retrospective study. Liver Int. 2018, 38, 2239–2247. [Google Scholar] [CrossRef]
- Shiba, S.; Shibuya, K.; Katoh, H.; Koyama, Y.; Okamoto, M.; Abe, T. No deterioration in the clinical outcomes of carbon ion radiotherapy for sarcopenia patients with hepatocellular carcinoma. Anticancer Res. 2018, 38, 3579–3586. [Google Scholar] [CrossRef]
- Shiba, S.; Shibuya, K.; Katoh, H.; Kaminuma, T.; Miyazaki, M.; Kakizaki, S.; Shirabe, K.; Ohno, T.; Nakano, T. A comparison of carbon ion radiotherapy and transarterial chemoembolization treatment outcomes for single hepatocellular carcinoma: A propensity score matching study. Radiat. Oncol. 2019, 14, 137. [Google Scholar] [CrossRef]
- Yasuda, S.; Kato, H.; Imada, H.; Isozaki, Y.; Kasuya, G.; Makishima, H.; Tsuji, H.; Ebner, D.K.; Yamada, S.; Kamada, T. Long-term results of high-dose 2-fraction carbon ion radiation therapy for hepatocellular carcinoma. Adv. Radiat. Oncol. 2020, 5, 196–203. [Google Scholar] [CrossRef]
- Shibuya, K.; Ohno, T.; Katoh, H.; Okamoto, M.; Shiba, S.; Koyama, Y.; Kakizaki, S.; Shirabe, K.; Nakano, T. A feasibility study of high-dose hypofractionated carbon ion radiation therapy using four fractions for localized hepatocellular carcinoma measuring 3 cm or larger. Radiother. Oncol. 2019, 132, 230–235. [Google Scholar] [CrossRef]
- Shiba, S.; Shibuya, K.; Okamoto, M.; Okazaki, S.; Komatsu, S.; Kubota, Y.; Nakano, T.; Ohno, T. Clinical impact of hypofractionated carbon ion radiotherapy on locally advanced hepatocellular carcinoma. Radiat. Oncol. 2020, 15, 195. [Google Scholar] [CrossRef]
- Shibuya, K.; Katoh, H.; Koyama, Y.; Shiba, S.; Okamoto, M.; Okazaki, S.; Araki, K.; Kakizaki, S.; Shirabe, K.; Ohno, T. Efficacy and safety of 4 fractions of carbon-ion radiation therapy for hepatocellular carcinoma: A prospective study. Liver Cancer 2021, 11, 61–74. [Google Scholar] [CrossRef]
- Fujita, N.; Kanogawa, N.; Makishima, H.; Ogasawara, S.; Maruta, S.; Iino, Y.; Shiko, Y.; Kanzaki, H.; Koroki, K.; Kobayashi, K. Carbon-ion radiotherapy versus radiofrequency ablation as initial treatment for early-stage hepatocellular carcinoma. Hepatol. Res. 2022, 52, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Wakatsuki, M.; Kaneko, T.; Makishima, H.; Okada, N.N.; Yasuda, S.; Ishikawa, H.; Tsuji, H. Clinical impact of carbon-ion radiotherapy on hepatocellular carcinoma with child-Pugh B cirrhosis. Cancer Med. 2023, 12, 14004–14014. [Google Scholar] [CrossRef]
- Tomizawa, K.; Shibuya, K.; Shiba, S.; Okazaki, S.; Miyasaka, Y.; Oishi, M.; Okamoto, M.; Ohno, T. Repeated carbon-ion radiation therapy for intrahepatic recurrent hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Zhang, W.; Cai, X.; Yu, Z.; Sun, J.; Wang, W.; Lin, L.; Zhao, J.; Cheng, J.; Zhang, G. Carbon ion radiotherapy with pencil beam scanning for hepatocellular carcinoma: Long-term outcomes from a phase I trial. Cancer Sci. 2023, 114, 976–983. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, X.; Sun, J.; Wang, W.; Zhao, J.; Zhang, Q.; Jiang, G.; Wang, Z. pencil beam scanning carbon ion radiotherapy for hepatocellular carcinoma. J. Hepatocell. Carcinoma 2023, 10, 2397–2409. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, O.; Takayama, T.; Matsuyama, Y.; Kubo, S.; Kokudo, N.; Kurosaki, M.; Murakami, T.; Shiina, S.; Kudo, M.; Sakamoto, M.; et al. Reevaluation of Makuuchi’s criteria for resecting hepatocellular carcinoma: A Japanese nationwide survey. Hepatol. Res. 2023, 53, 127–134. [Google Scholar] [CrossRef]
- Hasegawa, K.; Takemura, N.; Yamashita, T.; Watadani, T.; Kaibori, M.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Aikata, H. Clinical practice guidelines for hepatocellular carcinoma: The japan society of hepatology 2021 version (5th JSH-HCC guidelines). Hepatol. Res. 2023, 53, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef]
- Malagari, K.; Pomoni, M.; Moschouris, H.; Bouma, E.; Koskinas, J.; Stefaniotou, A.; Marinis, A.; Kelekis, A.; Alexopoulou, E.; Chatziioannou, A.; et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: Five-year survival analysis. Cardiovasc. Intervent. Radiol. 2012, 35, 1119–1128. [Google Scholar] [CrossRef]
- Inchingolo, R.; Posa, A.; Mariappan, M.; Spiliopoulos, S. Locoregional treatments for hepatocellular carcinoma: Current evidence and future directions. World. J. Gastroenterol. 2019, 25, 4614–4628. [Google Scholar] [CrossRef]
- Tanaka, T.; Takata, K.; Miyayama, T.; Shibata, K.; Fukuda, H.; Yamauchi, R.; Fukunaga, A.; Yokoyama, K.; Shakado, S.; Sakisaka, S.; et al. Long-term outcome and eligibility of radiofrequency ablation for hepatocellular carcinoma over 3.0 cm in diameter. Sci. Rep. 2023, 13, 16286. [Google Scholar] [CrossRef] [PubMed]
- Ruzzenente, A.; De Angelis, M.; Conci, S.; Campagnaro, T.; Isa, G.; Bagante, F.; Ciangherotti, A.; Pedrazzani, C.; Capelli, P.; Iacono, C.; et al. The albumin-bilirubin score stratifies the outcomes of Child-Pugh class A patients after resection of hepatocellular carcinoma. Transl. Cancer Res 2019, 8, S233–S244. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Sasaki, R.; Iwatsu, S.; Fukushima, M.; Haraguchi, M.; Yamamichi, S.; Miuma, S.; Miyaaki, H.; Taura, N.; Yamazaki, T.; et al. Long-term outcomes and prognostic factors of stereotactic body radiotherapy for hepatocellular carcinoma. Anticancer Res. 2022, 42, 5001–5007. [Google Scholar] [CrossRef] [PubMed]
- Shehta, A.; Elsabbagh, A.M.; Medhat, M.; Farouk, A.; Monier, A.; Said, R.; Salah, T.; Elshobari, M.; Fouad, A.; Elghawalby, A.N. Impact of tumor size on the outcomes of hepatic resection for hepatocellular carcinoma: A retrospective study. BMC Surg. 2024, 24, 7. [Google Scholar] [CrossRef]
- Macias, R.I.R.; Monte, M.J.; Serrano, M.A.; González-Santiago, J.M.; Martín-Arribas, I.; Simão, A.L.; Castro, R.E.; González-Gallego, J.; Mauriz, J.L.; Marin, J.J.G. Impact of aging on primary liver cancer: Epidemiology, pathogenesis and therapeutics. Aging 2021, 13, 23416–23434. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Lian, J.; Yue, Y.; Yu, W.; Zhang, Y. Immunosenescence: A key player in cancer development. J. Hematol. Oncol. 2020, 13, 151. [Google Scholar] [CrossRef]
- Teraoka, Y.; Kimura, T.; Aikata, H.; Daijo, K.; Osawa, M.; Honda, F.; Nakamura, Y.; Morio, K.; Morio, R.; Hatooka, M.; et al. Clinical outcomes of stereotactic body radiotherapy for elderly patients with hepatocellular carcinoma. Hepatol. Res. 2018, 48, 193–204. [Google Scholar] [CrossRef]
| CIRT (n = 38) | Recurrence (n = 17) | No Recurrence (n = 21) | p Value | |
|---|---|---|---|---|
| Age | Median: 80 (range: 56–93) | 84 (67–93) | 75 (56–91) | 0.0380 |
| Sex (male/female) | 29/9 | 12/5 | 17/4 | 0.1855 |
| Etiology (HBV/HCV/non-B, non-C/alcohol/MASH/PBC) | 5/10/7/8/6/2 | 4/6/0/3/3/1 | 1/4/7/5/3/1 | 0.0598 |
| mALBI grade (1/2a/2b/3) | 20/9/8/1 | 8/4/5/0 | 12/5/3/1 | 0.6716 |
| ALBI score | Median: −2.633 (−3.415 to −1.313) | −2.596 (−3.075 to −1.744) | −2.688 (−3.415 to −1.313) | 0.6873 |
| BCLC stage (A/B/C) | 30/3/5 | 11/2/4 | 19/1/1 | 0.1724 |
| First treatment/pretreatment (surgery/RFA/TACE/systemic therapy) | 26/12 | 9/8 | 17/4 | 0.0873 |
| Size (mm) | 53.5 (40–110) | 56.0 (42–101) | 50.0 (40–110) | 0.4081 |
| Total number of intrahepatic tumors (1/2) | 35/3 | 14/3 | 21/0 | 0.1935 |
| MVI (−/+) | 32/6 | 13/4 | 19/2 | 0.3776 |
| Blood biochemistry (median [range]) | ||||
| T-bil level (mg/dL) | 0.60 (0.40–2.2) | 0.70 (0.40–1.4) | 0.60 (0.40–2.2) | 0.9175 |
| Alb level (g/dL) | 3.9 (2.7–4.9) | 3.9 (2.7–4.5) | 4.0 (2.7–4.9) | 0.7185 |
| AST level (U/L) | 32.0 (12–222) | 36.0 (12–77) | 31.0 (13–222) | 0.3799 |
| ALT level (U/L) | 27.0 (5.0–135) | 26.0 (5–80) | 31.0 (9–135) | 0.5084 |
| PLT count (103/µL) | 176 (45–382) | 196.0 (45.0–346.0) | 166.0 (55.0–382.0) | 0.8904 |
| PT% | 94.0 (65–114) | 92.0 (65–114) | 96.0 (76–112) | 0.2655 |
| AFP level (ng/mL) | 4.8 (2.0–20,000) | 6.7 (2.8–20,000) | 4.0 (2.0–17,455) | 0.1770 |
| PIVKA-II level (mAU/mL) | 203.0 (13–81,886) | 552.0 (21–21,680) | 140.0 (13.0–81,886) | 0.5650 |
| CIRT (n = 38) | Hepatectomy (n = 13) | p Value | |
|---|---|---|---|
| Age | Median: 80 (range: 56–93) | 70 (58–77) | 0.0046 |
| Sex (male/female) | 29/9 | 11/2 | 0.7063 |
| Etiology (HBV/HCV/non-B, non-C/alcohol/MASH/PBC) | 5/10/7/8/6/2 | 1/1/4/6/1/0 | 0.5837 |
| mALBI grade (1/2a/2b/3) | 20/9/8/1 | 10/2/1/0 | 0.0450 |
| ALBI score | −2.633 (−3.415 to −1.313) | −2.786 (−3.186 to −1.800) | 0.1499 |
| BCLC stage (A/B/C) | 30/3/5 | 8/4/1 | 0.1403 |
| First treatment/pre-treatment (surgery/RFA/TACE/systemic therapy) | 26/12 | 8/5 | 0.7458 |
| Size (mm) | 53.5 (40–110) | 50.0 (40–130) | 0.2893 |
| Total number of intrahepatic tumors (1/2) | 35/3 | 9/4 | 0.0605 |
| MVI (−/+) | 32/6 | 12/1 | 0.6618 |
| Blood biochemistry (median [range]) | |||
| T-bil level (mg/dL) | 0.60 (0.40–2.2) | 0.70 (0.30–0.90) | 0.2348 |
| Alb level (g/dL) | 3.9 (2.7–4.9) | 4.1 (3.0–4.3) | 0.4139 |
| AST level (U/L) | 32.0 (12–222) | 24.0 (14–77) | 0.2399 |
| ALT level (U/L) | 27.0 (5.0–135) | 22.0 (13–107) | 0.6917 |
| PLT count (103/µL) | 176 (45–382) | 190 (102–252) | 0.7654 |
| PT% | 94.0 (65–114) | 93.0 (68–123) | 0.8252 |
| AFP level (ng/mL) | 4.8 (2.0–20,000) | 4.0(2.0–1273) | 0.2729 |
| PIVKA-II level (mAU/mL) | 203.0 (13–81,886) | 82.0(18.0–7719) | 0.4114 |
| CIRT (n = 19) | Hepatectomy (n = 13) | p Value | |
|---|---|---|---|
| Age | Median: 71 (range: 56–79) | 70 (58–77) | 0.5613 |
| Sex (male/female) | 18/1 | 11/2 | 0.1900 |
| Etiology (HBV/HCV/non-B, non-C/alcohol/MASH/PBC) | 2/3/3/7/3/1 | 1/1/4/6/1/0 | 0.0756 |
| mALBI grade (1/2a/2b/3) | 12/4/3/0 | 10/2/1/0 | 0.0577 |
| ALBI score | −2.648 (−3.415 to −1.313) | −2.786 (−3.186 to −1.800) | 0.3497 |
| BCLC stage (A/B/C) | 16/0/3 | 8/4/1 | 0.6220 |
| First treatment/pre-treatment (surgery/RFA/TACE/systemic therapy) | 15/4 | 8/5 | 0.5903 |
| Size (mm) | 50.0 (40–110) | 50.0 (40–130) | 0.4290 |
| Total number of intrahepatic tumors (1/2) | 19/0 | 9/4 | 0.7422 |
| MVI (−/+) | 16/3 | 12/1 | 0.2048 |
| Blood biochemistry (median [range]) | |||
| T-bil level (mg/dL) | 0.70 (0.40–2.2) | 0.70 (0.30–0.90) | 0.0771 |
| Alb level (g/dL) | 4.0 (2.7–4.9) | 4.1 (3.0–4.3) | 0.5634 |
| AST level (U/L) | 29.0 (13–83) | 24.0 (14–77) | 0.4416 |
| ALT level (U/L) | 26.0 (10–95) | 22.0 (13–107) | 0.9393 |
| PLT count (103/µL) | 162 (45–382) | 190 (102–252) | 0.8603 |
| PT% | 94.0 (65–112) | 93.0 (68–123) | 0.8019 |
| AFP level (ng/mL) | 4.0 (2.0–20,000) | 4.0 (2.0–1273) | 0.4414 |
| PIVKA-II level (mAU/mL) | 140.0 (13–1934) | 82.0 (18.0–7719) | 0.2008 |
| CIRT, <80 Years (n = 19) | CIRT, ≥80 Years (n = 19) | p Value | |
|---|---|---|---|
| Age | Median: 71 (range: 56–79) | 85 (81–93) | <0.0001 |
| Sex (male/female) | 18/1 | 11/8 | 0.0188 |
| Etiology (HBV/HCV/non-B, non-C/alcohol/MASH/PBC) | 2/3/3/7/3/1 | 3/7/4/1/3/1 | 0.2655 |
| mALBI grade (1/2a/2b/3) | 12/4/3/0 | 8/5/5/1 | 0.4916 |
| ALBI score | −2.648 (−3.415 to −1.313) | −2.563 (−3.075 to −1.744) | 0.6024 |
| BCLC stage (A/B/C) | 16/0/3 | 14/3/2 | 0.2647 |
| First treatment/pre-treatment (surgery/RFA/TACE/systemic therapy) | 15/4 | 11/8 | 0.1496 |
| Size (mm) | 50.0 (40–110) | 65.0 (40–105) | 0.1510 |
| Total number of intrahepatic tumors (1/2) | 19/0 | 16/3 | 0.1870 |
| MVI (−/+) | 16/3 | 16/3 | >0.9999 |
| Blood biochemistry (median [range]) | |||
| T-bil level (mg/dL) | 0.70 (0.40–2.2) | 0.60 (0.40–1.4) | 0.1052 |
| Alb level (g/dL) | 4.0 (2.7–4.9) | 3.8 (2.7–4.5) | 0.2510 |
| AST level (U/L) | 29.0 (13–83) | 36.0 (12–222) | 0.3784 |
| ALT level (U/L) | 26.0 (10–95) | 32.0 (5.0–135) | 0.9815 |
| PLT count (103/µL) | 162 (45–382) | 194 (70–371) | 0.4927 |
| PT% | 94.0 (65–112) | 94.0 (81–114) | 0.4667 |
| AFP level (ng/mL) | 4.0 (2.0–20,000) | 6.7 (2.0–20,000) | 0.3463 |
| PIVKA-II level (mAU/mL) | 140.0 (13–1934) | 785.0 (15.0–81,886) | 0.0542 |
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Time to Onset (Month) Median (Range) | ||
|---|---|---|---|---|---|---|---|
| Liver dysfunction | n = 13 (34.2%) | 11 | 2 | 0 | 0 | 0 | 2.2 (1.0–6.0) |
| Fatigue | n = 12 (31.6%) | 12 | 0 | 0 | 0 | 0 | 2.0 (0.5–5.0) |
| Skin redness/dermatitis | n = 11 (28.9%) | 9 | 2 | 0 | 0 | 0 | 1.5 (1.0–6.0) |
| Anorexia | n = 6 (15.8%) | 6 | 0 | 0 | 0 | 0 | 1.5 (0.5–3.0) |
| Localized pleural effusion | n = 3 (7.9%) | 3 | 0 | 0 | 0 | 0 | 4.5 (3.0–6.0) |
| Itchy skin | n = 3 (7.9%) | 3 | 0 | 0 | 0 | 0 | 2.5 (1.0–8.0) |
| Rib fracture/myositis | n = 3 (7.9%) | 1 | 2 | 0 | 0 | 0 | 6.0 (3.0–15.0) |
| Nausea | n = 1 (2.6%) | 1 | 0 | 0 | 0 | 0 | 3.0 (3.0–3.0) |
| Biliary stricture | n = 1 (2.6%) | 0 | 0 | 1 | 0 | 0 | 5.0 (5.0–5.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maki, K.; Haga, H.; Katsumi, T.; Hoshikawa, K.; Kaneko, T.; Takahashi, R.; Sugawara, S.; Koto, M.; Motoi, F.; Ueno, Y. Treatment Outcomes in Patients Receiving Carbon-Ion Radiotherapy Versus Hepatectomy for Hepatocellular Carcinoma (≥4 cm): A Retrospective Study in Japan. J. Clin. Med. 2025, 14, 5678. https://doi.org/10.3390/jcm14165678
Maki K, Haga H, Katsumi T, Hoshikawa K, Kaneko T, Takahashi R, Sugawara S, Koto M, Motoi F, Ueno Y. Treatment Outcomes in Patients Receiving Carbon-Ion Radiotherapy Versus Hepatectomy for Hepatocellular Carcinoma (≥4 cm): A Retrospective Study in Japan. Journal of Clinical Medicine. 2025; 14(16):5678. https://doi.org/10.3390/jcm14165678
Chicago/Turabian StyleMaki, Keita, Hiroaki Haga, Tomohiro Katsumi, Kyoko Hoshikawa, Takashi Kaneko, Ryosuke Takahashi, Shuichiro Sugawara, Masashi Koto, Fuyuhiko Motoi, and Yoshiyuki Ueno. 2025. "Treatment Outcomes in Patients Receiving Carbon-Ion Radiotherapy Versus Hepatectomy for Hepatocellular Carcinoma (≥4 cm): A Retrospective Study in Japan" Journal of Clinical Medicine 14, no. 16: 5678. https://doi.org/10.3390/jcm14165678
APA StyleMaki, K., Haga, H., Katsumi, T., Hoshikawa, K., Kaneko, T., Takahashi, R., Sugawara, S., Koto, M., Motoi, F., & Ueno, Y. (2025). Treatment Outcomes in Patients Receiving Carbon-Ion Radiotherapy Versus Hepatectomy for Hepatocellular Carcinoma (≥4 cm): A Retrospective Study in Japan. Journal of Clinical Medicine, 14(16), 5678. https://doi.org/10.3390/jcm14165678







