Dynamics of the Novel Cardiac Biomarkers sST2, H-FABP, GDF-15 and suPAR in HFrEF Patients Undergoing Heart Failure Therapy, a Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Blood Sampling
2.4. Biomarker Analysis
2.5. Ethics
2.6. Statistics
3. Results
3.1. Demographics and Baseline Characteristics
3.2. Descriptive Statistics
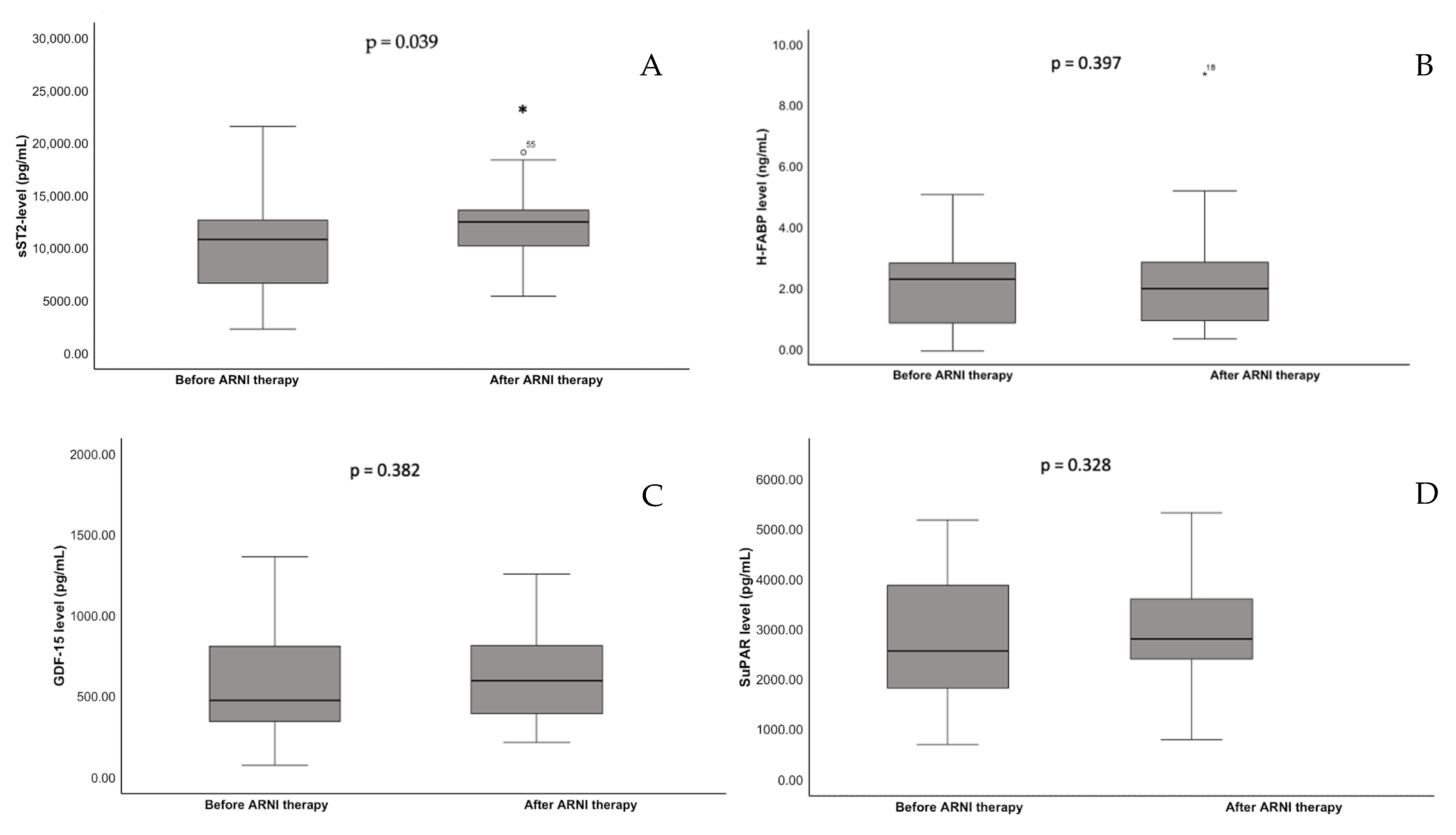
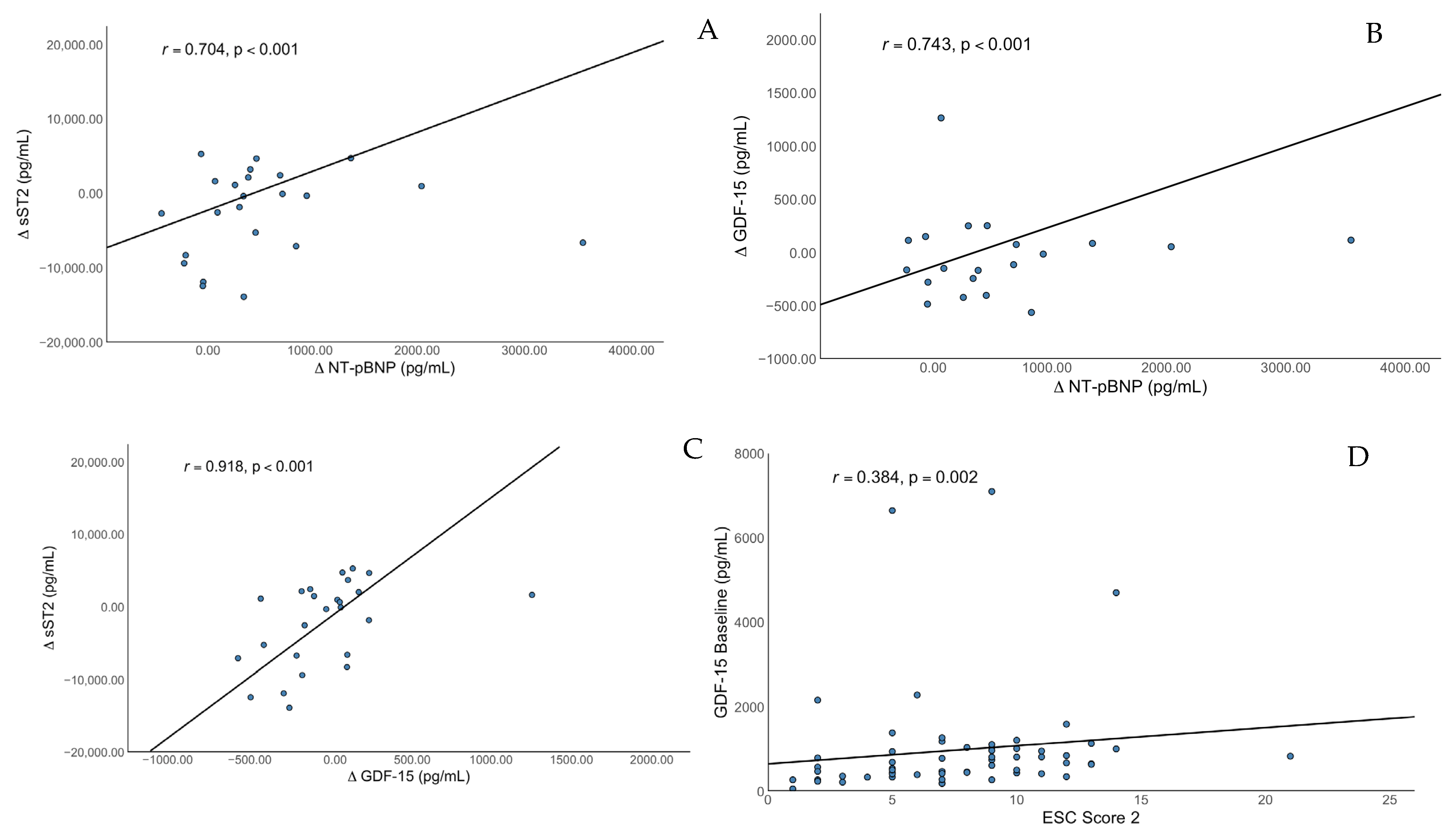
3.3. Main Findings
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Mensah, G.A.; Krish, V.; Glenn, S.D.; Miller-Petrie, M.K.; Lopez, A.D.; Murray, C.J.L. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Tsao, C.W.; Lyass, A.; Enserro, D.; Larson, M.G.; Ho, J.E.; Kizer, J.R.; Gottdiener, J.S.; Psaty, B.M.; Vasan, R.S. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail. 2018, 6, 678–685. [Google Scholar] [CrossRef]
- Barasa, A.; Schaufelberger, M.; Lappas, G.; Swedberg, K.; Dellborg, M.; Rosengren, A. Heart failure in young adults: 20-year trends in hospitalization, aetiology, and case fatality in Sweden. Eur. Heart J. 2014, 35, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.J.; DeFrances, C.J.; Williams, S.N.; Golosinskiy, A.; Schwartzman, A. National Hospital Discharge Survey: 2007 summary. In National Health Statistics Reports; National Center for Health Statistics: Hyattsville, MD, USA, 2010; Volume 24, pp. 1–20. [Google Scholar]
- Jhund, P.S.; Macintyre, K.; Simpson, C.R.; Lewsey, J.D.; Stewart, S.; Redpath, A.; Chalmers, J.W.; Capewell, S.; McMurray, J.J. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: A population study of 5.1 million people. Circulation 2009, 119, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.A.; Zaccardi, F.; Squire, I.; Ling, S.; Davies, M.J.; Lam, C.S.P.; Mamas, M.A.; Khunti, K.; Kadam, U.T. 20-year trends in cause-specific heart failure outcomes by sex, socioeconomic status, and place of diagnosis: A population-based study. Lancet Public Health 2019, 4, e406–e420. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Cook, C.; Cole, G.; Asaria, P.; Jabbour, R.; Francis, D.P. The annual global economic burden of heart failure. Int. J. Cardiol. 2014, 171, 368–376. [Google Scholar] [CrossRef]
- Angermann, C.E.; Assmus, B.; Anker, S.D.; Asselbergs, F.W.; Brachmann, J.; Brett, M.E.; Brugts, J.J.; Ertl, G.; Ginn, G.; Hilker, L.; et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: The CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF). Eur. J. Heart Fail. 2020, 22, 1891–1901. [Google Scholar] [CrossRef]
- Abraham, W.T.; Stevenson, L.W.; Bourge, R.C.; Lindenfeld, J.A.; Bauman, J.G.; Adamson, P.B. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow-up results from the CHAMPION randomised trial. Lancet 2016, 387, 453–461. [Google Scholar] [CrossRef]
- Störk, S.; Bernhardt, A.; Böhm, M.; Brachmann, J.; Dagres, N.; Frantz, S.; Hindricks, G.; Köhler, F.; Zeymer, U.; Rosenkranz, S.; et al. Pulmonary artery sensor system pressure monitoring to improve heart failure outcomes (PASSPORT-HF): Rationale and design of the PASSPORT-HF multicenter randomized clinical trial. Clin. Res. Cardiol. 2022, 111, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Ahmad, T.; Anstrom, K.J.; Adams, K.F.; Cooper, L.S.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.L.; Leifer, E.S.; et al. Rationale and design of the GUIDE-IT study: Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure. JACC Heart Fail. 2014, 2, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Porapakkham, P.; Porapakkham, P.; Zimmet, H.; Billah, B.; Krum, H. B-type natriuretic peptide-guided heart failure therapy: A meta-analysis. Arch. Intern. Med. 2010, 170, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Anstrom, K.J.; Adams, K.F.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.L., Jr.; Mark, D.B.; Piña, I.L.; Passmore, G.; et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2017, 318, 713–720. [Google Scholar] [CrossRef]
- Lichtenauer, M.; Jirak, P.; Wernly, B.; Paar, V.; Rohm, I.; Jung, C.; Schernthaner, C.; Kraus, J.; Motloch, L.J.; Yilmaz, A.; et al. A comparative analysis of novel cardiovascular biomarkers in patients with chronic heart failure. Eur. J. Intern. Med. 2017, 44, 31–38. [Google Scholar] [CrossRef]
- Jirak, P.; Pistulli, R.; Lichtenauer, M.; Wernly, B.; Paar, V.; Motloch, L.J.; Rezar, R.; Jung, C.; Hoppe, U.C.; Schulze, P.C.; et al. Expression of the Novel Cardiac Biomarkers sST2, GDF-15, suPAR, and H-FABP in HFpEF Patients Compared to ICM, DCM, and Controls. J. Clin. Med. 2020, 9, 1130. [Google Scholar] [CrossRef]
- Tominaga, S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989, 258, 301–304. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Weinberg, E.O.; Shimpo, M.; De Keulenaer, G.W.; MacGillivray, C.; Tominaga, S.; Solomon, S.D.; Rouleau, J.L.; Lee, R.T. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 2002, 106, 2961–2966. [Google Scholar] [CrossRef]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef]
- Reina-Couto, M.; Pereira-Terra, P.; Quelhas-Santos, J.; Silva-Pereira, C.; Albino-Teixeira, A.; Sousa, T. Inflammation in Human Heart Failure: Major Mediators and Therapeutic Targets. Front. Physiol. 2021, 12, 746494. [Google Scholar] [CrossRef]
- Weinberg, E.O.; Shimpo, M.; Hurwitz, S.; Tominaga, S.; Rouleau, J.L.; Lee, R.T. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation 2003, 107, 721–726. [Google Scholar] [CrossRef]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef] [PubMed]
- Böttner, M.; Suter-Crazzolara, C.; Schober, A.; Unsicker, K. Expression of a novel member of the TGF-beta superfamily, growth/differentiation factor-15/macrophage-inhibiting cytokine-1 (GDF-15/MIC-1) in adult rat tissues. Cell Tissue Res. 1999, 297, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Kempf, T.; Wollert, K.C. Growth-differentiation factor-15 in heart failure. Heart Fail. Clin. 2009, 5, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Ago, T.; Sadoshima, J. GDF15, a cardioprotective TGF-beta superfamily protein. Circ. Res. 2006, 98, 294–297. [Google Scholar] [CrossRef]
- Brown, D.A.; Breit, S.N.; Buring, J.; Fairlie, W.D.; Bauskin, A.R.; Liu, T.; Ridker, P.M. Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: A nested case-control study. Lancet 2002, 359, 2159–2163. [Google Scholar] [CrossRef]
- Damman, P.; Kempf, T.; Windhausen, F.; van Straalen, J.P.; Guba-Quint, A.; Fischer, J.; Tijssen, J.G.; Wollert, K.C.; de Winter, R.J.; Hirsch, A. Growth-differentiation factor 15 for long-term prognostication in patients with non-ST-elevation acute coronary syndrome: An Invasive versus Conservative Treatment in Unstable coronary Syndromes (ICTUS) substudy. Int. J. Cardiol. 2014, 172, 356–363. [Google Scholar] [CrossRef]
- Kempf, T.; Sinning, J.M.; Quint, A.; Bickel, C.; Sinning, C.; Wild, P.S.; Schnabel, R.; Lubos, E.; Rupprecht, H.J.; Münzel, T.; et al. Growth-differentiation factor-15 for risk stratification in patients with stable and unstable coronary heart disease: Results from the AtheroGene study. Circ. Cardiovasc. Genet. 2009, 2, 286–292. [Google Scholar] [CrossRef]
- Ploug, M.; Rønne, E.; Behrendt, N.; Jensen, A.L.; Blasi, F.; Danø, K. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J. Biol. Chem. 1991, 266, 1926–1933. [Google Scholar] [CrossRef]
- Thunø, M.; Macho, B.; Eugen-Olsen, J. suPAR: The molecular crystal ball. Dis. Markers 2009, 27, 157–172. [Google Scholar] [CrossRef]
- Sehestedt, T.; Lyngbæk, S.; Eugen-Olsen, J.; Jeppesen, J.; Andersen, O.; Hansen, T.W.; Linneberg, A.; Jørgensen, T.; Haugaard, S.B.; Olsen, M.H. Soluble urokinase plasminogen activator receptor is associated with subclinical organ damage and cardiovascular events. Atherosclerosis 2011, 216, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.J.H.; Petersen, J.E.V.; Eugen-Olsen, J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a Biomarker of Systemic Chronic Inflammation. Front. Immunol. 2021, 12, 780641. [Google Scholar] [CrossRef] [PubMed]
- Velissaris, D.; Zareifopoulos, N.; Koniari, I.; Karamouzos, V.; Bousis, D.; Gerakaris, A.; Platanaki, C.; Kounis, N. Soluble Urokinase Plasminogen Activator Receptor as a Diagnostic and Prognostic Biomarker in Cardiac Disease. J. Clin. Med. Res. 2021, 13, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Jirak, P.; Mirna, M.; Wernly, B.; Paar, V.; Thieme, M.; Betge, S.; Franz, M.; Hoppe, U.; Lauten, A.; Kammler, J.; et al. Analysis of novel cardiovascular biomarkers in patients with peripheral artery disease. Minerva Med. 2018, 109, 443–450. [Google Scholar] [CrossRef]
- Koller, L.; Stojkovic, S.; Richter, B.; Sulzgruber, P.; Potolidis, C.; Liebhart, F.; Mörtl, D.; Berger, R.; Goliasch, G.; Wojta, J.; et al. Soluble Urokinase-Type Plasminogen Activator Receptor Improves Risk Prediction in Patients With Chronic Heart Failure. JACC Heart Fail. 2017, 5, 268–277. [Google Scholar] [CrossRef]
- Ockner, R.K.; Manning, J.A.; Poppenhausen, R.B.; Ho, W.K. A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science 1972, 177, 56–58. [Google Scholar] [CrossRef]
- Chmurzyńska, A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J. Appl. Genet. 2006, 47, 39–48. [Google Scholar] [CrossRef]
- Liebetrau, C.; Nef, H.M.; Dörr, O.; Gaede, L.; Hoffmann, J.; Hahnel, A.; Rolf, A.; Troidl, C.; Lackner, K.J.; Keller, T.; et al. Release kinetics of early ischaemic biomarkers in a clinical model of acute myocardial infarction. Heart 2014, 100, 652–657. [Google Scholar] [CrossRef]
- Rezar, R.; Jirak, P.; Gschwandtner, M.; Derler, R.; Felder, T.K.; Haslinger, M.; Kopp, K.; Seelmaier, C.; Granitz, C.; Hoppe, U.C.; et al. Heart-Type Fatty Acid-Binding Protein (H-FABP) and its Role as a Biomarker in Heart Failure: What Do We Know So Far? J. Clin. Med. 2020, 9, 164. [Google Scholar] [CrossRef]
- Niizeki, T.; Takeishi, Y.; Arimoto, T.; Takabatake, N.; Nozaki, N.; Hirono, O.; Watanabe, T.; Nitobe, J.; Harada, M.; Suzuki, S.; et al. Heart-type fatty acid-binding protein is more sensitive than troponin T to detect the ongoing myocardial damage in chronic heart failure patients. J. Card. Fail. 2007, 13, 120–127. [Google Scholar] [CrossRef]
- Markousis-Mavrogenis, G.; Tromp, J.; Ouwerkerk, W.; Devalaraja, M.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.S.; van der Harst, P.; Lang, C.C.; et al. The clinical significance of interleukin-6 in heart failure: Results from the BIOSTAT-CHF study. Eur. J. Heart Fail. 2019, 21, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Redfield, M.M.; Chen, H.H.; Borlaug, B.A.; Semigran, M.J.; Lee, K.L.; Lewis, G.; LeWinter, M.M.; Rouleau, J.L.; Bull, D.A.; Mann, D.L.; et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA 2013, 309, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Liu, P.; Gao, X.; Shi, S.; Li, Y.; Zhang, B.; Wu, H.; Song, Q. Association of systemic inflammatory markers with clinical adverse prognosis and outcomes in HFpEF: A systematic review and meta-analysis of cohort studies. Front. Cardiovasc. Med. 2024, 11, 1461073. [Google Scholar] [CrossRef] [PubMed]
- von Lueder, T.G.; Wang, B.H.; Kompa, A.R.; Huang, L.; Webb, R.; Jordaan, P.; Atar, D.; Krum, H. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ. Heart Fail. 2015, 8, 71–78. [Google Scholar] [CrossRef]
- Pu, Q.; Amiri, F.; Gannon, P.; Schiffrin, E.L. Dual angiotensin-converting enzyme/neutral endopeptidase inhibition on cardiac and renal fibrosis and inflammation in DOCA-salt hypertensive rats. J. Hypertens. 2005, 23, 401–409. [Google Scholar] [CrossRef]
- Bozkurt, B.; Nair, A.P.; Misra, A.; Scott, C.Z.; Mahar, J.H.; Fedson, S. Neprilysin Inhibitors in Heart Failure: The Science, Mechanism of Action, Clinical Studies, and Unanswered Questions. JACC Basic. Transl. Sci. 2023, 8, 88–105. [Google Scholar] [CrossRef]
- Ohnewein, B.; Shomanova, Z.; Paar, V.; Topf, A.; Jirak, P.; Fiedler, L.; Granitz, C.; Van Almsick, V.; Semo, D.; Zagidullin, N.; et al. Effects of Angiotensin Receptor-Neprilysin Inhibitors (ARNIs) on the Glucose and Fat Metabolism Biomarkers Leptin and Fructosamine. J. Clin. Med. 2023, 12, 3083. [Google Scholar] [CrossRef]
- O’Meara, E.; Prescott, M.F.; Claggett, B.; Rouleau, J.L.; Chiang, L.M.; Solomon, S.D.; Packer, M.; McMurray, J.J.V.; Zile, M.R. Independent Prognostic Value of Serum Soluble ST2 Measurements in Patients With Heart Failure and a Reduced Ejection Fraction in the PARADIGM-HF Trial (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ. Heart Fail. 2018, 11, e004446. [Google Scholar] [CrossRef]
- Ge, Z.; Li, C.; Liu, Y.; Sun, X. The Effect of Recombinant Human Brain Natriuretic Peptide Combined with Xinmailong on Heart Failure and Its Impact on Cardiac Function and Inflammatory Response. Int. J. Gen. Med. 2025, 18, 1999–2008. [Google Scholar] [CrossRef]
- Mezzasoma, L.; Talesa, V.N.; Romani, R.; Bellezza, I. ANP and BNP Exert Anti-Inflammatory Action via NPR-1/cGMP Axis by Interfering with Canonical, Non-Canonical, and Alternative Routes of Inflammasome Activation in Human THP1 Cells. Int. J. Mol. Sci. 2021, 22, 24. [Google Scholar] [CrossRef]
- Fish-Trotter, H.; Ferguson, J.F.; Patel, N.; Arora, P.; Allen, N.B.; Bachmann, K.N.; Daniels, L.B.; Reilly, M.P.; Lima, J.A.C.; Wang, T.J.; et al. Inflammation and Circulating Natriuretic Peptide Levels. Circ. Heart Fail. 2020, 13, e006570. [Google Scholar] [CrossRef]
- Kiemer, A.K.; Hartung, T.; Vollmar, A.M. cGMP-Mediated Inhibition of TNF-α Production by the Atrial Natriuretic Peptide in Murine Macrophages1. J. Immunol. 2000, 165, 175–181. [Google Scholar] [CrossRef]
- Mirna, M.; Lichtenauer, M.; Wernly, B.; Paar, V.; Jung, C.; Kretzschmar, D.; Uhlemann, M.; Franz, M.; Hoppe, U.C.; Schulze, P.C.; et al. Novel cardiovascular biomarkers in patients with cardiovascular diseases undergoing intensive physical exercise. Panminerva Med. 2020, 62, 135–142. [Google Scholar] [CrossRef]
- MacDonald, M.R.; Petrie, M.C.; Varyani, F.; Östergren, J.; Michelson, E.L.; Young, J.B.; Solomon, S.D.; Granger, C.B.; Swedberg, K.; Yusuf, S.; et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: An analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur. Heart J. 2008, 29, 1377–1385. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Voors, A.A.; Zannad, F.; Januzzi, J.L.; Mark Richards, A.; Díez, J. Transitioning from usual care to biomarker-based personalized and precision medicine in heart failure: Call for action. Eur. Heart J. 2017, 39, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Specification | Results | Q1; Q3/SD or % |
|---|---|---|---|
| Demographics | Age (years) | 62.3 | (±12.4) |
| Gender (male/female) | 49/21 | (70.0; 30.0) | |
| Medical history | Ischemic cardiomyopathy | 34 | (48.6) |
| Non-ischemic cardiomyopathy | 36 | (51.4) | |
| Atrial fibrillation * | 20 | (28.6) | |
| Dyslipidemia | 39 | (58.2) | |
| Diabetes mellitus | 12 | (17.9) | |
| Hypertension | 37 | (55.2) | |
| Chronic kidney disease ** | 26 | (37.0) | |
| History of smoking | 31 | (46.2) | |
| Clinical measurement | BMI | 26.7 | (23.2; 30.9) |
| SBP (mmHg) | 126 | (±18.0) | |
| Heart rate (bpm) | 70 | (60; 82) | |
| LVEF (%) | 29.9 | (23.0; 36.25) | |
| LVEDD (mm) | 60.2 | (±8.6) | |
| Treatment | ACI/ARB (before ARNI) | 60 | (85.7) |
| BB | 60 | (85.7) | |
| MRA | 48 | (68.6) | |
| Statin | 44 | (58.6) | |
| Ezetimib | 9 | (12.9) | |
| Loop Diuretics | 35 | (55.0) | |
| Thiacids | 1 | (1.4) | |
| Metformin | 10 | (14.3) | |
| SGLT-2 inhibitors | 2 | (2.9) | |
| Insulin | 4 | (5.7) | |
| Laboratory | NT-proBNP (ng/L) | 1402 | (475; 2636) |
| Hemoglobin g/L | 14.0 | (±1.9) | |
| eGFR (mL/min/1.73 m2) | 64.8 | (±16.3) | |
| LDL (mg/dL) | 86.0 | (62.8; 98.0) | |
| HbA1c (%) | 5.7 | (5.4; 6.1) | |
| CRP (mg/dL) | 0.5 | (0.2; 1.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohnewein, B.; Shomanova, Z.; Jirak, P.; Paar, V.; Topf, A.; Pylypenko, L.; Schäbinger, M.; Volg, F.; Hoppe, U.C.; Pistulli, R.; et al. Dynamics of the Novel Cardiac Biomarkers sST2, H-FABP, GDF-15 and suPAR in HFrEF Patients Undergoing Heart Failure Therapy, a Pilot Study. J. Clin. Med. 2025, 14, 5668. https://doi.org/10.3390/jcm14165668
Ohnewein B, Shomanova Z, Jirak P, Paar V, Topf A, Pylypenko L, Schäbinger M, Volg F, Hoppe UC, Pistulli R, et al. Dynamics of the Novel Cardiac Biomarkers sST2, H-FABP, GDF-15 and suPAR in HFrEF Patients Undergoing Heart Failure Therapy, a Pilot Study. Journal of Clinical Medicine. 2025; 14(16):5668. https://doi.org/10.3390/jcm14165668
Chicago/Turabian StyleOhnewein, Bernhard, Zornitsa Shomanova, Peter Jirak, Vera Paar, Albert Topf, Lidia Pylypenko, Max Schäbinger, Fabian Volg, Uta C. Hoppe, Rudin Pistulli, and et al. 2025. "Dynamics of the Novel Cardiac Biomarkers sST2, H-FABP, GDF-15 and suPAR in HFrEF Patients Undergoing Heart Failure Therapy, a Pilot Study" Journal of Clinical Medicine 14, no. 16: 5668. https://doi.org/10.3390/jcm14165668
APA StyleOhnewein, B., Shomanova, Z., Jirak, P., Paar, V., Topf, A., Pylypenko, L., Schäbinger, M., Volg, F., Hoppe, U. C., Pistulli, R., Zagidullin, N., Lichtenauer, M., & Motloch, L. J. (2025). Dynamics of the Novel Cardiac Biomarkers sST2, H-FABP, GDF-15 and suPAR in HFrEF Patients Undergoing Heart Failure Therapy, a Pilot Study. Journal of Clinical Medicine, 14(16), 5668. https://doi.org/10.3390/jcm14165668






