Mesopic and Low-Contrast Visual Acuity Deficits in Retinitis Pigmentosa: Clinical Markers for Early Functional Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedures
- Photopic conditions 100 cd/m2.
- Mesopic conditions 1 cd/m2, obtained by placing a 2.0 log unit neutral density filter (Rosco e-colour 211; Rosco-Ibérica S.A., Madrid, Spain) over the backlit ETDRS cabinet panel.
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VA | Visual acuity |
| RP | Retinitis pigmentosa |
| LLD | Low-luminance deficit |
| LCD | Low-contrast deficit |
| AMD | Age-related macular degeneration |
| HCVAP | High-contrast visual acuity under photopic conditions |
| HCVAM | High-contrast visual acuity under mesopic conditions |
| LCVAP | Low-contrast visual acuity under photopic conditions |
| LCVAM | Low-contrast visual acuity under mesopic conditions |
| LLDHC | Low-luminance deficit with high contrast |
| LLDLC | Low-luminance deficit with low contrast |
| HCVAP | High-contrast VA photopic |
| HCVAM | High-contrast VA mesopic |
| RPE | Retinal pigment epithelium |
| OCT | Optical coherence tomography |
| DR | Diabetic retinopathy |
| DME | Diabetic macular edema |
| CHM | Choroideremia |
| PXE | Pseudoxanthoma elasticum |
| MacTel | Macular telangiectasia |
| ETDRS | Early Treatment Diabetic Retinopathy Study |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
| VAR | Visual acuity rating |
| ND | Neutral density |
References
- Finger, R.P.; Fenwick, E.; Marella, M.; Holz, F.G.; Lamoureux, E.L. The Impact of Vision Impairment on Vision-Specific Quality of Life in Germany. Invest Ophthalmol. Vis. Sci. 2011, 52, 3613–3619. [Google Scholar] [CrossRef] [PubMed]
- Altinbay, D.; Taskin, I. Evaluation of vision-related quality of life in retinitis pigmentosa patients with low vision. Jpn. J. Ophthalmol. 2021, 65, 777–785. [Google Scholar] [CrossRef]
- Hamel, C. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Li, P.; Yao, K. Retinitis Pigmentosa: Progress in Molecular Pathology and Biotherapeutical Strategies. Int. J. Mol. Sci. 2022, 23, 4883. [Google Scholar] [CrossRef]
- Verbakel, S.K.; Van Huet, R.A.C.; Boon, C.J.F.; Den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Tomita, Y.; Nagai, N.; Suzuki, M.; Kurihara, T.; Minami, S.; Tsubota, K.; Ozawa, Y. Functional Visual Acuity in Age-Related Macular Degeneration. Optom. Vis. Sci. 2016, 93, 70–76. [Google Scholar] [CrossRef]
- Lin, R.J.; Ng, J.S.; Nguyen, A.L. Determinants and standardization of mesopic visual acuity. Optom. Vis. Sci. 2015, 92, 559–565. [Google Scholar] [CrossRef]
- Wood, L.J.; Jolly, J.K.; Andrews, C.D.; MacLaren, R.E. Low-contrast visual acuity versus low-luminance visual acuity in choroideremia. Clin. Exp. Optom. 2020, 103, 386–392. [Google Scholar] [CrossRef]
- Regan, D.M. The Charles F. Prentice Award Lecture 1990: Specific Tests and Specific Blindnesses: Keys, Locks, and Parallel Processing. Optom. Vis. Sci. 1991, 68, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Regan, D.; Giaschi, D.E.; Fresco, B.B. Measurement of glare susceptibility using low-contrast letter charts. Optom. Vis. Sci. 1993, 70, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Linden, A. Measuring diagnostic and predictive accuracy in disease management: An introduction to receiver operating characteristic (ROC) analysis. J. Eval. Clin. Pract. 2006, 12, 132–139. [Google Scholar] [CrossRef]
- Yuan, R.; Yager, D.; Guethlein, M.; Oliver, G.; Kapoor, N.; Zhong, R. Controlling unwanted sources of threshold change in disability glare studies: A prototype apparatus and procedure. Optom. Vis. Sci. 1993, 70, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.R.; Fishman, G.A.; Derlacki, D.J. Intraocular light scatter in patients with retinitis pigmentosa. Vision Res. 1996, 36, 3703–3709. [Google Scholar] [CrossRef]
- Hong, Y.; Li, H.; Sun, Y.; Ji, Y. A Review of Complicated Cataract in Retinitis Pigmentosa: Pathogenesis and Cataract Surgery. J. Ophthalmol. 2020, 2020, 6699103. [Google Scholar] [CrossRef]
- Szamier, R.B.; Berson, E.L.; Klein, R.; Meyers, S. Sex-linked retinitis pigmentosa: Ultrastructure of photoreceptors and pigment epithelium. Invest. Ophthalmol. Vis. Sci. 1979, 18, 145–160. [Google Scholar]
- Schmidt, S.Y.; Peisch, R.D. Melanin concentration in normal human retinal pigment epithelium: Regional variation and age-related reduction. Invest. Ophthalmol. Vis. Sci. 1986, 27, 1063–1067. [Google Scholar] [PubMed]
- Szamier, R.B.; Berson, E.L. Histopathologic study of an unusual form of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 1982, 22, 559–570. [Google Scholar]
- Van den Berg, T.J.; Ijspeert, J.K.; de Waard, P.W. Dependence of intraocular straylight on pigmentation and light transmission through the ocular wall. Vision Res. 1991, 31, 1361–1367. [Google Scholar] [CrossRef]
- Kruijt, B.; Franssen, L.; Prick, L.J.; van Vliet, J.M.; van den Berg, T.J. Ocular straylight in albinism. Optom. Vis. Sci. 2011, 88, E585–E592. [Google Scholar] [CrossRef]
- Barrio, A.; Antona, B.; Puell, M.C. Repeatability of mesopic visual acuity measurements using high- and low-contrast ETDRS letter charts. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.J.; Jolly, J.K.; Josan, A.S.; Buckley, T.M.W.; MacLaren, R.E. Low luminance visual acuity and low luminance deficit in choroideremia and RPGR-associated retinitis pigmentosa. Transl. Vis. Sci. Technol. 2021, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.M.W.; Jolly, J.K.; Menghini, M.; Wood, L.J.; Nanda, A.; MacLaren, R.E. Test-retest repeatability of microperimetry in patients with retinitis pigmentosa caused by mutations in RPGR. Clin. Exp. Ophthalmol. 2020, 48, 714–715. [Google Scholar] [CrossRef]
- Menghini, M.; Jolly, J.K.; Nanda, A.; Wood, L.; Cehajic-Kapetanovic, J.; MacLaren, R.E. Early cone photoreceptor outer segment length shortening in RPGR X-linked retinitis pigmentosa. Ophthalmologica 2021, 244, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Guymer, R.H.; Finger, R.P. Low luminance deficit and night vision symptoms in intermediate age-related macular degeneration. Br. J. Ophthalmol. 2016, 100, 395. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.J.; Jolly, J.K.; Buckley, T.M.; Josan, A.S.; MacLaren, R.E. Low luminance visual acuity as a clinical measure and clinical trial outcome measure: A scoping review. Ophthalmic Physiol. Opt. 2021, 41, 213–223. [Google Scholar] [CrossRef]
- Hess, K.; Gliem, M.; Birtel, J.; Müller, P.L.; Wolters, D.; Holz, F.G.; Charbel Issa, P. Impaired dark adaptation associated with a diseased Bruch membrane in pseudoxanthoma elasticum. Retina 2020, 40, 1988–1995. [Google Scholar] [CrossRef]
- Müller, S.; Heeren, T.F.C.; Bonelli, R.; Pfau, M.; Thiele, S.; Holz, F.G.; Charbel Issa, P. Contrast sensitivity and visual acuity under low light conditions in macular telangiectasia type 2. Br. J. Ophthalmol. 2019, 103, 398. [Google Scholar] [CrossRef]
- Hsu, S.T.; Thompson, A.C.; Stinnett, S.S.; O’Connell, R.V.; Jaffe, G.J.; Toth, C.A. Longitudinal study of visual function in dry age-related macular degeneration at 12 months. Ophthalmol. Retina 2019, 3, 637–648. [Google Scholar] [CrossRef]
- Lad, E.M.; Fang, V.; Tessier, M.; Cao, X.; Lin, Y.; Yu, H.; Wang, L.; Birch, D.G. Longitudinal evaluation of visual function impairments in early and intermediate age-related macular degeneration patients. Ophthalmol. Sci. 2022, 2, 100173. [Google Scholar] [CrossRef]
- Grewal, M.K.; Sivapathasuntharam, C.; Chandra, S.; Moosajee, M. A pilot study evaluating the effects of 670 nm photobiomodulation in healthy ageing and age-related macular degeneration. J. Clin. Med. 2020, 9, 1001. [Google Scholar] [CrossRef]
- Cocce, K.J.; Stinnett, S.S.; Luhmann, U.F.O.; Yehoshua, Z.; Curcio, C.A.; Farsiu, S.; Toth, C.A. Visual function metrics in early and intermediate dry age-related macular degeneration for use as clinical trial endpoints. Am. J. Ophthalmol. 2018, 189, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Puell, M.C.; Hurtado-Ceña, F.J.; Pérez-Carrasco, M.J.; Contreras, I. Association between central retinal thickness and low luminance visual acuity in early age-related macular degeneration. Eur. J. Ophthalmol. 2020, 31, 2467–2473. [Google Scholar] [CrossRef] [PubMed]
- Sukha, A.Y.; Rubin, G.S. High, medium, and low contrast visual acuities in diabetic retinal disease. Optom. Vis. Sci. 2009, 86, 1086–1095. [Google Scholar] [CrossRef]
- Puell, M.C.; Barrio, A.R.; Palomo-Álvarez, C.; Gómez-Sanz, F.J.; Clement-Corral, A.; Pérez-Carrasco, M.J. Impaired mesopic visual acuity in eyes with early age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2012, 53, 7310–7314. [Google Scholar] [CrossRef]
- Legras, R.; Gaudric, A.; Woog, K. Distribution of cone density, spacing and arrangement in adult healthy retinas with adaptive optics flood illumination. PLoS ONE 2018, 13, e0191141. [Google Scholar] [CrossRef] [PubMed]
- Remis-Gonzalez, M.; Moreira-Estebaranz, C.; Puell, M.C.; Cedrún-Sánchez, J. Inner retinal layers’ thickness changes in relation to age in healthy eyes. Acta Ophthalmol. 2022, 100, S275. [Google Scholar] [CrossRef]
- Van den Berg, T.J.; Van Rijn, L.J.; Michael, R.; Heine, C.; Coppens, J.E.; Vrensen, G.F.; Grabner, G. Straylight effects with aging and lens extraction. Am. J. Ophthalmol. 2007, 144, 358–363. [Google Scholar] [CrossRef]
- Stifter, E.; Sacu, S.; Thaler, A.; Weghaupt, H. Contrast acuity in cataracts of different morphology and association to self-reported visual function. Invest. Ophthalmol. Vis. Sci. 2006, 47, 5412–5422. [Google Scholar] [CrossRef]
- Wood, J.M.; Owens, D.A. Standard measures of visual acuity do not predict drivers’ recognition performance under day or night conditions. Optom. Vis. Sci. 2005, 82, 698–705. [Google Scholar] [CrossRef]

| Control | RP | Mann–Whitney U Test | |
|---|---|---|---|
| No. of subjects (n) | 54 | 57 | |
| Age groups (<50 years, >50 years) | (32, 22) | (32, 25) | 0.2327 |
| Age, median (range) | 30.0 (20, 87) | 48.0 (23, 71) | 0.1701 |
| Female, n (%) | 34 (63%) | 31 (54.4%) | 0.9682 |
| Spherical Equivalent RE (D) | 0.00 (−2.25, 0.00) | −0.38 (−1.13, 0.25) | 0.3135 |
| Spherical Equivalent LE (D) | −0.38 (−2.88, 0.00) | −0.13 (−0.63, 0.75) | 0.0073 |
| Stereopsis (arcseconds) | 60 (60, 120) | 240 (120, 1000) | <0.0001 |
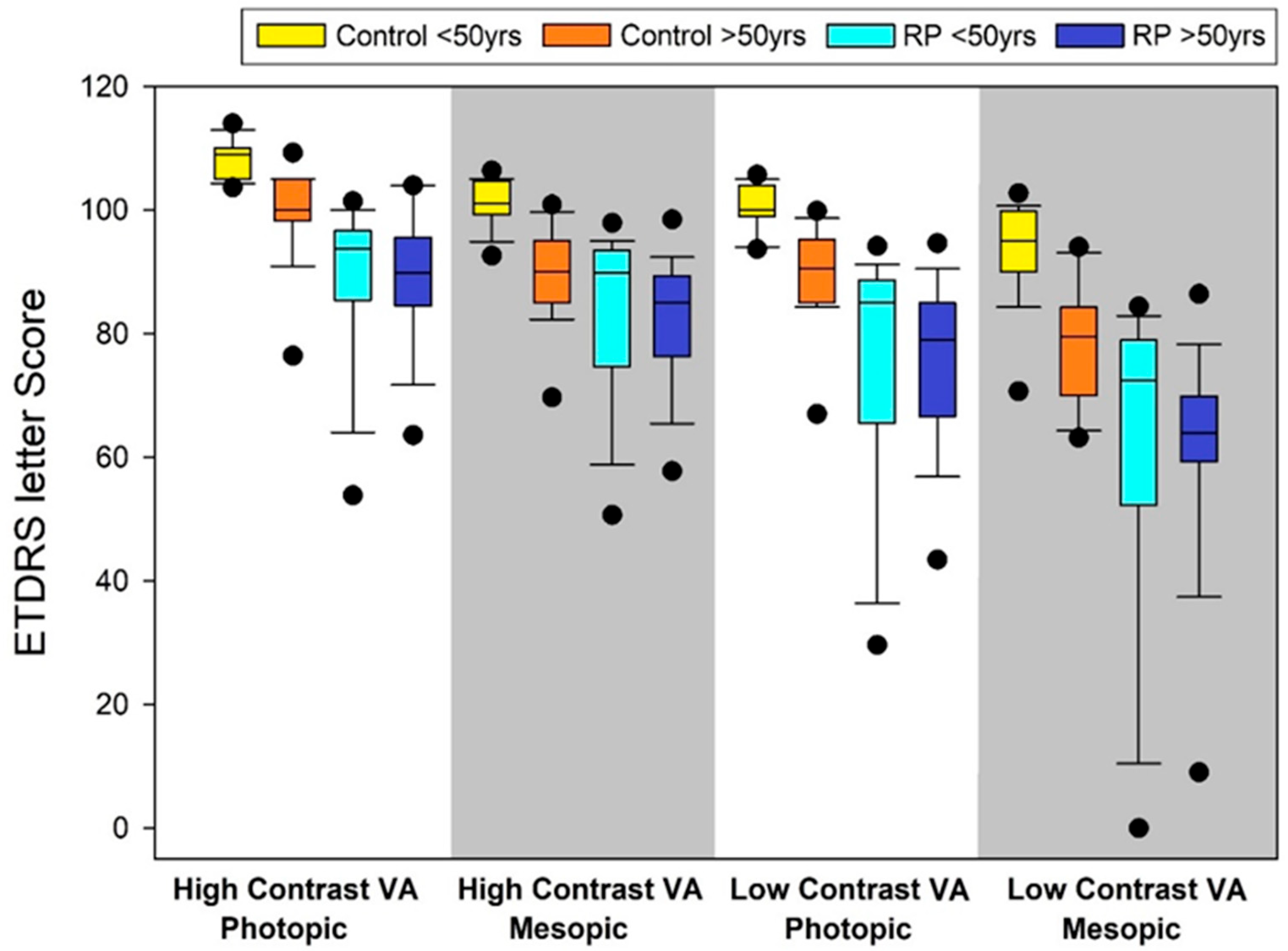
| Control (n = 54) | RP (n = 57) | Mann–Whitney U Test | |
|---|---|---|---|
| ETDRS letter score | |||
|
High-contrast VA Photopic (HCVAP) | 105 (103, 109) | 89 (85, 96) | <0.001 |
|
High-contrast VA Mesopic (HCVAM) | 99 (90, 102) | 87 (75, 91) | <0.001 |
|
10% Low-contrast VA photopic (10% LCVAP) | 99 (93, 102) | 80 (67, 87) | <0.001 |
|
10% Low-contrast VA mesopic (10% LCVAM) | 90 (80, 96) | 67 (59, 77) | <0.001 |
| Control (n = 54) | RP (n = 57) | Mann–Whitney U Test | |
|---|---|---|---|
| ETDRS letter difference score | |||
|
Low-luminance deficit with high contrast (LLDHC) | 8.0 (5.0, 10.0) | 4.9 (4.0, 7.4) | <0.001 |
|
Low-luminance deficit with low contrast (LLDLC) | 6.0 (4.0, 12.0) | 11.8 (7.6, 16.2) | <0.001 |
|
Low-contrast deficit in photopic (LCDP) | 9.0 (5.0, 11.0) | 12.0 (9.7, 16.8) | <0.001 |
|
Low-contrast deficit in mesopic (LCDM) | 8.0 (5.5, 12.0) | 18.0 (15.0, 24.0) | <0.001 |
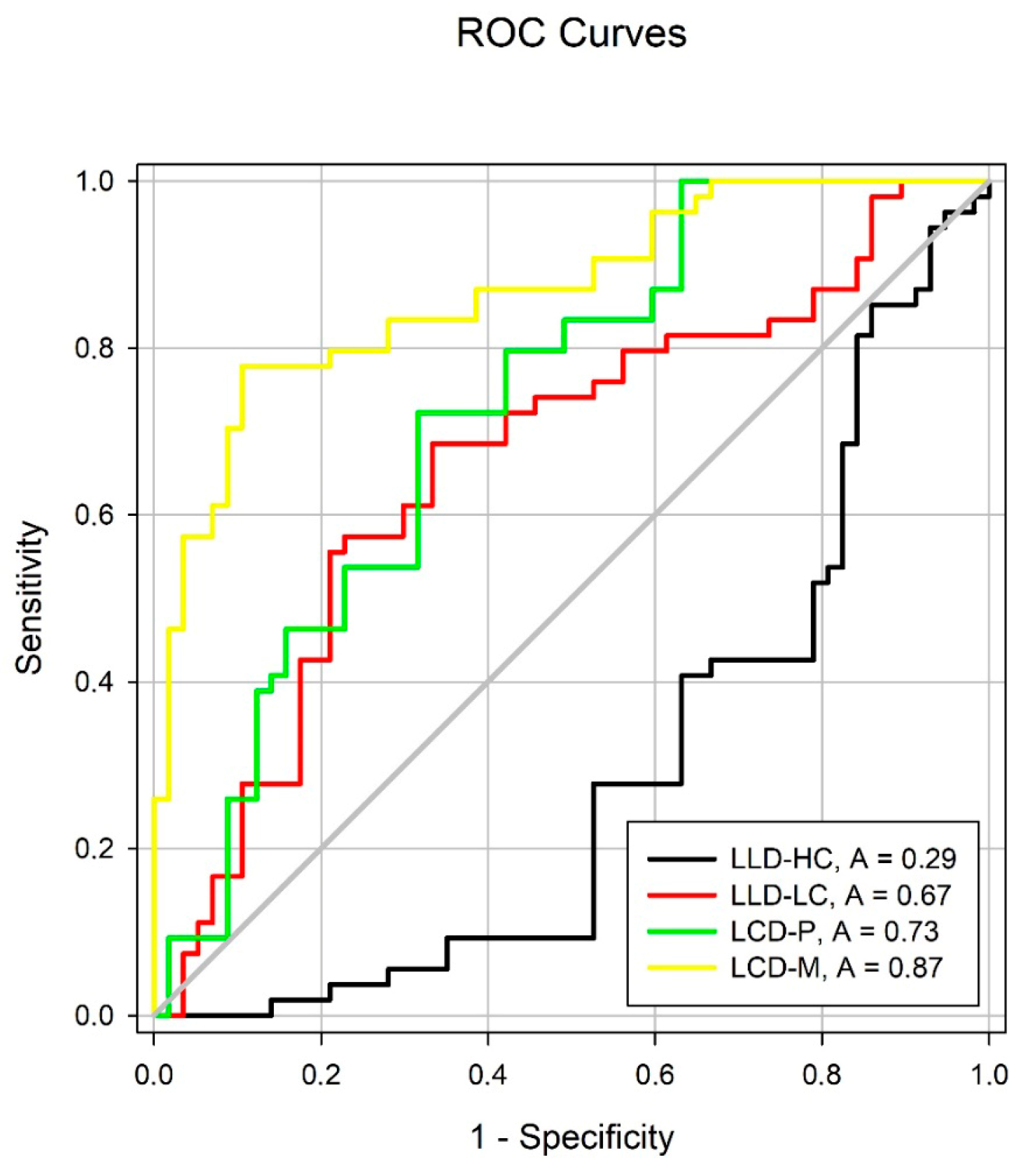
| Variable | Control vs. RP AUC |
|---|---|
| Low-luminance deficit with high contrast (LLDHC) | |
| Mean ± standard error | 0.29 ± 0.05 |
| 95% confidence interval | 0.20, 0.39 |
| p value | >0.05 |
| Low-luminance deficit with low contrast (LLDLC) | |
| Mean ± standard error | 0.67 ± 0.05 |
| 95% confidence interval | 0.56, 0.77 |
| p value | 0.0025 |
| Low-contrast deficit in photopic (LCDP) | |
| Mean ± standard error | 0.73 ± 0.05 |
| 95% confidence interval | 0.63, 0.82 |
| p value | <0.0001 |
| Low-contrast deficit in mesopic (LCDM) | |
| Mean ± standard error | 0.87 ± 0.03 |
| 95% confidence interval | 0.80, 0.94 |
| p value | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedrún-Sánchez, J.E.; Povedano-Montero, F.J.; Chamorro, E.; Sánchez-Ramos, C.; Puell, M.C. Mesopic and Low-Contrast Visual Acuity Deficits in Retinitis Pigmentosa: Clinical Markers for Early Functional Impairment. J. Clin. Med. 2025, 14, 5659. https://doi.org/10.3390/jcm14165659
Cedrún-Sánchez JE, Povedano-Montero FJ, Chamorro E, Sánchez-Ramos C, Puell MC. Mesopic and Low-Contrast Visual Acuity Deficits in Retinitis Pigmentosa: Clinical Markers for Early Functional Impairment. Journal of Clinical Medicine. 2025; 14(16):5659. https://doi.org/10.3390/jcm14165659
Chicago/Turabian StyleCedrún-Sánchez, Juan E., F. Javier Povedano-Montero, Eva Chamorro, Celia Sánchez-Ramos, and María C. Puell. 2025. "Mesopic and Low-Contrast Visual Acuity Deficits in Retinitis Pigmentosa: Clinical Markers for Early Functional Impairment" Journal of Clinical Medicine 14, no. 16: 5659. https://doi.org/10.3390/jcm14165659
APA StyleCedrún-Sánchez, J. E., Povedano-Montero, F. J., Chamorro, E., Sánchez-Ramos, C., & Puell, M. C. (2025). Mesopic and Low-Contrast Visual Acuity Deficits in Retinitis Pigmentosa: Clinical Markers for Early Functional Impairment. Journal of Clinical Medicine, 14(16), 5659. https://doi.org/10.3390/jcm14165659








