Obesity as a Part of Polycysric Ovary Syndrome (PCOS)—A Review of Pathophysiology and Comprehensive Therapeutic Strategies
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. PCOS Clinical Aspects
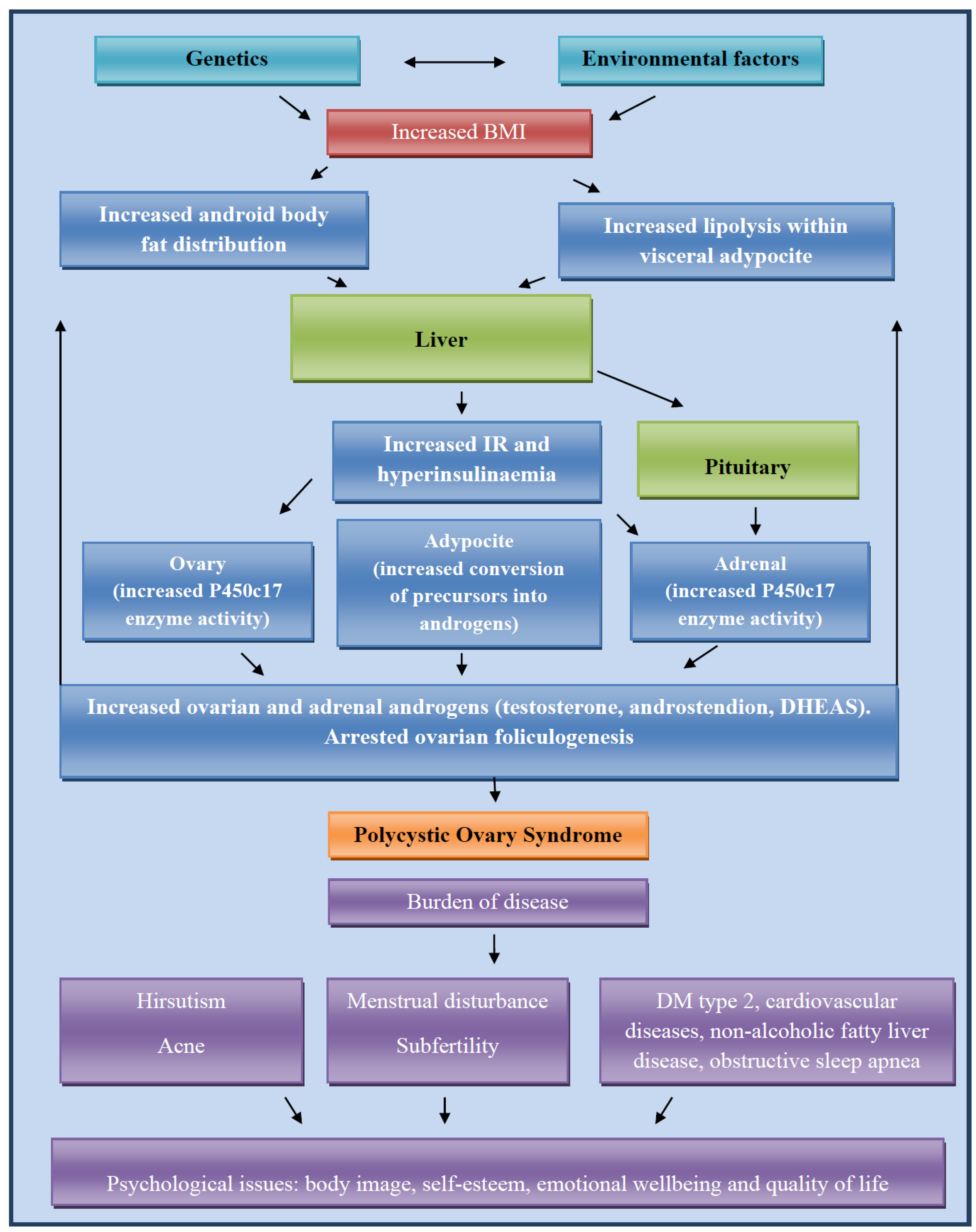
3.2. Molecular Basis of PCOS Development
3.3. Obesity and PCOS
3.4. Management of Obesity in PCOS
3.5. Medications for PCOS in Patients with Obesity
3.6. Differences in Treatment Between Obese and Lean Patients with PCOS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teede, H.; Tay, C.T.; Laven, J.; Dokras, A.; Moran, L.; Piltonen, T.; Costello, M.; Boivin, J.; Redman, L.; Boyle, J.; et al. International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome 2023; ESHRE: Melbourne, Australia, 2023. [Google Scholar]
- Meng, Y.; Zhao, T.; Zhang, R.; Zhu, X.; Ma, C.; Shi, Q. Global burden of polycystic ovary syndrome among women of childbearing age, 1990–2021: A systematic analysis using the global burden of disease study 2021. Front. Public Health 2025, 13, 1514250. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef]
- Glueck, C.J.; Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism 2019, 92, 108–120. [Google Scholar] [CrossRef]
- Smigoc, K.; Kawwass, J.F. Polycystic ovarian syndrome, obesity, and insulin resistance: Intertwined comorbidities that impact ART success. Fertil. Steril. 2024, 123, 65–66. [Google Scholar] [CrossRef]
- Treister-Goltzman, Y.; Nemet, D.; Menashe, I. Associations of adolescent obesity with hypertension, diabetes mellitus and polycystic ovaries in Arabs and Jews in Israel—A nationwide study. Front. Public Health 2024, 12, 1443756. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, J.; Cheng, X.; Nie, X.; He, B. Insulin resistance in polycystic ovary syndrome across various tissues: An updated review of pathogenesis, evaluation, and treatment. J. Ovarian Res. 2023, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiao, Z.; Cai, Y.; Pan, Y. Reproductive hormone characteristics of obese Chinese patients with polycystic ovarian syndrome: A meta-analysis. Gynecol. Endocrinol. 2025, 41, 2497854. [Google Scholar] [CrossRef]
- Venkatesh, S.S.; Ferreira, T.; Benonisdottir, S.; Rahmioglu, N.; Becker, C.M.; Granne, I.; Zondervan, K.T.; Holmes, M.V.; Lindgren, C.M.; Wittemans, L.B.L. Obesity and risk of female reproductive conditions: A Mendelian randomisation study. PLoS Med. 2022, 19, e1003679. [Google Scholar]
- Feng, L.; Yao, L.; Hong, W. Comparative observation of metabolic changes in obese and non-obese patients with polycystic ovary syndrome. J. Henan Med. Coll. 2017, 29, 46–48. [Google Scholar]
- Dobbie, L.J.; Pittam, B.; Zhao, S.S.; Alam, U.; Hydes, T.J.; Barber, T.M.; Cuthbertson, D.J. Childhood, adolescent, and adulthood adiposity are associated with risk of PCOS: A Mendelian randomization study with meta-analysis. Hum. Reprod. 2023, 38, 1168–1182. [Google Scholar] [CrossRef]
- Burns, K.; Mullin, B.H.; Moolhuijsen, L.M.E.; Laisk, T.; Tyrmi, J.S.; Cui, J.; Actkins, K.V.; Louwers, Y.V.; Davis, L.K.; Dudbridge, F.; et al. Body mass index stratified meta-analysis of genome-wide association studies of polycystic ovary syndrome in women of European ancestry. BMC Genom. 2024, 25, 208. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, Z.; Kraft, P.; Deng, Q.; Stener-Victorin, E.; Jiang, X. Genomic correlation, shared loci, and causal relationship between obesity and polycystic ovary syndrome: A large-scale genome-wide cross-trait analysis. BMC Med. 2022, 20, 66. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Lobo, R.A. Comparing Lean and Obese PCOS in Different PCOS Phenotypes: Evidence That the Body Weight Is More Important than the Rotterdam Phenotype in Influencing the Metabolic Status. Diagnostics 2022, 12, 2313. [Google Scholar] [CrossRef] [PubMed]
- Christ, J.P.; Cedars, M.I. Current Guidelines for Diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef]
- Myers, S.H.; Russo, M.; Dinicola, S.; Forte, G.; Unfer, V. Questioning PCOS phenotypes for reclassification and tailored therapy. Trends Endocrinol. Metab. 2023, 34, 694–703. [Google Scholar] [CrossRef]
- Głuszak, O.; Stopińska-Głuszak, U.; Glinicki, P.; Kapuscinska, R.; Snochowska, H.; Zgliczynski, W.; Debski, R. Phenotype and metabolic disorders in polycystic ovary syndrome. ISRN Endocrinol. 2012, 2012, 569862. [Google Scholar] [CrossRef]
- Carruba, M.O.; Busetto, L.; Bryant, S.; Caretto, A.; Farpour-Lambert, N.J.; Fatati, G.; Foschi, D.; Giorgino, F.; Halford, J.C.; Lenzi, A.; et al. The European Association for the Study of Obesity (EASO) Endorses the Milan Charter on Urban Obesity. Obes. Facts 2021, 14, 163–168. [Google Scholar] [CrossRef]
- Jena, S.; Mishra, I.; Baliarsinha, A.K.; Jena, D.; Debata, M. Variation in Clinical Presentation, Metabolic Profile, Hormonal Parameters and Inflammatory Markers in Polycystic Ovary Syndrome Women with and without Polycystic Ovary Morphology Appearance. J. Hum. Reprod. Sci. 2023, 16, 132–139. [Google Scholar] [CrossRef]
- Khan, M.J.; Ullah, A.; Basit, S. Genetic Basis of Polycystic Ovary Syndrome (PCOS): Current Perspectives. Appl. Clin. Genet. 2019, 12, 249–260. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, B.; Zhu, Z.; Kraft, P.; Deng, Q.; Stener-Victorin, E.; Jiang, X. A genome-wide cross-trait analysis identifies shared loci and causal relationships of type 2 diabetes and glycaemic traits with polycystic ovary syndrome. Diabetologia 2022, 65, 1483–1494. [Google Scholar] [CrossRef]
- Zhao, S.; Tian, Y.; Gao, X.; Zhang, X.; Liu, H.; You, L.; Cao, Y.; Su, S.; Chan, W.-Y.; Sun, Y.; et al. Family-based analysis of eight susceptibility loci in polycystic ovary syndrome. Sci. Rep. 2015, 5, 12619. [Google Scholar] [CrossRef]
- Laven, J.S.E. Follicle Stimulating Hormone Receptor (FSHR) Polymorphisms and Polycystic Ovary Syndrome (PCOS). Front. Endocrinol. 2019, 10, 23. [Google Scholar] [CrossRef]
- Singh, S.; Pal, N.; Shubham, S.; Sarma, D.K.; Verma, V.; Marotta, F.; Kumar, M. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J. Clin. Med. 2023, 12, 1454. [Google Scholar] [CrossRef]
- Gholinezhad, M.; Gholsorkhtabaramiri, M.; Esmaeilzadeh, S.; Ghanbarpour, A. Insulin resistance and adverse metabolic profile in overweight/obese and normal weight of young women with polycystic ovary syndrome. Casp. J. Intern. Med. 2018, 9, 260–267. [Google Scholar]
- Stepto, N.K.; Cassar, S.; Joham, A.E.; Hutchison, S.K.; Harrison, C.L.; Goldstein, R.F.; Teede, H.J. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum. Reprod. 2013, 28, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.P.; Hawkins, L.K.; Missmer, S.A.; Correia, K.F.; Yanushpolsky, E.H. Effect of body mass index on in vitro fertilization outcomes in women with polycystic ovary syndrome. Am. J. Obstet. Gynecol. 2014, 211, 163.e1–163.e6. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.E.; Daley, D.; Tarta, C.; Stanciu, P.I. Risk of endometrial cancer in patients with polycystic ovarian syndrome: A meta-analysis. Oncol. Lett. 2023, 25, 168. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Sung, Y.A.; Hong, Y.S.; Song, D.K.; Jung, H.; Jeong, K.; Chung, H.; Lee, H. Non-alcoholic fatty liver disease is associated with hyperandrogenism in women with polycystic ovary syndrome. Sci. Rep. 2023, 13, 13397. [Google Scholar] [CrossRef]
- Toosy, S.; Sodi, R.; Pappachan, J.M. Lean polycystic ovary syndrome (PCOS): An evidence-based practical approach. J. Diabetes Metab. Disord. 2018, 17, 277–285. [Google Scholar] [CrossRef]
- Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Barrea, L.; Frias-Toral, E.; Verde, L.; Ceriani, F.; Cucalón, G.; Garcia-Velasquez, E.; Moretti, D.; Savastano, S.; Colao, A.; Muscogiuri, G. PCOS and nutritional approaches: Differences between lean and obese phenotype. Metab. Open 2021, 12, 100123. [Google Scholar] [CrossRef]
- Tong, C.; Wu, Y.; Zhang, L.; Yu, Y. Insulin resistance, autophagy and apoptosis in patients with polycystic ovary syndrome: Association with PI3K signaling pathway. Front. Endocrinol. 2022, 13, 1091147. [Google Scholar] [CrossRef]
- Barber, T.M.; Kyrou, I.; Randeva, H.S.; Weickert, M.O. Mechanisms of Insulin Resistance at the Crossroad of Obesity with Associated Metabolic Abnormalities and Cognitive Dysfunction. Int. J. Mol. Sci. 2021, 22, 546. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Ma, Y.; Xiao, J.; Luo, G.; Li, Y.; Wu, D. Multi-system reproductive metabolic disorder: Significance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS). Life Sci. 2019, 228, 167–175. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Szczuko, U.; Szydłowska, I.; Nawrocka-Rutkowska, J.; Ziętek, M.; Verbanac, D.; Saso, L. Nutrition Strategy and Life Style in Polycystic Ovary Syndrome—Narrative Review. Nutrients 2021, 13, 2452. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhou, H.; Hu, M.; Feng, H. Effect of Diet on Insulin Resistance in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, 3346–3360. [Google Scholar] [CrossRef] [PubMed]
- Porchia, L.M.; Hernandez-Garcia, S.C.; Gonzalez-Mejia, M.E.; Lopez-Bayghen, E. Diets with lower carbohydrate concentrations improve insulin sensitivity in women with polycystic ovary syndrome: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Patten, R.K.; Boyle, R.A.; Moholdt, T.; Kiel, I.; Hopkins, W.G.; Harrison, C.L.; Stepto, N.K. Exercise Interventions in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Physiol. 2020, 11, 606. [Google Scholar] [CrossRef]
- Li, Y.J.; Han, Y.; He, B. Effects of bariatric surgery on obese polycystic ovary syndrome: A systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2019, 15, 942–950. [Google Scholar] [CrossRef]
- Chen, M.; Jia, Q.; Chen, Y.; Shan, W.; Tang, H.; Xing, T.; Wei, W.; Zheng, H.; Xue, W.; Shi, R.; et al. A meta-analysis of bariatric surgery in patients with obesity and polycystic ovary syndrome. Asian J. Surg. 2024, 47, 5083–5087. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, D.; Bu, H.; Zhao, T.; Wang, H. The Effect of Metformin on Polycystic Ovary Syndrome in Overweight Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Endocrinol. 2020, 2020, 5150684. [Google Scholar] [CrossRef] [PubMed]
- Greff, D.; Juhász, A.E.; Váncsa, S.; Váradi, A.; Sipos, Z.; Szinte, J.; Park, S.; Hegyi, P.; Nyirády, P.; Ács, N.; et al. Inositol is an effective and safe treatment in polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Reprod. Biol. Endocrinol. 2023, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Ali Fadlalmola, H.; Elhusein, A.M.; Al-Sayaghi, K.M.; Albadrani, M.S.; Swamy, D.V.; Mamanao, D.M.; El-Amin, E.I.; Ibrahim, S.E.; Abbas, S.M. Efficacy of resveratrol in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized clinical trials. Pan Afr. Med. J. 2023, 44, 134. [Google Scholar] [CrossRef]
- Wang, X.T.; Hou, Y.S.; Zhao, H.L.; Wang, J.; Guo, C.H.; Guan, J.; Lv, Z.G.; Ma, P.; Han, J.L. Effect of laparoscopic sleeve gastrectomy on related variables of obesity complicated with polycystic ovary syndrome. World J. Gastrointest. Surg. 2023, 15, 2423–2429. [Google Scholar] [CrossRef]
- Jensterle, M.; Kravos, N.A.; Ferjan, S.; Goricar, K.; Dolzan, V.; Janez, A. Long-term efficacy of metformin in overweight-obese PCOS: Longitudinal follow-up of retrospective cohort. Endocr. Connect. 2020, 9, 44–54. [Google Scholar] [CrossRef]
- Duca, F.A.; Côté, C.D.; Rasmussen, B.A.; Zadeh-Tahmasebi, M.; Rutter, G.A.; Filippi, B.M.; Lam, T.K. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 2015, 21, 506–511. [Google Scholar] [CrossRef]
- Stark, R.; Ashley, S.E.; Andrews, Z.B. AMPK and the neuroendocrine regulation of appetite and energy expenditure. Mol. Cell. Endocrinol. 2013, 366, 215–223. [Google Scholar] [CrossRef]
- Han, Y.; Li, Y.; He, B. GLP-1 receptor agonists versus metformin in PCOS: A systematic review and meta-analysis. Reprod. Biomed. Online 2019, 39, 332–342. [Google Scholar] [CrossRef]
- Malin, S.K.; Kashyap, S.R. Effects of metformin on weight loss: Potential mechanisms. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Kamenov, Z.; Gateva, A. Inositols in PCOS. Molecules 2020, 25, 5566. [Google Scholar] [CrossRef] [PubMed]
- Nazirudeen, R.; Sridhar, S.; Priyanka, R.; Sumathi, B.; Natarajan, V.; Subbiah, E.; Raghavan, K.S.; Sangumani, J. A randomized controlled trial comparing myoinositol with metformin versus metformin monotherapy in polycystic ovary syndrome. Clin. Endocrinol. 2023, 99, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Soldat-Stanković, V.; Popović-Pejičić, S.; Stanković, S.; Prtina, A.; Malešević, G.; Bjekić-Macut, J.; Livadas, S.; Ognjanović, S.; Mastorakos, G.; Micić, D.; et al. The effect of metformin and myoinositol on metabolic outcomes in women with polycystic ovary syndrome: Role of body mass and adiponectin in a randomized controlled trial. J. Endocrinol. Investig. 2022, 45, 583–595. [Google Scholar] [CrossRef]
- Hassan, S.; Shah, M.; Malik, M.O.; Ehtesham, E.; Habib, S.H.; Rauf, B. Treatment with combined resveratrol and myoinositol ameliorates endocrine, metabolic alterations and perceived stress response in women with PCOS: A double-blind randomized clinical trial. Endocrine 2023, 79, 208–220. [Google Scholar] [CrossRef]
- Xing, C.; Li, C.; He, B. Insulin Sensitizers for Improving the Endocrine and Metabolic Profile in Overweight Women With PCOS. J. Clin. Endocrinol. Metab. 2020, 105, 2950–2963. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Austregésilo de Athayde De Hollanda Morais, B.; Martins Prizão, V.; de Moura de Souza, M.; Ximenes Mendes, B.; Rodrigues Defante, M.L.; Cosendey Martins, O.; Rodrigues, A.M. The Efficacy and Safety of GLP-1 Agonists in PCOS Women Living with Obesity in Promoting Weight Loss and Hormonal Regulation: A Meta-Analysis of Randomized Controlled Trials. J. Diabetes Its Complicat. 2024, 38, 108834. [Google Scholar] [CrossRef]
- Fitz, V.; Graca, S.; Mahalingaiah, S.; Liu, J.; Lai, L.; Butt, A.; Armour, M.; Rao, V.; Naidoo, D.; Maunder, A.; et al. Inositol for Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis to Inform the 2023 Update of the International Evidence-Based PCOS Guidelines. J. Clin. Endocrinol. Metab. 2024, 109, 1630–1655. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations from the 2023 International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, M.P.A.; Di Tomo, P.; Centorame, G.; Pandolfi, A.; Di Pietro, N.; Consoli, A.; Formoso, G. Myoinositol Reduces Inflammation and Oxidative Stress in Human Endothelial Cells Exposed In Vivo to Chronic Hyperglycemia. Nutrients 2021, 13, 2210. [Google Scholar] [CrossRef] [PubMed]
- Bednarz, K.; Kowalczyk, K.; Cwynar, M.; Czapla, D.; Czarkowski, W.; Kmita, D.; Nowak, A.; Madej, P. The Role of GLP-1 Receptor Agonists in Insulin Resistance with Concomitant Obesity Treatment in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2022, 23, 4334. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, P.; Wang, X.; Lai, S.; Qiu, H.; Chen, Z.; Hu, S.; Yao, J.; Shen, J. GLP-1/GLP-1R Signaling Regulates Ovarian PCOS-Associated Granulosa Cells Proliferation and Antiapoptosis by Modification of Forkhead Box Protein O1 Phosphorylation Sites. Int. J. Endocrinol. 2020, 2020, 1484321. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Buse, J.B.; Kim, T.; Burns, C.; Skare, S.; Baron, A.; Fineman, M. Once-daily delayed-release metformin lowers plasma glucose and enhances fasting and postprandial GLP-1 and PYY: Results from two randomised trials. Diabetologia 2016, 59, 1645–1654. [Google Scholar] [CrossRef]
- Tilinca, M.C.; Tiuca, R.A.; Burlacu, A.; Varga, A. A 2021 Update on the Use of Liraglutide in the Modern Treatment of ‘Diabesity’: A Narrative Review. Medicina 2021, 57, 669. [Google Scholar] [CrossRef]
- Ma, R.L.; Deng, Y.; Wang, Y.F.; Zhu, S.Y.; Ding, X.S.; Sun, A.J. Short-term combined treatment with exenatide and metformin for overweight/obese women with polycystic ovary syndrome. Chin. Med. J. 2021, 134, 2882–2889. [Google Scholar] [CrossRef]
- Xing, C.; Zhao, H.; Zhang, J.; He, B. Effect of metformin versus metformin plus liraglutide on gonadal and metabolic profiles in overweight patients with polycystic ovary syndrome. Front. Endocrinol. 2022, 13, 945609. [Google Scholar] [CrossRef]
- Spremović Rađenović, S.; Pupovac, M.; Andjić, M.; Bila, J.; Srećković, S.; Gudović, A.; Dragaš, B.; Radunović, N. Prevalence, Risk Factors, and Pathophysiology of Nonalcoholic Fatty Liver Disease (NAFLD) in Women with Polycystic Ovary Syndrome (PCOS). Biomedicines 2022, 10, 131. [Google Scholar] [CrossRef]
- Elnashar, A. Lean Polycystic Ovary Syndrome: A Narrative Review. Clin. Exp. Obstet. Gynecol. 2024, 51, 142. [Google Scholar] [CrossRef]
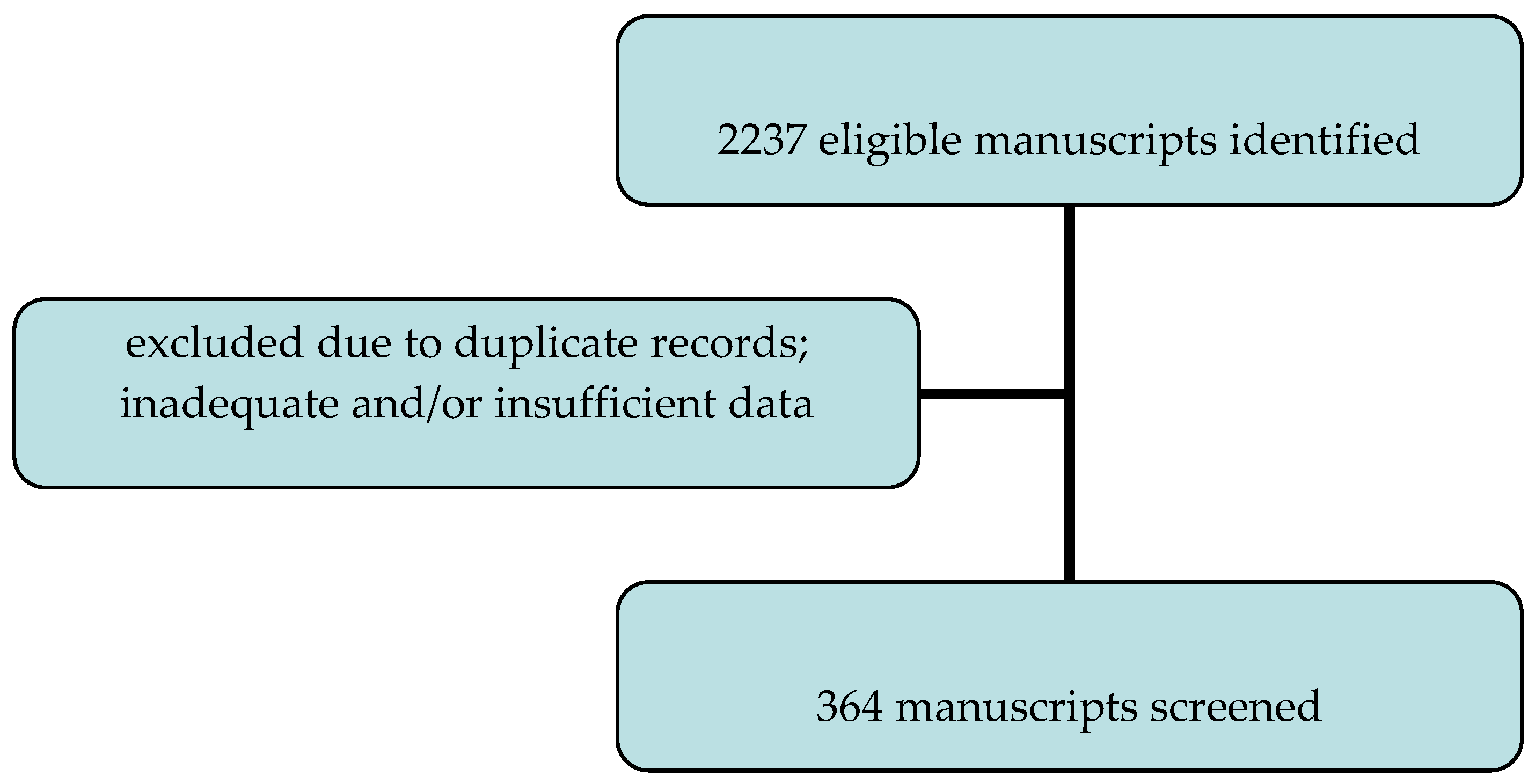
| Studies Comparing Obese and Non-Obese Patients | Population | Main Results and Conclusions |
|---|---|---|
| Gholinezhad M et al. (2018) cross-sectional study [27] | 27 normal weight (18 < BMI < 25) and 85 overweight/obese (BMI ≥ 25) patients aged 18 and 35 underwent clinical measures of HOMA as insulin resistance and QUICKI as insulin sensitivity tools | BMI was significantly positively correlated with insulin resistance (p < 0.001) and negatively with insulin sensitivity (p < 0.001). BMI showed stronger reverse relationship with SHBG (p < 0.001). In the overweight/obese group, 91.7% of the women showed insulin resistance (HOMA > 3.15) vs. 8.3% in the normal weight group (p < 0.001). Low insulin sensitivity (QUICKI < 0.34) had 17.6% of the lean women and 82.4% of the overweight/obese patients (p < 0.001). |
| Stepto NK et al. (2013) cross-sectional study [28] | 20 overweight and 20 lean PCOS patients (Rotterdam criteria) | Women with PCOS had more IR than BMI-matched controls (main effect for BMI and PCOS; p < 0.001). IR was present in 75% of lean PCOS, 62% of overweight controls and 95% of overweight PCOS. Lean controls (mean + SD; GIR 339 + 76 mg) were less IR than lean PCOS (270 + 66 mg), overweight controls (264 + 66 mg) and overweight PCOS (175 + 96 mg). The negative relationship between BMI and IR reflected by GIR was more marked in PCOS (p < 0.0001) than controls (p < 0.01). |
| Bailey AP et al. (2014) retrospective cohort study [29] | 79 women with clinically documented diagnosis of PCOS by Rotterdam criteria undergoing IVF | Women with obesity and PCOS had 69% lower odds of clinical pregnancy per started cycle (OR 0.31; 95% CI, 0.11–0.86; p = 0.02) and 77% lower odds per embryo transfer (OR, 0.23; 95% CI, −0.08 to 0.68; p = 0.008) compared with lean women with PCOS. Among women with obesity and PCOS, the odds of live birth were 71% lower per started cycle (OR, 0.29; 95% CI, 0.10–0.84; p = 0.02) and 77% lower per embryo transfer (OR, 0.23; 95% CI, 0.07–0.71; p = 0.01) compared with lean women with PCOS. Ovarian hyperstimulation syndrome odds were decreased with increasing BMI among PCOS patients: 19.6% in lean, 10.5% overweight and 3.2% obese women. |
| Johnson JE et al. (2023) meta-analysis [30] | 10 relevant studies were identified and included (12,248 patients with PCOS and 54,120 controls) | Women with PCOS had a significantly increased odds of developing endometrial cancer as compared to those without PCOS [OR, 4.07; 95% confidence interval (CI), 2.13–7.78; p < 0.0001]. When postmenopausal subjects (age > 54 years) were excluded from the meta-analysis, the odds increased further (OR, 5.14; 95% CI, 3.22–8.21; p < 0.00001). Patients with PCOS are up to 5 times more likely to develop endometrial cancer compared to those without PCOS. |
| Hong SH et al. (2023) cross sectional observational study [31] | 667 patients with PCOS and 289 women with regular menstrual cycles as control | The prevalence of NAFLD in women with PCOS evaluated by LFS, FLI, and HIS were 19.9, 10.3, and 32.2%, respectively. In the control group, the incidence was 2.1, 0.7, and 4.2%, respectively. Both FT and FAI levels showed significant association with increased NAFLD-related indices, after adjusting for insulin resistance and other factors (LFS (OR 3.18 (95% CI 1.53–6.63) in FT; 1.12 (1.04–1.22) in FAI), FLI (OR 2.68 (95% CI 1.43–5.03) in FT; 1.13 (1.06–1.20) in FAI), and HSI (OR 3.29 (95% CI 2.08–5.21) in FT; 1.5 (1.09–1.21) in FAI). TT did not exhibit association with any NAFLD index. |
| Studies | Population | Main Results and Conclusions |
|---|---|---|
| Shang Y et al. (2020) Meta-analysis [40] | 19 trials with 1193 patients | Diet leads to more pronounced improvement in the homeostasis model assessment of IR, fasting insulin, fasting plasma glucose, BMI, weight, and waist circumference in PCOS patients compared to the metformin group. |
| Porchia LM et al. (2020) Meta-analysis [41] | 25 studies with 486 patients | Diet leads to the significant improvement in the IR in women with PCOS. |
| Patten TK et al. (2020) Meta-analysis [44] | 19 studies with 777 patients | The physical activity leads to the small reductions in HOMA-IR and waist circumference. |
| Chen M et al. (2024) Meta-analysis [44] | 9 studies with 1330 patients | Bariatric surgery lowers menstrual irregularity, BMI, ovarian volume hypertrichosis and free testosterone levels in women with obesity and PCOS. |
| Guan Y et al. (2020) Meta-analysis [45] | 12 studies with 683 patients | The metformin leads to the significant decrease in the BMI, waist circumference, LDL, FSH, LH, and testosterone levels in women with obesity and PCOS. |
| Greff D et al. (2023) Meta-analysis [46] | 26 studies with 1691 patients | The inositol leads to the significant decrease in free testosterone, total testosterone, androsenedione, glucose, AUC insulin as well as the BMI and increase in the SHBG compared to the placebo. |
| Ali Fadlalmola H et al. (2023) Meta-analysis [47] | 4 studies with 218 patients | The resveratrol significantly decreases the LH, testosterone and DHEAS levels in women with PCOS compared to the placebo in women with PCOS. |
| Studies | Population | Main Results and Conclusions |
|---|---|---|
| Nazirudeen R et al. (2023) Randomized controlled trial [55] | 53 patients (27 treated with metformin 1500 mg/day and 26 treated with metformin 1500 mg/day and myoinositol 4 g/day) | In comparison to the metfomin monoterapy, combination of the metformin and mioinositol leads to the significant improvement in the menstrual regularity. There is no significant difference in the anthropometric parameters, modified Ferriman Gallwey score, global acne score, Fasting insulin, HOMA-IR, fasting lipid profile, serum testosterone, SHBG, LH, FSH, AMH, and pelvic ultrasound to assess ovarian volume. |
| Soldat Stankovic V et al. (2022) Randomized controlled trial [56] | 66 patients treated with metformin 1500 mg/day and myoinositol 4 g/day | There is no difference in total cholesterol, HDL, LDL cholesterol and triglycerdies between treatment with metformin and myoinositol. Metformin treatment significantly reduces the BMI, waist circumference, Ferriman Gallwey score testosterone and FAI. |
| Hassan S et al. (2023) Randomized controlled trial [57] | 110 patients (55 treated with metformin 1000 mg/day + pioglitazone 30 mg/day and 55 treated with myoinositol 2000 mg/day + resveratrol 2000 mg/day) | The treatment with the combination of the myoinositol and resveratrol is more efficient in the lower of the testosterone, LH, FSH, ovarian volume, BMI, WH ratio and Ferrimen-Gllwey score when compare to the treatment with the combination of the metformin and pioglitazone. |
| Xing C et al. (2020) Meta-analysis [58] | 14 studies with 619 patients | The combination of the GLP-1 receptor agonists and metformin is more effective in decreasing of the free testosterone, androstenedine, fasting blood glucose when compare to the metformin monoterapy while the combination of the metformin and GLP-1 receptor agonists is more effective in increasing SHBG when compared to the GLP-1 RA monotherapy. |
| Obese Patients with PCOS | Lean Patients with PCOS |
|---|---|
| 1. weight reduction and lifestyle changes | 1. inositol; resveratrol |
| 2. metformin (depending on metabolic status) | 2. hormonal contraception |
| 3. inositol; resveratrol(with or without metformin) | 3. antiandrogens |
| 4. GLP-1 receptor agonists liraglutide for obesity treatment of patients with no metabolic disorders semaglutide for obesity treatment of patients with DM type II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bila, J.; Dotlic, J.; Andjic, M.; Ivanovic, K.; Micic, J.; Tulic, L.; Pupovac, M.; Stojnic, J.; Vukovic, I.; Ivanovic, S. Obesity as a Part of Polycysric Ovary Syndrome (PCOS)—A Review of Pathophysiology and Comprehensive Therapeutic Strategies. J. Clin. Med. 2025, 14, 5642. https://doi.org/10.3390/jcm14165642
Bila J, Dotlic J, Andjic M, Ivanovic K, Micic J, Tulic L, Pupovac M, Stojnic J, Vukovic I, Ivanovic S. Obesity as a Part of Polycysric Ovary Syndrome (PCOS)—A Review of Pathophysiology and Comprehensive Therapeutic Strategies. Journal of Clinical Medicine. 2025; 14(16):5642. https://doi.org/10.3390/jcm14165642
Chicago/Turabian StyleBila, Jovan, Jelena Dotlic, Mladen Andjic, Katarina Ivanovic, Jelena Micic, Lidija Tulic, Miljan Pupovac, Jelena Stojnic, Ivana Vukovic, and Stefan Ivanovic. 2025. "Obesity as a Part of Polycysric Ovary Syndrome (PCOS)—A Review of Pathophysiology and Comprehensive Therapeutic Strategies" Journal of Clinical Medicine 14, no. 16: 5642. https://doi.org/10.3390/jcm14165642
APA StyleBila, J., Dotlic, J., Andjic, M., Ivanovic, K., Micic, J., Tulic, L., Pupovac, M., Stojnic, J., Vukovic, I., & Ivanovic, S. (2025). Obesity as a Part of Polycysric Ovary Syndrome (PCOS)—A Review of Pathophysiology and Comprehensive Therapeutic Strategies. Journal of Clinical Medicine, 14(16), 5642. https://doi.org/10.3390/jcm14165642





