1. Introduction
Pathologies of the aortic root have been a significant challenge for cardiologists and cardiac surgeons due to its complex anatomy and function.
Aortic root replacement is usually indicated based on size criteria, as informed by recently updated European and American guidelines [
1,
2]. Concomitant aortic valve disease, either stenosis or incompetence, is a common consideration during root replacement, first described in 1967 by Bentall and De Bono, with a composite valve graft root replacement. Although less commonly performed, aortic valve-sparing techniques for root replacement were published in the early nineties by David [
3] and Yacoub [
4] involving aortic valve resuspension or remodeling, respectively. Preserving the native aortic valve, while more technically demanding, avoids the long-term complications of prosthetic valves: namely structural degeneration in biological valves and anticoagulation related complications of mechanical valves.
Patient specificity is key when determining the optimum treatment strategy in aortic valve disease, with or without root pathology. Controversies about whether to replace the mildly dilated aortic root, where AVR is indicated, are present in the literature [
5,
6,
7,
8]. Replacing the root (in addition to AVR for hemodynamically significant valve disease) adds to the operative time and thus to the overall risk of open-heart surgery. Therefore, when the root diameter is short of the replacement threshold, many surgeons opt for AVR only and preserve the native root. Despite this, aortic dilatation is becoming a more recognized complication after AVR, affecting 0.6% of all AVR routine procedures [
9] and occurring in 10% of cases after BAV replacement [
10]. Given the increased risk of type A dissection in these patients with post-AVR root dilatation, redo surgery and root replacement become a necessary procedure. Although not previously well-described, this type of redo aortic root replacement is inevitably higher risk and technically more challenging than an index root replacement.
Due to the complexity of these situations, the accepted treatment is a formal Bentall procedure with explant and replacement of the former prosthetic aortic valve, even if well-functioning, as well as the dilated aortic root using a composite valve graft.
This article aims to show the operative technique and to summarize the results of a new, advantageous, delayed two-stage Bentall procedure where the well-functioning prosthetic aortic valve is maintained, and the dilated aortic root is replaced.
2. Materials and Methods
This is a case series paper that describes a redo operative technique and presents the outcome of 11 patients who underwent this procedure: all patients have had one or more cardiac procedures in the past. Since the previous operations took place in different institutes, the operation techniques and the course of hospitalization after these previous operations are incomplete.
2.1. Data Collection
Data were collected retrospectively from database units at Groningen Institute, Maastricht Institute (the Netherlands), and Achen Institute (Germany). All cases were performed by a single operator (E.N).
From March 2012 to May 2017, 11 patients were admitted to the hospital due to dilated aortic root or ascending aorta after aortic valve replacement surgery in the past. In all 11 patients, a delayed two-stage Bentall procedure was performed.
The surgical procedure, the rationale for performing this procedure, and detailed outcomes are presented. The follow-up to date was made by medical and nursery personnel.
2.2. Definition
The delayed two-stage Bentall procedure was defined as replacing the dilated aortic root with a vascular prosthetic graft and re-implanting the coronary buttons while restoring the well-functioning, previously implanted, mechanical aortic valve.
2.3. Pre-Operative Assessment
Choosing suitable patients for a delayed two-stage Bentall procedure is essential since it usually involves high-risk reoperation. Therefore, the decision whether to preserve the previously implanted mechanical aortic valve or not is made by a heart team, including a cardiac surgeon, a cardiologist, an anesthesiologist, and a radiologist.
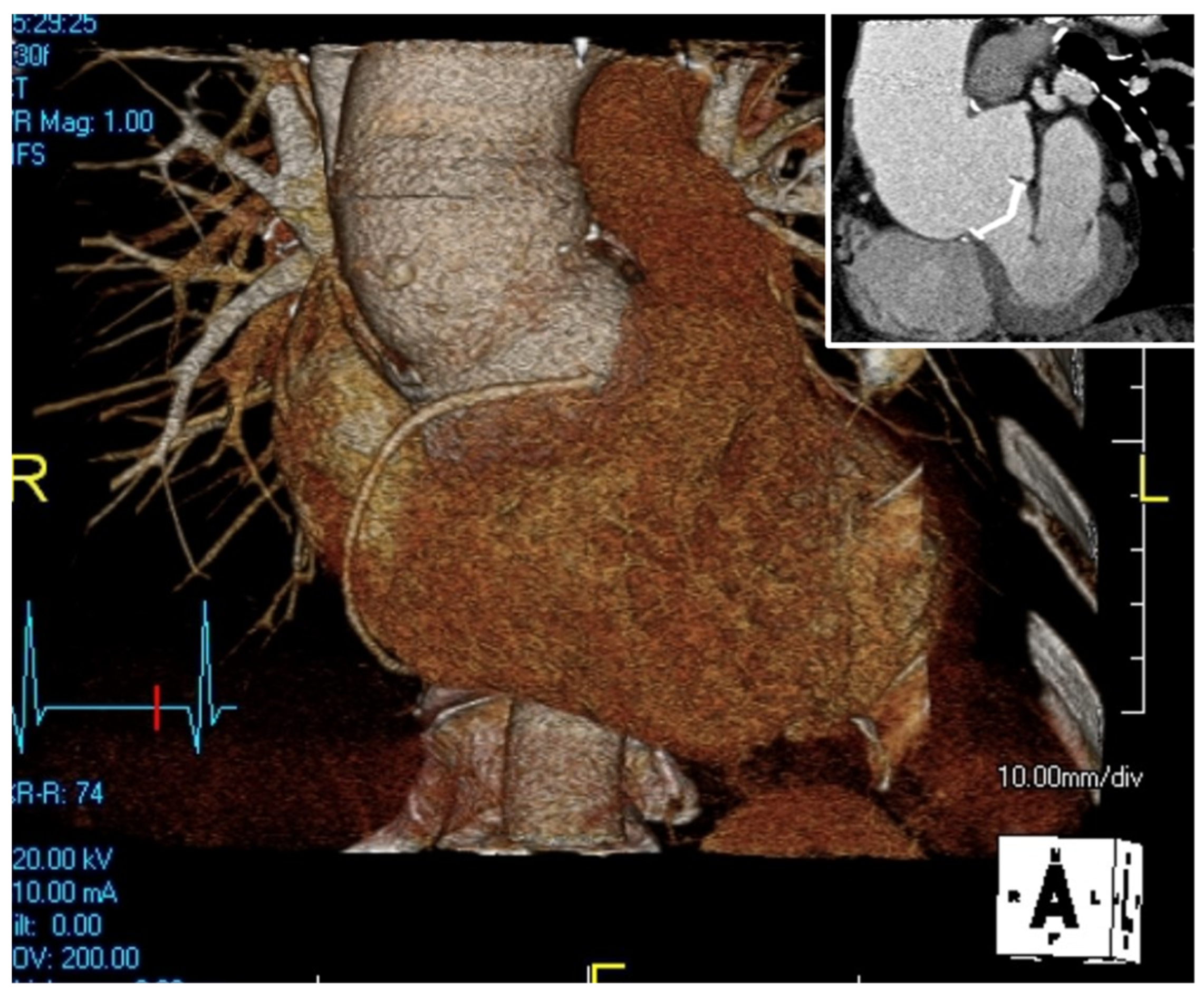
The pre-operative assessment starts with imaging to estimate the severity of the disease, and the structures involved. It starts with echocardiography, including trans-thoracic and trans-esophageal tests, to visualize the aortic valve and assure its well-functionality and hemodynamics, and is followed by a computed tomography angiography (CTA) or a magnetic resonance imaging (MRI) and a 3D image reconstruction to understand the anatomy of the mediastinum, especially in a redo-operation (
Figure 1).
The anesthesiologist is then asked to estimate the operative risk, mainly in patients with diminished cardiac function or other high perioperative risk factors.
After vigorous investigations and multi-disciplinary team discussions, the decision whether to preserve the valve or to replace it is made. However, only after opening the aorta and inspecting the valve is the final decision made.
2.4. Operative Technique
All patients underwent a re-sternotomy approach to access the mediastinum using an oscillating saw to minimize the risk of injuring vital retrosternal structures. The cardio-pulmonary bypass (CPB) machine was centrally connected in all patients via the right atrium to the inferior vena cava, using the two-stage venous cannula (Thin-flex dual stage venous cannulae-Edwards
®) and via the aorta (EZ glide aortic cannulae-Edwards
®). Right after cannulation extracorporeal blood flow was achieved, and moderate hypothermia was initiated. An aortic cross-clamp was then applied, and cardioplegia was administered antegradely to the aortic root via an aortic needle (Antegrade cardioplegia cannulae-Edwards
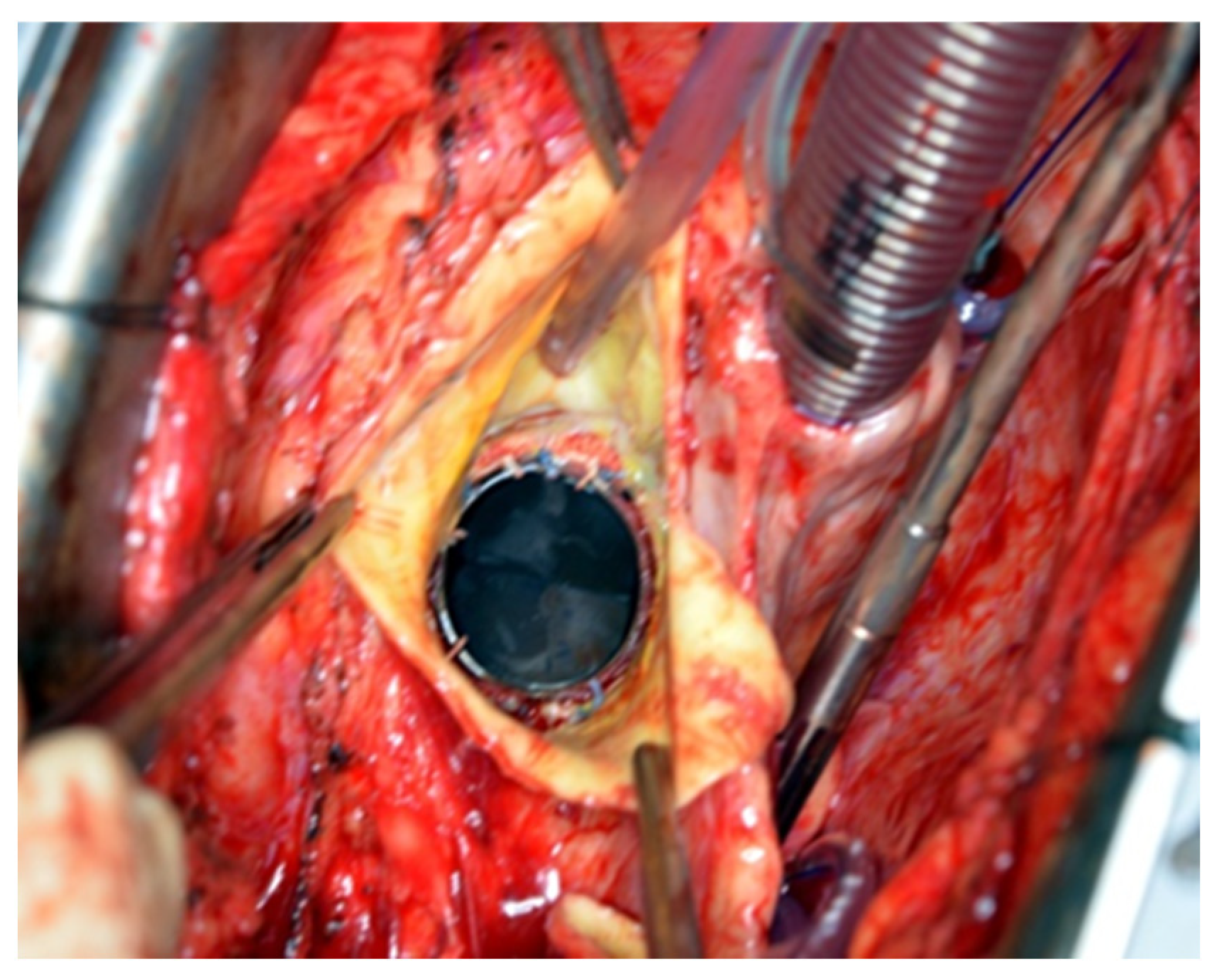
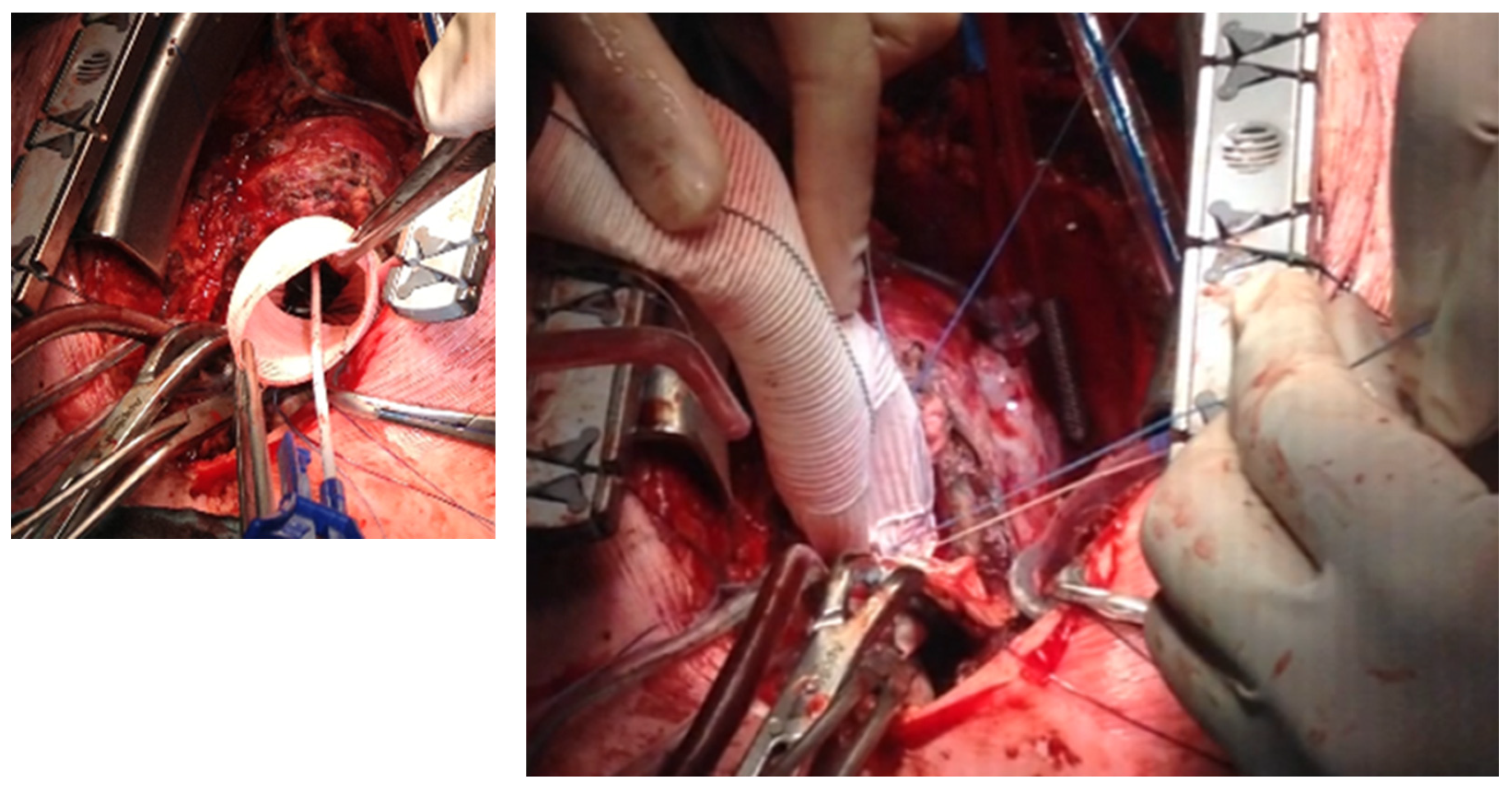
®) to induce cardiac arrest. The dilated aortic root and ascending aorta were then resected, and the coronary ostia were carefully detached from the aortic root. Direct inspection of the prosthetic aortic valve was performed to rule out any visible pathology (
Figure 2). The size of the aortic Dacron graft (Vascutek
® Gelweave Valsalva™ Grafts) was selected to be 5 mm larger than the prosthetic aortic valve. Separate 2-0 Ti-Cron
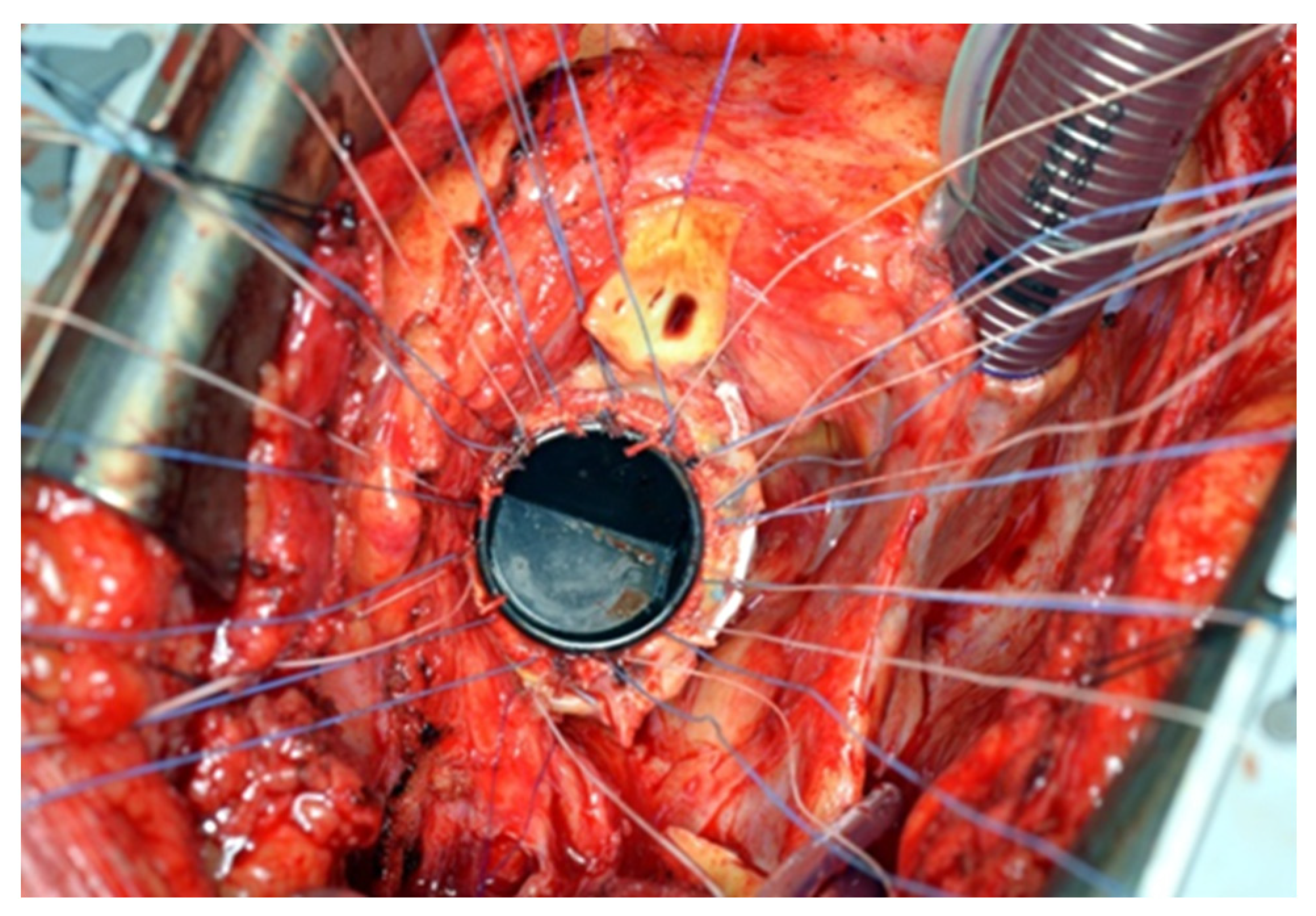
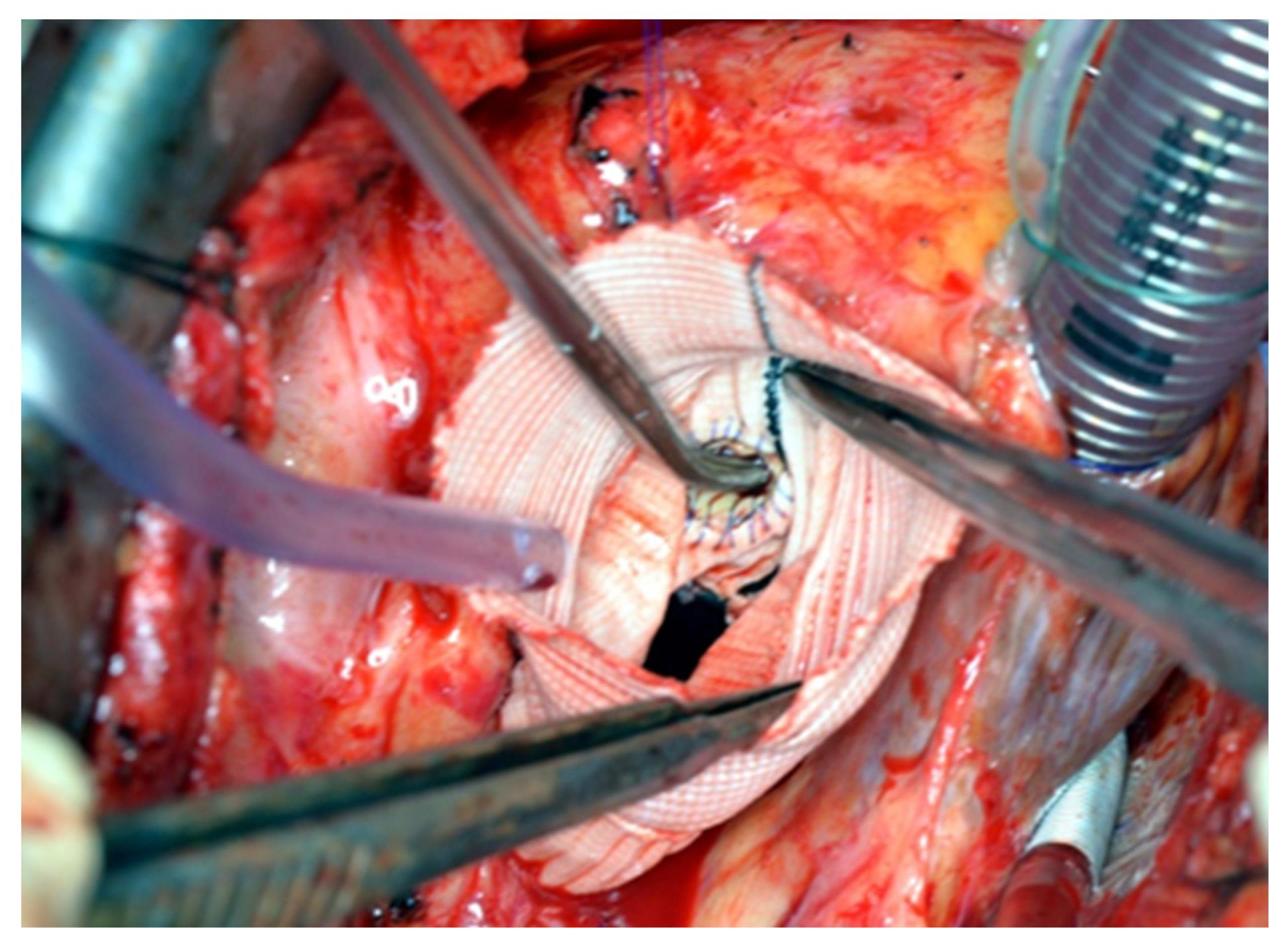
® sutures with pledgets were placed to connect the fibrotic aortic annulus and the prosthetic valvular annulus to the aortic graft (
Figure 3,
Figure 4 and
Figure 5). The coronary buttons were then reattached to the graft using 5-0 Prolene
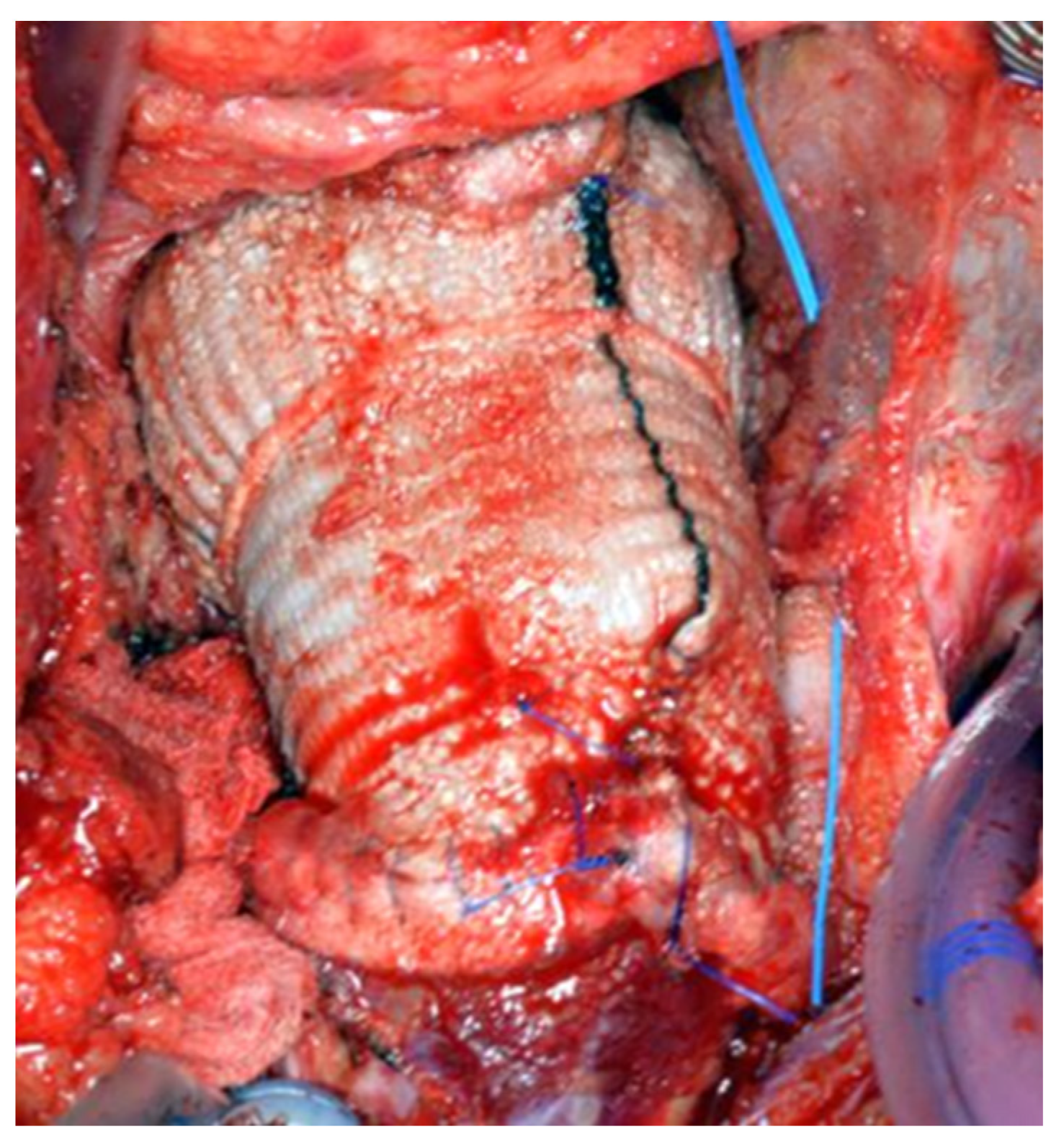
®, paying particular attention to the geometry of the vessels (
Figure 6). A continuous 4-0 Prolene
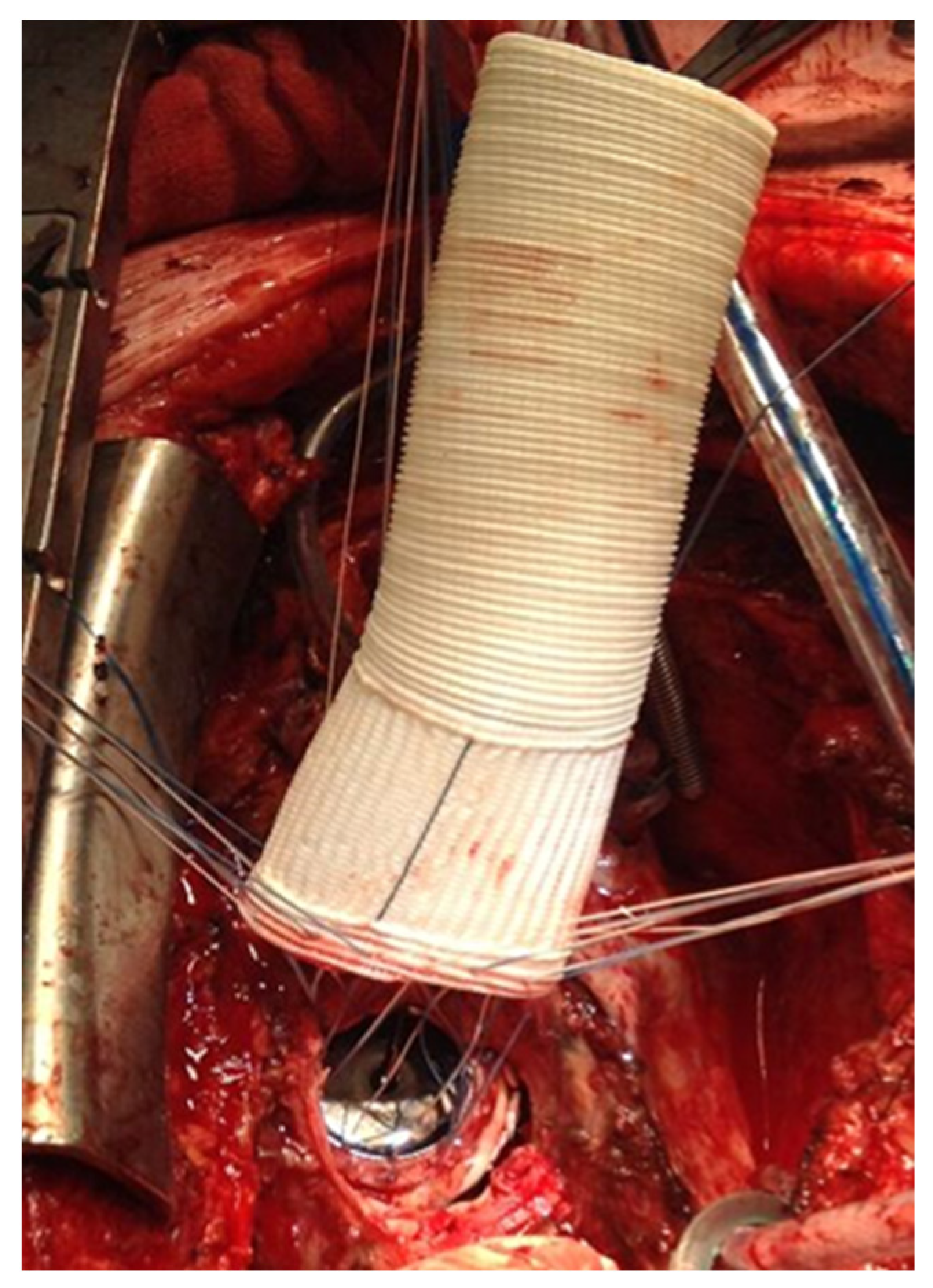
® suture was chosen with pledgets to perform the distal anastomosis (
Figure 7).
In two patients (cases 10 and 11), a delayed two-stage Bentall procedure was performed with circulatory arrest to replace the aortic root, ascending aorta, and hemi-arch. Re-sternotomy, cannulation, and aortic resection were performed as previously described. Deep hypothermia was targeted at 25 degrees Celsius. Once hypothermia was achieved, circulatory arrest was started to allow direct inspection of the aortic arch, and cerebral protection was started immediately. After performing the distal anastomosis, the aortic cannula was inserted into the neo-aorta to restart CPB and re-warm the patient. Proximal anastomosis, reconnecting the coronary ostia, de-airing and de-clamping of the aorta, and weaning from CPB was performed routinely.
The Brachiocephalic trunk (BCT) was debranched in two patients (cases 4 and 10). The Dacron aortic graft was distally anastomosed to the aortic arch between the common carotid artery and the BCT, as in hemi-arch replacement, using a 4-0 Prolene® suture with pledgets. A 16 mm vascular graft (Vascutek® Gelweave™ Straight Grafts) was chosen to connect the BCT to the prosthetic ascending aorta. The end-to-side anastomosis was performed using 4-0 Prolene® to connect the vascular tube to the neo-aorta and end-to-end anastomosis, using 4-0 Prolene® with pledgets, to connect the vascular tube to the BCT.
Temporary atrial and ventricular pacemakers were implanted in all patients. Synthetic glue—Coseal®—was used prophylactically in all patients to ensure hemostasis. The sternum was closed using steel wires, and the patient was transferred to the cardiothoracic intensive care unit.
3. Results
3.1. Demographics and Outcome
Altogether, 11 “delayed two-stage Bentall procedure” reoperations took place in three institutes. All patients had open cardiac surgery before this operation, where single or combined cardiac procedures were performed. Among the other procedures, mechanical aortic valves were implanted in all patients, and anticoagulation was prescribed for life. Demographics and cardiac surgical history of the patients are presented in
Table 1.
In all cases, the aortic root was severely dilated (53 mm–65 mm). The average time between the first operation and the “delayed two-stage Bentall procedure” was 169 months.
Survival to hospital discharge was 100%, and no major adverse cardiac or cardiovascular events (MACCEs) occurred during the post-operative period.
According to follow-up registries, all patients are in good health. They were all able to answer the phone and report feeling well by themselves at the time of writing this article. The median follow-up time was 61.1 months.
Table 2 summarizes the operative and ICU outcomes of the 11 delayed two-stage Bentall procedure patients.
3.2. Two-Stage Bentall Procedure Patients: Pre- and Intra-Operative Considerations
As previously noted, all patients underwent comprehensive pre-operative evaluation, including CTA with 3D reconstruction and echocardiography, to assess chest anatomy before reoperation and evaluate prosthetic valve function. Despite favorable imaging results and confirmed suitability, the final decision to preserve the aortic valve was made intra-operatively after direct inspection of the valve and the annulus.
Preservation criteria included:
- (1)
Intact valve annulus following dissection,
- (2)
Proper valve positioning,
- (3)
Full mobility of the valve leaflets.
In most cases, a two-stage Bentall procedure was performed where the aortic root was replaced while the well-functioning previously implanted mechanical aortic valve was preserved. During surgery, the ascending aorta and aortic root were removed after detaching the coronary ostia. The previously implanted mechanical aortic valve was directly inspected to reassure its adequate function, and a Dacron graft, usually 5 mm larger than the aortic valve diameter, was connected to the prosthetic valve annulus proximally and the distal ascending aorta or as a hemi-ach distally. The coronary ostia were then connected to the aortic graft.
Two cases included BCT debranching, of which one was performed with circulatory arrest. And in one case, the aortic arch needed to be replaced together with the ascending aorta and the root. This was also performed with circulatory arrest (
Table 2).
4. Discussion
Aortic root dilatation is a known entity after AVR. Many authors describe surgical techniques to replace the dilated aortic root with the prosthetic aortic valve, including the most commonly used, the Bentall procedure [
11]. Other authors published their experiences as a single case report or an idea describing the completion of the Bentall procedure and valve-sparing aortic root replacement after AVR [
12,
13]. However, the delayed two-stage Bentall technique described in this article was never published before in a series of patients.
While aortic root replacement techniques are well-established in cardiac surgery, many smaller centers or younger surgeons avoid performing them. On the one hand, the Bentall procedure and valve-sparing root replacement procedures are surgically demanding; on the other hand, they are life-saving procedures. Nevertheless, in redo settings it is more complicated. Here comes the main advantage of the two-stage Bentall procedure.
Careful patient selection is essential before performing the delayed two-stage Bentall procedure; the prosthetic aortic valve patency is a significant concern. The final decision on whether to proceed with this technique or not can only be made after the surgeon directly inspects the prosthetic aortic valve based on the echocardiographic results previously performed.
Mortality rates after root replacement techniques are low, as are significant complications like bleeding and stroke [
14,
15]. These data are comparable with the data published in our experience, where the post-operative mortality rate up to 4 years follow-up was 0 and the major post-operative complication rate was 0. Nevertheless, we acknowledge that this case series is limited by the small sample size, the retrospective design, and the absence of control group. For that, further studies are needed to confirm these findings.
Preserving a well-functioning previously implanted prosthetic aortic valve during a delayed two-stage Bentall procedure has several advantages when compared to other aortic root replacement surgeries after a previous mechanical AVR: avoidance of further trauma to the already distressed and fibrosed aortic annulus from previous surgery means a lower chance of inducing atrio-ventricular block. Furthermore, attaching the prosthetic aortic graft to the previously implanted prosthetic mechanical valve is more likely to be a stronger connection than attaching it to the patient’s native tissue. Since less surgery is required in the aortic annulus during the two-stage Bentall technique, there is expected to be less post-operative bleeding. It is worth mentioning that even subvalvular pannus formation could be used as reinforcement tissue with this technique.
Considering the operative timing, preserving the valvular prosthesis would inevitably reduce CPB time and aortic cross-clamp time, yet another important advantage for the patient overall. In fact, according to published papers on Bentall procedure experiences [
16,
17,
18,
19], the mean CPB time varied between 161.2 ± 83 and 246.9 ± 89.8 min, and the mean aortic cross-clamp (ACC) time varied between 115 ± 60.4 and 170.3 ± 63 min. According to our experience, the mean CPB time was 200.1 ± 19.7 min, and the mean ACC time was 153.8 ± 17.2 min.
5. Conclusions
A delayed two-stage Bentall operation is a safe and feasible procedure. It could replace the formal root replacement techniques when the aortic valve prosthesis is in good condition.
Author Contributions
Conceptualization, M.M., N.N., and E.N.; methodology, E.N.; software, M.M.; validation, J.S.J., N.N., and R.L.; formal analysis, M.M.; investigation, J.S.J.; resources, E.N.; data curation, M.M.; writing—original draft preparation, J.S.J.; writing—review and editing, M.M., M.Y.S., and A.S.; visualization, N.N.; supervision, R.L., E.B.; project administration, E.N.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the institutional medical ethical review board of the University Medical Center, Groningen, the Netherlands (Project identification code METc2015/033; date of approval 2015-02).
Informed Consent Statement
Patient consent was waived due to retrospective study design.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AVR | Aortic valve replacement |
| CTA | Computed tomography angiography |
| MRI | Magnetic resonance imaging |
| CPB | Cardio-pulmonary bypass |
| BCT | Brachio-cephalic branch |
| MVP | Mitral valve prolapse |
| AVP | Aortic valve prolapse |
| MVR | Mitral valve replacement |
| MACCE | Major cardiac and cardiovascular event |
| ICU | Intensive care unit |
| ACC | Aortic cross clamp |
| RBCs | Red blood cells |
References
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J., III; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 acc/aha guideline for the diagnosis and management of aortic disease: A report of the American heart association/American college of cardiology joint committee on clinical practice guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef] [PubMed]
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Rivière, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. 2024 ESC Guidelines for the management of peripheral arterial and aortic diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar] [CrossRef] [PubMed]
- David, T.E.; Feindel, C.M. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J. Thorac. Cardiovasc. Surg. 1992, 103, 617–621; discussion 22. [Google Scholar] [CrossRef] [PubMed]
- Sarsam, M.A.; Yacoub, M. Remodeling of the aortic valve anulus. J. Thorac. Cardiovasc. Surg. 1993, 105, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Edwards, W.D.; Leaf, D.S.; Edwards, J.E. Dissecting aortic aneurysm associated with congenital bicuspid aortic valve. Circulation 1978, 57, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E., Jr.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: A report of the American college of cardiology foundation/American heart association task force on practice guidelines, American association for thoracic surgery, American college of radiology, American stroke association, society of cardiovascular anesthesiologists, society for cardiovascular angiography and interventions, society of interventional radiology, society of thoracic surgeons, and society for vascular medicine. Circulation 2010, 121, e266–e369. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, S.; Chikwe, J.P.; Chiang, Y.P.; Egorova, N.N.; Adams, D.H. Long-term risk for aortic complications after aortic valve replacement in patients with bicuspid aortic valve versus marfan syndrome. J. Am. Coll. Cardiol. 2015, 65, 2363–2369. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.F.; Mazzetti, S.; Garatti, A.; Ribera, E.; Milazzo, A.; Bruschi, G.; Lanfranconi, M.; Colombo, T.; Vitali, E. Aortic complications after bicuspid aortic valve replacement: Long-term results. Ann. Thorac. Surg. 2002, 74, S1773–S1776; discussion S92–S99. [Google Scholar] [CrossRef] [PubMed]
- von Kodolitsch, Y.; Loose, R.; Ostermeyer, J.; Aydin, A.; Koschyk, D.H.; Haverich, A.; Nienaber, C.A. Proximal aortic dissection late after aortic valve surgery: 119 cases of a distinct clinical entity. Thorac. Cardiovasc. Surg. 2000, 48, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Nakatani, S.; Stugaard, M.; Tsujita-Kuroda, Y.; Bando, K.; Kobayashi, J.; Yamagishi, M.; Kitakaze, M.; Kitamura, S.; Miyatake, K. Failure to prevent progressive dilation of ascending aorta by aortic valve replacement in patients with bicuspid aortic valve: Comparison with tricuspid aortic valve. Circulation 2003, 108 (Suppl. 1), Ii291–Ii294. [Google Scholar] [CrossRef] [PubMed]
- Bentall, H.; De Bono, A. A technique for complete replacement of the ascending aorta. Thorax 1968, 23, 338–339. [Google Scholar] [CrossRef] [PubMed]
- Kugai, T.; Mabuni, K.; Morishima, Y.; Abe, N.; Yamazato, T.; Nishioka, M. Completion bentall procedure after aortic valve replacement. Kyobu Geka Jpn. J. Thorac. Surg. 2014, 67, 207–210. [Google Scholar]
- Malekan, R.; Spielvogel, D.; Saunders, P.C.; Lansman, S.L.; Griepp, R.B. The completion bentall procedure. Ann. Thorac. Surg. 2011, 92, 362–363. [Google Scholar] [CrossRef] [PubMed]
- El-Hamamsy, I.; Ibrahim, M.; Stevens, L.-M.; Witzke, H.; Clark, L.; Yacoub, M.H. Early and long-term results of reoperative total aortic root replacement with reimplantation of the coronary arteries. J. Thorac. Cardiovasc. Surg. 2011, 142, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Kvitting, J.P.; Kari, F.A.; Fischbein, M.P.; Liang, D.H.; Beraud, A.S.; Stephens, E.H.; Mitchell, R.S.; Miller, D.C. David valve-sparing aortic root replacement: Equivalent mid-term outcome for different valve types with or without connective tissue disorder. J. Thorac. Cardiovasc. Surg. 2013, 145, 117–127; discussion 26–27. [Google Scholar] [CrossRef] [PubMed]
- Etz, C.D.; Bischoff, M.S.; Bodian, C.; Roder, F.; Brenner, R.; Griepp, R.B.; Di Luozzo, G. The bentall procedure: Is it the gold standard? A series of 597 consecutive cases. J. Thorac. Cardiovasc. Surg. 2010, 140, S64–S70; discussion S86–S91. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.C.; Chang, B.C.; Youn, Y.N.; Yoo, K.J.; Lee, S. Clinical experience with the bentall procedure: 28 years. Yonsei Med. J. 2012, 53, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.D.; Weiss, E.S.; Alejo, D.E.; Nwakanma, L.U.; Williams, J.A.; Dietz, H.C.; Spevak, P.J.; Gott, V.L.; Vricella, L.A.; Cameron, D.E. Aortic root operations for marfan syndrome: A comparison of the bentall and valve-sparing procedures. Ann. Thorac. Surg. 2008, 85, 2003–2010; discussion 10–11. [Google Scholar] [CrossRef] [PubMed]
- Skripochnik, E.; Michler, R.E.; Hentschel, V.; Neragi-Miandoab, S. Repair of aortic root in patients with aneurysm or dissection: Comparing the outcomes of valve-sparing root replacement with those from the bentall procedure. Rev. Bras. Cir. Cardiovasc. Orgao Of. Soc. Bras. Cir. Cardiovasc. 2013, 28, 435–441. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).