Bispecific Radioligands (BRLs): Two Is Better Than One
Abstract
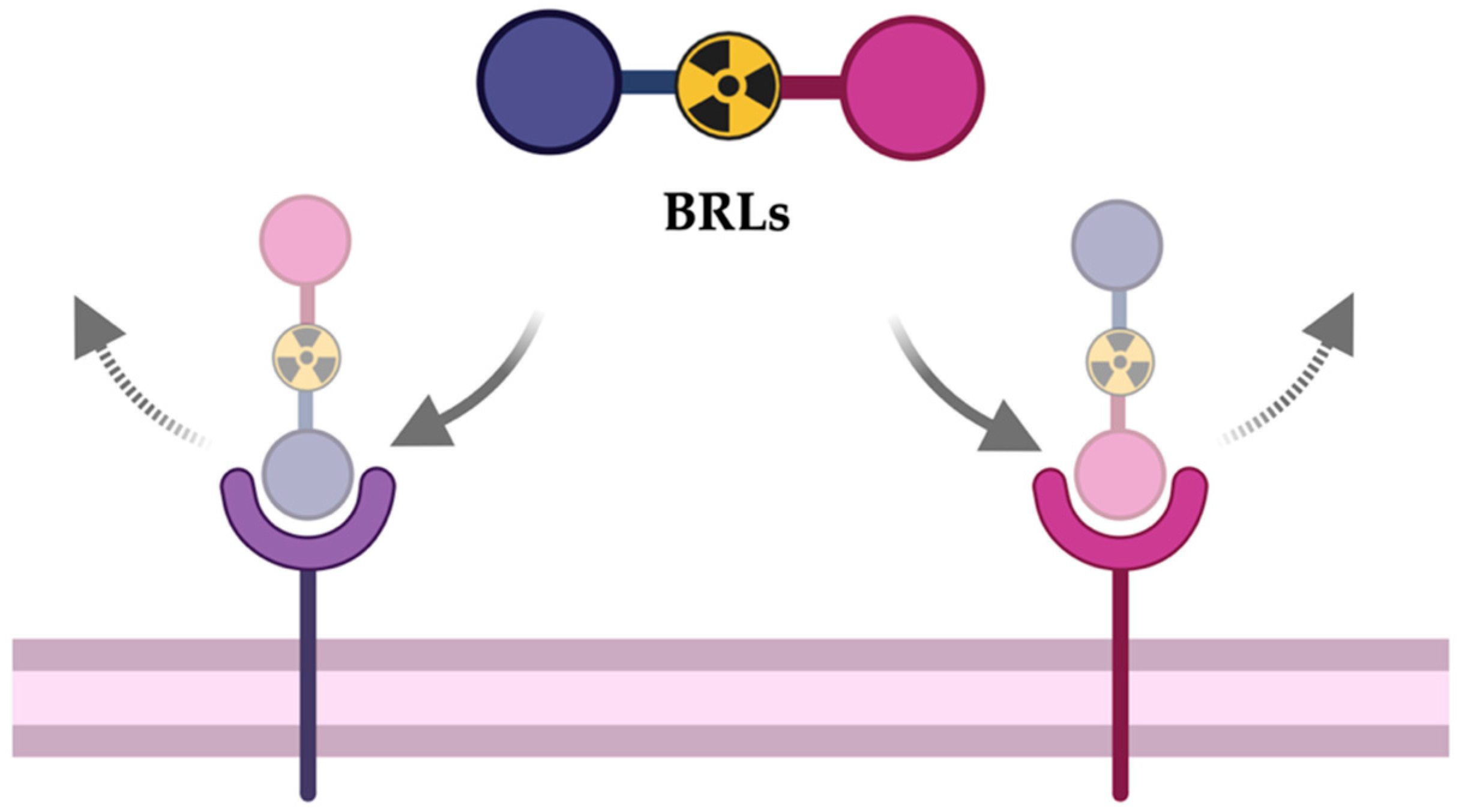
1. Introduction
2. BRLs for SPECT Use
2.1. Radiolabelling with Technetium-99-Metastable
2.2. Radiolabelling with Indium-111
2.3. Radiolabelling with Iodine-125
3. BRLs for PET Use
3.1. Radiolabelling with Fluorine-18
3.2. Radiolabelling with Gallium-68
3.3. Radiolabelling with Copper-64
4. BRLs for Theranostic Use
4.1. Radiolabelling with Lutetium-177
4.2. Radiolabelling with Other Radioisotopes
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kashyap, A.; Rapsomaniki, M.A.; Barros, V.; Fomitcheva-Khartchenko, A.; Martinelli, A.L.; Rodriguez, A.F.; Gabrani, M.; Rosen-Zvi, M.; Kaigala, G. Quantification of tumor heterogeneity: From data acquisition to metric generation. Trends Biotechnol. 2022, 40, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, D.C.; Shevde, L.A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019, 79, 4557–4567. [Google Scholar] [CrossRef]
- Arneth, B. Tumor microenvironment. Medicina 2020, 56, 15. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Schottelius, M.; Wester, H.J. Molecular imaging targeting peptide receptors. Methods 2009, 48, 161–177. [Google Scholar] [CrossRef]
- Judmann, B.; Braun, D.; Wängler, B.; Schirrmacher, R.; Fricker, G.; Wängler, C. Current state of radiolabeled heterobivalent peptidic ligands in tumor imaging and therapy. Pharmaceuticals 2020, 13, 173. [Google Scholar] [CrossRef]
- Javid, H.; Oryani, M.A.; Rezagholinejad, N.; Esparham, A.; Tajaldini, M.; Karimi-Shahri, M. RGD peptide in cancer targeting: Benefits, challenges, solutions, and possible integrin–RGD interactions. Cancer Med. 2024, 13, e6800. [Google Scholar] [CrossRef]
- Rodrigues Toledo, C.; Tantawy, A.A.; Lima Fuscaldi, L.; Malavolta, L.; de Aguiar Ferreira, C. EGFR- and Integrin αVβ3-Targeting Peptides as Potential Radiometal-Labeled Radiopharmaceuticals for Cancer Theranostics. Int. J. Mol. Sci. 2024, 25, 8553. [Google Scholar] [CrossRef] [PubMed]
- Ohki-Hamazaki, H.; Iwabuchi, M.; Maekawa, F. Development and function of bombesin-like peptides and their receptors. Int. J. Dev. Biol. 2005, 49, 293–300. [Google Scholar] [CrossRef]
- Mansi, R.; Nock, B.A.; Dalm, S.U.; Busstra, M.B.; van Weerden, W.M.; Maina, T. Radiolabeled bombesin analogs. Cancers 2021, 13, 5766. [Google Scholar] [CrossRef] [PubMed]
- Gomena, J.; Vári, B.; Oláh-Szabó, R.; Biri-Kovács, B.; Bősze, S.; Borbély, A.; Soós, Á.; Ranđelović, I.; Tóvári, J.; Mező, G. Targeting the Gastrin-Releasing Peptide Receptor (GRP-R) in Cancer Therapy: Development of Bombesin-Based Peptide–Drug Conjugates. Int. J. Mol. Sci. 2023, 24, 3400. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.H.; Real, C.C.; Malafaia, O. Heterobivalent Dual-Target Peptide for Integrin-αvβ3 and Neuropeptide Y Receptors on Breast Tumor. Pharmaceuticals 2024, 17, 1328. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Lara, L.; Ferro-Flores, G.; De María Ramírez, F.; Ocampo-García, B.; Santos-Cuevas, C.; Díaz-Nieto, L.; Isaac-Olivé, K. Improved radiopharmaceutical based on 99mTc-Bombesin-folate for breast tumour imaging. Nucl. Med. Commun. 2016, 37, 377–386. [Google Scholar] [CrossRef]
- Ji, T.; Sun, Y.; Chen, B.; Ji, B.; Gao, S.; Ma, Q.; Cheng, G.; Zhang, H. The diagnostic role of 99mTc-dual receptor targeted probe and targeted peptide bombesin (RGD-BBN) SPET/CT in the detection of malignant and benign breast tumors and axillary lymph nodes compared to ultrasound. Hell. J. Nucl. Med. 2015, 18, 108–113. [Google Scholar]
- Santos-Cuevas, C.L.; Ferro-Flores, G.; de Murphy, C.A.; Ramírez, F.D.M.; Luna-Gutiérrez, M.A.; Pedraza-López, M.; García-Becerra, R.; Ordaz-Rosado, D. Design, preparation, in vitro and in vivo evaluation of 99mTc-N2S2-Tat(49-57)-bombesin: A target-specific hybrid radiopharmaceutical. Int. J. Pharm. 2009, 375, 75–83. [Google Scholar] [CrossRef]
- Bajwa, D.E.; Salvanou, E.A.; Theodosiou, M.; Koutsikou, T.S.; Efthimiadou, E.K.; Bouziotis, P.; Liolios, C. Radiolabeled iron oxide nanoparticles functionalized with PSMA/BN ligands for dual-targeting of prostate cancer. Front. Nucl. Med. 2023, 3, 1184309. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Dong, C.; Cui, L.; Jin, X.; Jia, B.; Zhu, Z.; Li, F.; Wang, F. 99mTc-labeled RGD-BBN peptide for small-animal SPECT/CT of lung carcinoma. Mol. Pharm. 2012, 9, 1409–1417. [Google Scholar] [CrossRef]
- De Oliveira, É.A.; Faintuch, B.L.; Targino, R.C.; Moro, A.M.; Martinez, R.C.R.; Pagano, R.L.; Fonoff, E.T.; Carneiro, C.D.G.; Garcez, A.T.; Faria, D.D.P.; et al. Evaluation of GX1 and RGD-GX1 peptides as new radiotracers for angiogenesis evaluation in experimental glioma models. Amino Acids 2016, 48, 821–831. [Google Scholar] [CrossRef]
- Yang, J.; Guo, H.; Gallazzi, F.; Berwick, M.; Padilla, R.S.; Miao, Y. Evaluation of a novel Arg-Gly-Asp-conjugated α-melanocyte stimulating hormone hybrid peptide for potential melanoma therapy. Bioconjug Chem. 2009, 20, 1634–1642. [Google Scholar] [CrossRef][Green Version]
- Bandari, R.P.; Carmack, T.L.; Malhotra, A.; Watkinson, L.; Fergason Cantrell, E.A.; Lewis, M.R.; Smith, C.J. Development of Heterobivalent Theranostic Probes Having High Affinity/Selectivity for the GRPR/PSMA. J. Med. Chem. 2021, 64, 2151–2166. [Google Scholar] [CrossRef] [PubMed]
- Mitran, B.; Varasteh, Z.; Abouzayed, A.; Rinne, S.S.; Puuvuori, E.; De Rosa, M.; Larhed, M.; Tolmachev, V.; Orlova, A.; Rosenström, U. Bispecific GRPR-antagonistic anti-PSMA/GRPR heterodimer for PET and SPECT diagnostic imaging of prostate cancer. Cancers 2019, 11, 1371. [Google Scholar] [CrossRef]
- Stott Reynolds, T.J.; Schehr, R.; Liu, D.; Xu, J.; Miao, Y.; Hoffman, T.J.; Rold, T.L.; Lewis, M.R.; Smith, C.J. Characterization and evaluation of DOTA-conjugated Bombesin/RGD-antagonists for prostate cancer tumor imaging and therapy. Nucl. Med. Biol. 2015, 42, 99–108. [Google Scholar] [CrossRef]
- Ellerby, H.M.; Arap, W.; Ellerby, L.M.; Kain, R.; Andrusiak, R.; Rio, G.D.; Krajewski, S.; Lombardo, C.R.; Rao, R.; Ruoslahti, E.; et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat. Med. 1999, 5, 1032–1038. [Google Scholar] [CrossRef]
- Bernard, B.; Capello, A.; Van Hagen, M.; Breeman, W.; Srinivasan, A.; Schmidt, M.; Erion, J.; Van Gameren, A.; Krenning, E.; De Jongz, M. Radiolabeled RGD-DTPA-Tyr 3-Octreotate for Receptor-Targeted Radionuclide Therapy. Cancer Biother. Radiopharm. 2004, 19, 173–180. [Google Scholar]
- Kim, E.M.; Jeong, M.H.; Kim, D.W.; Jeong, H.J.; Lim, S.T.; Sohn, M.H. Iodine 125-labeled mesenchymal-epithelial transition factor binding peptide-click-cRGDyk heterodimer for glioma imaging. Cancer Sci. 2011, 102, 1516–1521. [Google Scholar] [CrossRef]
- Abouzayed, A.; Yim, C.B.; Mitran, B.; Rinne, S.S.; Tolmachev, V.; Larhed, M.; Rosenström, U.; Orlova, A. Synthesis and preclinical evaluation of radio-iodinated GRPR/PSMA bispecific heterodimers for the theranostics application in prostate cancer. Pharmaceutics 2019, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Hübner, R.; von Kiedrowski, V.; Fricker, G.; Schirrmacher, R.; Wängler, C.; Wängler, B. Design, synthesis, in vitro and in vivo evaluation of heterobivalent sifalin-modified peptidic radioligands targeting both integrin αvβ3 and the mc1 receptor—Suitable for the specific visualization of melanomas? Pharmaceuticals 2021, 14, 547. [Google Scholar] [CrossRef]
- Ma, X.; Wang, M.; Wang, H.; Zhang, T.; Wu, Z.; Sutton, M.V.; Popik, V.V.; Jiang, G.; Li, Z. Development of bispecific NT-PSMA heterodimer for prostate cancer imaging: A potential approach to address tumor heterogeneity. Bioconjug Chem. 2019, 30, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, Y.; Liu, S.; Wang, F.; Chen, X. 18F, 64Cu, and68Ga labeled RGD-bombesin heterodimeric peptides for PET imaging of breast cancer. Bioconjug Chem. 2009, 20, 1016–1025. [Google Scholar] [CrossRef]
- Wu, H.; Chen, H.; Pan, D.; Ma, Y.; Liang, S.; Wan, Y.; Fang, Y. Imaging Integrin αvβ3 and NRP-1 Positive Gliomas with a Novel Fluorine-18 Labeled RGD-ATWLPPR Heterodimeric Peptide Probe. Mol. Imaging Biol. 2014, 16, 781–792. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, S.; Guo, J.; Guo, R.; Wang, H. 18F labeled RGD-A7R peptide for dual integrin and VEGF-targeted tumor imaging in mice bearing U87MG tumors. J. Label. Comp. Radiopharm. 2014, 57, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.B.; Wu, Z.; Chen, K.; Eun, K.R.; Chen, X. 18F-labeled BBN-RGD heterodimer for prostate cancer imaging. J. Nucl. Med. 2008, 49, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, S.; Zhu, D.; Bao, G.; Wang, Z.; Deng, X.; Liu, X.; Ma, X.; Zhao, J.; Zhu, L.; et al. Synthesis and Preclinical Evaluation of Dual-Specific Probe Targeting Glypican-3 and Prostate-Specific Membrane Antigen for Hepatocellular Carcinoma PET Imaging. Mol. Pharm. 2025, 22, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xia, D.; Zeng, X.; Meng, L.; Wang, Y.; Li, H.; Zhang, J.; Zhao, Z.; Zhuang, R.; Fang, J.; et al. Rational Design and Pharmacomodulation of 18F-Labeled Biotin/FAPI-Conjugated Heterodimers. J. Med. Chem. 2024, 67, 8361–8371. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, Y.; Chin, F.T.; Wang, F.; Chen, X. Dual integrin and gastrin-releasing peptide receptor targeted tumor imaging using 18F-Labeled PEGylated RGD-bombesin heterodimer 18F-FB-PEG 3-Glu-RGD-BBN. J. Med. Chem. 2009, 52, 425–432. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, K.; Yang, M.; Sun, X.; Liu, S.; Chen, X. A new 18F-labeled BBN-RGD peptide heterodimer with a symmetric linker for prostate cancer imaging. Amino Acids 2011, 41, 439–447. [Google Scholar] [CrossRef]
- Liu, N.; Wan, Q.; Wu, X.; Zhao, T.; Jakobsson, V.; Yuan, H.; Chen, X.; Zhang, J.; Zhang, W. A comparison of [18F]AlF- and 68Ga-labeled dual targeting heterodimer FAPI-RGD in malignant tumor: Preclinical evaluation and pilot clinical PET/CT imaging. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 1685–1697. [Google Scholar] [CrossRef]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Haberkorn, U.; Eisenhut, M.; Kopka, K. Preclinical evaluation of a bispecific low-molecular heterodimer targeting both PSMA and GRPR for improved PET imaging and therapy of prostate cancer. Prostate 2014, 74, 659–668. [Google Scholar] [CrossRef]
- Liolios, C.; Schäfer, M.; Haberkorn, U.; Eder, M.; Kopka, K. Novel Bispecific PSMA/GRPr Targeting Radioligands with Optimized Pharmacokinetics for Improved PET Imaging of Prostate Cancer. Bioconjug Chem. 2016, 27, 737–751. [Google Scholar] [CrossRef]
- Mendoza-Figueroa, M.J.; Escudero-Castellanos, A.; Ramirez-Nava, G.J.; Ocampo-García, B.E.; Santos-Cuevas, C.L.; Ferro-Flores, G.; Pedraza-Lopez, M.; Avila-Rodriguez, M.A. Preparation and preclinical evaluation of 68Ga-iPSMA-BN as a potential heterodimeric radiotracer for PET-imaging of prostate cancer. J. Radioanal. Nucl. Chem. 2018, 318, 2097–2105. [Google Scholar] [CrossRef]
- Liolios, C.; Bouziotis, D.; Sihver, W.; Schäfer, M.; Lambrinidis, G.; Salvanou, E.A.; Bauder-Wüst, U.; Benesova, M.; Kopka, K.; Kolocouris, A.; et al. Synthesis and Preclinical Evaluation of a Bispecific PSMA-617/RM2 Heterodimer Targeting Prostate Cancer. ACS Med. Chem. Lett. 2024, 15, 1970–1978. [Google Scholar] [CrossRef]
- Liu, Z.; Niu, G.; Wang, F.; Chen, X. 68Ga-labeled NOTA-RGD-BBN peptide for dual integrin and GRPR-targeted tumor imaging. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1483–1494. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, G.; Lang, L.; Li, F.; Fan, X.; Yan, X.; Yao, S.; Yan, W.; Huo, L.; Chen, L.; et al. Clinical translation of a dual integrin αvβ3- and gastrin-releasing peptide receptor-targeting PET radiotracer, 68Ga-BBN-RGD. J. Nucl. Med. 2017, 58, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mao, F.; Niu, G.; Peng, L.; Lang, L.; Li, F.; Ying, H.; Wu, H.; Pan, B.; Zhu, Z.; et al. 68Ga-BBN-RGD PET/CT for GRPR and integrin αvβ3 imaging in patients with breast cancer. Theranostics 2018, 8, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Amraee, N.; Alirezapour, B.; Hosntalab, M.; Yousefnia, H. Human dose assessment of 68 Ga-NODAGA-RGD-BBN heterodimer peptide based on animal data. J. Med. Phys. 2022, 47, 287–293. [Google Scholar] [CrossRef]
- Zang, J.; Wen, X.; Lin, R.; Zeng, X.; Wang, C.; Shi, M.; Zeng, X.; Zhang, J.; Wu, X.; Zhang, X.; et al. Synthesis, preclinical evaluation and radiation dosimetry of a dual targeting PET tracer [68Ga]Ga-FAPI-RGD. Theranostics 2022, 12, 7180–7190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wen, X.; Xu, W.; Pang, Y.; Sun, L.; Wu, X.; Xu, P.; Zhang, J.; Guo, Z.; Lin, Q.; et al. Clinical Evaluation of 68Ga-FAPI-RGD for Imaging of Fibroblast Activation Protein and Integrin avb3 in Various Cancer Types. J. Nucl. Med. 2023, 64, 1210–1217. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhang, X.; Guan, L.; Gao, X.; Xu, L.; Pang, H.; Du, J.; Zhang, J.; Cui, M. Design, Preclinical Evaluation, and First-in-Human PET Study of [68 Ga]Ga-PSFA-01: A PSMA/FAP Heterobivalent Tracer. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 1166–1176. [Google Scholar] [CrossRef]
- Chen, J.; Luo, D.; Dai, Y.; Zhou, Y.; Pang, Y.; Wu, H.; Sun, L.; Su, G.; Lin, Q.; Zhao, L.; et al. Enhanced Detection of Early Pulmonary Fibrosis Disease Using 68Ga-FAPI-LM3 PET. Mol. Pharm. 2024, 21, 3684–3692. [Google Scholar] [CrossRef]
- Bodin, S.; Previti, S.; Jestin, E.; Rémond, E.; Vimont, D.; Lamare, F.; Ait-Arsa, I.; Hindié, E.; Cavelier, F.; Morgat, C. Design and Synthesis of 68Ga-Labeled Peptide-Based Heterodimers for Dual Targeting of NTS1 and GRPR. Chem. Med. Chem. 2025, 20, e202400843. [Google Scholar] [CrossRef]
- Vall-Sagarra, A.; Litau, S.; Decristoforo, C.; Wängler, B.; Schirrmacher, R.; Fricker, G.; Wängler, C. Design, synthesis, in vitro, and initial in vivo evaluation of heterobivalent peptidic ligands targeting both NPY(Y1)-and GRP-receptors—An improvement for breast cancer imaging? Pharmaceuticals 2018, 11, 65. [Google Scholar] [CrossRef]
- Lindner, S.; Fiedler, L.; Wängler, B.; Bartenstein, P.; Schirrmacher, R.; Wängler, C. Design, synthesis and in vitro evaluation of heterobivalent peptidic radioligands targeting both GRP- and VPAC1-Receptors concomitantly overexpressed on various malignancies—Is the concept feasible? Eur. J. Med. Chem. 2018, 155, 84–95. [Google Scholar] [CrossRef]
- Lindner, S.; Rudolf, H.; Palumbo, G.; Oos, R.; Antons, M.; Hübner, R.; Bartenstein, P.; Schirrmacher, R.; Wängler, B.; Wängler, C. Are heterobivalent GRPR- and VPAC1R-bispecific radiopeptides suitable for efficient in vivo tumor imaging of prostate carcinomas? Bioorg Med. Chem. Lett. 2021, 155, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.; Judmann, B.; Cheng, X.; Wängler, B.; Schirrmacher, R.; Fricker, G.; Wängler, C. Synthesis, Radiolabeling, and in Vitro and in Vivo Characterization of Heterobivalent Peptidic Agents for Bispecific EGFR and Integrin αvβ3 Targeting. ACS Omega 2022, 8, 2793–2807. [Google Scholar] [CrossRef]
- Yu, H.M.; Chen, J.H.; Lin, K.L.; Lin, W.J. Synthesis of 68Ga-labeled NOTA-RGD-GE11 heterodimeric peptide for dual integrin and epidermal growth factor receptor-targeted tumor imaging. J. Label. Comp. Radiopharm. 2015, 58, 299–303. [Google Scholar] [CrossRef]
- Wen, X.; Wang, R.; Xu, P.; Shi, M.; Shang, Q.; Zeng, X.; Zeng, X.; Liu, J.; Wang, X.; Zhu, Z.; et al. Synthesis, preclinical, and initial clinical evaluation of integrin αVβ3 and gastrin-releasing peptide receptor (GRPR) dual-targeting radiotracer [68Ga]Ga-RGD-RM26-03. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2023–2035. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.B.; Cao, Q.; Liu, S.; Wang, F.; Chen, X. Small-animal PET of tumors with 64Cu-labeled RGD-bombesin heterodimer. J. Nucl. Med. 2009, 50, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.B.; Nanda, P.K.; Rold, T.L.; Sieckman, G.L.; Szczodroski, A.F.; Hoffman, T.J.; Chen, X.; Smith, C.J. 64Cu-NO2A-RGD-Glu-6-Ahx-BBN(7-14)NH 2: A heterodimeric targeting vector for positron emission tomography imaging of prostate cancer. Nucl. Med. Biol. 2012, 39, 377–387. [Google Scholar] [CrossRef]
- Lucente, E.; Liu, H.; Liu, Y.; Hu, X.; Lacivita, E.; Leopoldo, M.; Cheng, Z. Novel 64Cu Labeled RGD2-BBN Heterotrimers for PET Imaging of Prostate Cancer. Bioconjug Chem. 2018, 29, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Amraee, N.; Alirezaour, B.; Hosntalab, M.; Hadadi, A.; Yousefnia, H. Development of [64Cu]Cu-NODAGA-RGD-BBN as a Novel Radiotracer for Dual Integrin and GRPR-targeted Tumor PET Imaging. Curr. Radiopharm. 2025, 18, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Durkan, K.; Jiang, Z.; Rold, T.L.; Sieckman, G.L.; Hoffman, T.J.; Bandari, R.P.; Szczodroski, A.F.; Liu, L.; Miao, Y.; Reynolds, T.S.; et al. A heterodimeric [RGD-Glu-[64Cu-NO2A]-6-Ahx-RM2] αvβ3/GRPr-targeting antagonist radiotracer for PET imaging of prostate tumors. Nucl. Med. Biol. 2014, 41, 133–139. [Google Scholar] [CrossRef]
- Li, H.; Peng, W.; Zhen, Z.; Zhang, W.; Liao, S.; Wu, X.; Wang, L.; Xuan, A.; Gao, Y.; Xu, J. Integrin αvβ3 and EGFR dual-targeted [64Cu]Cu-NOTA-RGD-GE11 heterodimer for PET imaging in pancreatic cancer mouse model. Nucl. Med. Biol. 2023, 124–125, 108364. [Google Scholar] [CrossRef]
- Escudero-Castellanos, A.; Ocampo-García, B.; Ferro-Flores, G.; Santos-Cuevas, C.; Morales-Ávila, E.; Luna-Gutiérrez, M.; Isaac-Olivé, K. Synthesis and preclinical evaluation of the 177 Lu-DOTA-PSMA(inhibitor)-Lys 3 -bombesin heterodimer designed as a radiotheranostic probe for prostate cancer. Nucl. Med. Commun. 2019, 40, 278–286. [Google Scholar] [CrossRef]
- Escudero-Castellanos, A.; Ocampo-García, B.E.; Ferro-Flores, G.; Isaac-Olivé, K.; Santos-Cuevas, C.L.; Olmos-Ortiz, A.; García-Quiroz, J.; García-Becerra, R.; Díaz, L. Preparation and in vitro evaluation of 177Lu-iPSMA-RGD as a new heterobivalent radiopharmaceutical. J. Radioanal. Nucl. Chem. 2017, 314, 2201–2207. [Google Scholar] [CrossRef]
- Jiang, L.; Miao, Z.; Liu, H.; Ren, G.; Bao, A.; Cutler, C.S.; Shi, H.; Cheng, Z. 177Lu-labeled RGD-BBN heterodimeric peptide for targeting prostate carcinoma. Nucl. Med. Commun. 2013, 34, 909–914. [Google Scholar] [CrossRef]
- Aranda-Lara, L.; Ferro-Flores, G.; Azorín-Vega, E.; Ramírez Fde, M.; Jiménez-Mancilla, N.; Ocampo-García, B.; Santos-Cuevas, C.; Isaac-Olivé, K. Synthesis and evaluation of Lys1(α,γ-Folate)Lys3(177Lu-DOTA)-Bombesin(1-14) as a potential theranostic radiopharmaceutical for breast cancer. Appl. Radiat. Isot. 2016, 107, 214–219. [Google Scholar] [CrossRef]
- Cui, L.; Liu, Z.; Jin, X.; Jia, B.; Li, F.; Wang, F. Evaluation of 188Re-MAG2-RGD-bombesin for potential prostate cancer therapy. Nucl. Med. Biol. 2013, 40, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Bandara, N.; Stott Reynolds, T.J.; Schehr, R.; Bandari, R.P.; Diebolder, P.J.; Krieger, S.; Xu, J.; Miao, Y.; Rogers, B.E.; Smith, C.J. Matched-pair, 86Y/90Y-labeled, bivalent RGD/bombesin antagonist, [RGD-Glu-[DO3A]-6-Ahx-RM2], as a potential theranostic agent for prostate cancer. Nucl. Med. Biol. 2018, 62–63, 71–77. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, R.; Guo, J.; Wang, X. The role and future prospects of artificial intelligence algorithms in peptide drug development. Biomed. Pharmacother. 2024, 175, 116709. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Liu, T.; Lin, S.; Li, D.; Liu, H.; Yao, X.; Hou, T. Artificial intelligence in peptide-based drug design. Drug Discov. Today 2025, 30, 104300. [Google Scholar] [CrossRef] [PubMed]
- Tu, N.P.; Searle, P.A.; Sarris, K. An Automated Microwave-Assisted Synthesis Purification System for Rapid Generation of Compound Libraries. J. Lab. Autom. 2016, 21, 459–469. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; Carney, R.P.; Liu, R.; Fan, J.; Zhao, S.; Chen, Y.; Lam, K.S.; Pan, T. Microfluidic Print-to-Synthesis Platform for Efficient Preparation and Screening of Combinatorial Peptide Microarrays. Anal. Chem. 2018, 90, 5833–5840. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, M.; Zhang, H. Microfluidics for synthesis of peptide-based PET tracers. Biomed. Res. Int. 2013, 2013, 839683. [Google Scholar] [CrossRef] [PubMed]
- Passariello, M.; Yoshioka, A.; Takahashi, K.; Hashimoto, S.I.; Inoue, T.; Nakamura, K.; De Lorenzo, C. Novel tri-specific tribodies induce strong T cell activation and anti-tumor effects in vitro and in vivo. J. Exp. Clin. Cancer Res. 2022, 41, 269. [Google Scholar] [CrossRef]
- Alas, M.; Saghaeidehkordi, A.; Kaur, K. Peptide-Drug Conjugates with Different Linkers for Cancer Therapy. J. Med. Chem. 2021, 64, 216–232. [Google Scholar] [CrossRef]

| 99mTc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BRL | Pathology | Peptide 1 | Target 1 | Peptide 2 | Target 2 | Chelator | Linker | Development Phase | Limitations | Ref. |
| [99mTc]HYNIC-cRGDfk NPY | Breast cancer | NPY | Y1R and Y2R | cRDGfK | Integrin αvβ4 | HYNIC-tricine-EDDA | 8-amino-3,6-dioxaoctanoic acid | Pre-Clinical trial | High uptake in the stomach and intestines | [13] |
| 99mTc-Bombesin-Folate | Breast cancer | BBN | GRPR | Folate | FRα | HYNIC-tricine-TPPTS | Glu | Pre-Clinical trial | High renal and pancreatic uptake | [14] |
| 99mTc-RGD-BBN | Lung Carcinoma | BBN | GRPR | RGD | Integrin αvβ13 | Cys(Acm)-Gly-Cys(Acm) | Gly-Gly-Cys-Gly | Pre-Clinical trial | Cancer model used was not dual-receptor | [18] |
| 99mTc-RGD-BBN | Breast cancer and axillary lymph nodes | BBN | GRPR | RGD | Integrin αvβ3 | HYNIC-tricine-EDDA | Lys-Lys | Clinical trial | Low sensitivity for lesions with sizes less than 10 mm | [15] |
| 99mTc-Tat-BN | Different types of cancer | BBN | GRPR | Tat | Internalization | Glucoheptonate | Lys-Cys-Cys | Pre-Clinical trial | High uptake in kidneys and in non-target organs | [16] |
| 99mTc-IONs-PSMA/BN | Prostate cancer | BBN | GRPR | Lys-CO-Glu | PSMA | HYNIC-tricine-TPPTS | Glu | In vitro cell studies | Very preliminary data | [17] |
| 99mTc-HYNIC-E-[c(RGDfk)-c(GX1)] | Gliomas | GX1 | Integrin α3β1 | RGD | Integrin αvβ3 | [Tc(H2O)3(CO)3]+ | - | Pre-Clinical trial | Studied in a nonanatomical site for human tumors | [19] |
| 99mTc-RGD-Lys-(Arg(11))CCMSH | Melanoma | α-MSH | MC1 | RGD | Integrin αvβ15 | HYNIC-tricine-EDDA | - | Pre-Clinical trial | High renal uptake | [20] |
| 111In | ||||||||||
| BRL | Pathology | Peptide 1 | Target 1 | Peptide 2 | Target 2 | Chelator | Linker | Development phase | Limitations | Ref. |
| [DUPA-6-Ahx-([111In]In-DO3A)-8-Aoc-BBN ANT] | Prostate cancer | BBN | GRPR | DUPA | PSMA | DO3A | 6-Ahx-8-Aoc | Pre-Clinical trial | High renal uptake | [21] |
| 111In-Glu-Urea-Glu-Aoc-Lys(NOTA)-(PEG)6-RM26 | Prostate cancer | RM26 | GRPR | Glu-Urea-Glu-Aoc-Lys | PSMA | NOTA | Polyethylene glycol (PEG6) | Pre-Clinical trial | Rapid activity washout from tumors | [22] |
| RGD-Glu-[111In-DO3A]-6-Ahx-RM2 | Prostate cancer | RM2 | GRPR | RGD | Integrin αvβ11 | DOTA | Glu-6Ahx | Pre-Clinical trial | High pancreatic uptake | [23] |
| RGD-111In-DTPA-octreotate | Different types of cancer | Octreotate | Sst2r | RGD | Integrin αvβ13 | DTPA | - | Pre-Clinical trial | High renal uptake | [24] |
| RGD-111In-DTpA-Tyr3-octretoate | Different types of cancer | Octreotate | Sst2r | RGD | Integrin αvβ14 | DTPA | - | Pre-Clinical trial | High renal uptake | [25] |
| 125I | ||||||||||
| BRL | Pathology | Peptide 1 | Target 1 | Peptide 2 | Target 2 | Chelator | Linker | Development phase | Limitations | Ref. |
| 125I-cMBP-click-cRGDyk | Gliomas | cMBP | c-Met | RGD | Integrin αvβ3 | Chloramine-T | 1 + 3 cyclo addition | Pre-Clinical trial | Low tumor uptake | [26] |
| [125I]I-BO530 | Prostate cancer | RM26 | GRPR | PSMA-617 | PSMA | IodoGen | Cu(I)-catalyzed cycloaddition | Pre-Clinical trial | High renal uptake | [27] |
| 18F | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BRL | Pathology | Peptide 1 | Target 1 | Peptide 2 | Target 2 | Chelator | Linker | Development Phase | Limitations | Ref. |
| 18F-N3-NT-PSMA | Prostate cancer | NT20.3 | NTR1 | Glu-urea-lys(Ahx) | PSMA | via azide–alkyne click reaction | - | Pre-Clinical trial | Low affinity to PSMA | [29] |
| [18F]HBPL | Melanoma | GG-Nle-c(DHfRWK) | MC1R | (c(RGDfK) | Integrin αvβ3 | SiFAlin | Different linkers | Pre-Clinical trial | Low radiochemical yield | [28] |
| 18F-FB-PEG3-RGD-BBN | Breast cancer | BBN | GRPR | RGD | Integrin αvβ4 | -SFB | PEG3 | Pre-Clinical trial | Rapid wash-out | [30] |
| 18FAl-NOTA-RGD-ATWLPPR | Gliomas | ATWLPPR | NRP-1 | c(RGDyK) | Integrin αvβ15 | -SFB | glutamic acid. | Pre-Clinical trial | low receptor-binding affinity | [31] |
| 18F-RGD-A7R | Different types of cancer | ATWLPPR A7R | VEGFR | RGD | Integrin αvβ14 | -SFB | glutamic acid. | Pre-Clinical trial | Unfavorable uptakes in stomach and intestine | [32] |
| 18F-RGD-BBN | Different types of cancer | BBN | GRPR | RGD | Integrin αvβ3 | -SFB | glutamic acid. | Pre-Clinical trial | Low radiochemical yield | [33] |
| 18F-T2P | Prostate cancer | TJ12P2 | Glypican-3 (GPC3) | 2-PMPA | PSMA | NOTA | PEG | Pre-Clinical trial | Short half-life | [34] |
| [18F]AlF-NSFB, [18F]AlF-NSFBP2, [18F]AlF-NSFBP4 | Different types of cancer | Onco-FAP | FAP | Biotin | Biotin receptor | DFO | PEG-MAL | Pre-Clinical trial | Necessity of rational design and pharmacomodulation | [35] |
| 18F-FB-PEG(3)-Glu-RGD-BBN | Different types of cancer | BBN | GRPR | RGD | Integrin αvβ3 | EG3 spacer | glutamate linker | Pre-Clinical trial | Prominent uptake in kidneys at early time points | [36] |
| 18F-FB-AEADP-BBN-RGD | Prostate cancer | BBN | GRPR | RGD | Integrin αvβ3 | -SFB | glutamate linker | Pre-Clinical trial | Low specificity | [37] |
| [18F]AlF-LNC1007 | Different types of cancer | FAP-2286 | FAP | RGD | Integrin αvβ3 | NOTA | - | Clinical trial | Fast clearance and short retention time | [38] |
| 64Ga | ||||||||||
| BRL | Pathology | Peptide 1 | Target 1 | Peptide 2 | Target 2 | Chelator | Linker | Development phase | Limitations | Ref. |
| 68Ga-Glu-urea-Lys-HBED-CC-BZH3 | Prostate cancer | BZH3 | GRPR | Glu-urea-Lys(Ahx)-HBED-CC | PSMA | HBED-CC | Pre-Clinical trial | High kidney and pancreatic uptake | [39] | |
| 68Ga-HE2 | Prostate cancer | H2N-PEG2-BN(6–14) | GRPR | Glu-ureido-Lys | PSMA | HBED-CC | Pre-Clinical trial | Tracer accumulation in nontarget organs | [40] | |
| 68Ga-iPSMA-BN | Prostate cancer | Lys3-BBN | GRPR | Nal-Lys-CO-Glu-OH | PSMA | DOTA | GMBS | Pre-Clinical trial | High renal uptake | [41] |
| 68Ga-Glu-Urea-Glu-Aoc-Lys(NOTA)-(PEG)6-RM26 | Prostate cancer | RM26 | GRPR | Glu-Urea-Glu-Aoc-Lys | PSMA | NOTA | PEG | Pre-Clinical trial | Low tumor-to-nontumor ratios | [22] |
| 68Ga-PSMa-617/RM2 | Prostate cancer | RM2 | GRPR | PSMA-617 | PSMA | NOTA | PEG/Aoc-Phe | Pre-Clinical trial | Pharmacokinetics to be improved | [42] |
| 68Ga-FB-PEG3-RGD-BBN | Breast cancer | BBN | GRPR | RGD | Integrin αvβ4 | -SFB | PEG3 | Pre-Clinical trial | Low affinity to GPPR | [30] |
| 68Ga-BBN-RGD | Prostate cancer | BBN | GRPR | RGD | Integrin αvβ4 | NOTA | - | Clinical trial | Expression status in metastases unknown | [44] |
| 68Ga-BBN-RGD | Breast cancer | BBN | GRPR | RGD | Integrin αvβ3 | NOTA | glutamic acid. | Clinical trial | False-positive cases | [45] |
| 68Ga-NODAGA-RGD-BBN | Prostate cancer | BBN | GRPR | RGD | Integrin αvβ3 | NODAGA | - | Pre-Clinical trial | High renal uptake | [46] |
| 68Ga-FAPI-RGD | Different types of cancer | FAPI-02 | FAP | RGD | Integrin αvβ3 | NOTA | PEG | Clinical trial | Limited number of patients and no healthy subjects involved | [47] |
| 68Ga-FAPI-RGD | Different types of cancer | FAPI-02 | FAP | RGD | Integrin αvβ3 | NOTA | PEG | Clinical trial | High uptake in thyroid and pancreas | [48] |
| [68Ga]Ga-PSFA-01 | Prostate cancer | FAPI-04 | FAP | EuK (PSMA11) | PSMA | HBED-CC | Clinical trial | Only one patient studied | [49] | |
| 68Ga-FAPI-LM3 | Pulmonary fibrosis | FAPI-46 | FAP | LM3 | SSTR2 | - | Pre-Clinical trial | The mice model do not replicate the human pathology | [50] | |
| 68Ga-JMV 7110 | Breast cancer | BBN analogs | GRPR | NT analogs | NTS1 | DOTA | βAla | In vitro cell studies | Loss of NTS1-specific internalization | [51] |
| 68Ga-JMV 7253 | ||||||||||
| 64Ga-JMV 7266 | No specific binding | |||||||||
| 68Ga-HBPL | Breast cancer | BBN7–14 | GRPR | [Lys4, Trp5, Nle7] BVD15 | NPY (Y1)R | NODAGA | PEG | Pre-Clinical trial | High kidney and liver uptake | [52] |
| [68Ga]19–[68Ga]23 | Different types of cancer | Aminooxy-PESIN | GRPR | Aminooxy-TP3805 | VPAC 1R | NODAGA | PEG | In vitro evaluation | High renal uptake | [53] |
| [68Ga]Ga-9 | Prostate cancer | PESIN | GRPR | PACAP-27 | VPAC 1R | NODAGA | (PEG)3 | Pre-Clinical trial | Suboptimal pharmacokinetic | [54] |
| 68Ga-NODAGA-PEG3-GE11-PEG3-c(RGDyK) | (NODAGA-PEG 3-GE11-PEG3-c(RGDyK) | GE11 | EFGR | RGD | Integrin αvβ3 | NODAGA | (PEG)3 | Pre-Clinical trial | Low affinity to EGFR | [55] |
| 68Ga-NODAGA-PEG5-GE11-PEG5-c(RGDfK) | NODA GA-PEG5-GE11-PEG5-c(RGDfK) | (PEG)5 | ||||||||
| 68Ga-NOTA-RGD-cys-6-Ahx-GE11 | (68)Ga-NOTA-RGD-cys-6-Ahx-GE11 | GE11 | EFGR | RGD | Integrin αvβ3 | NOTA | 6-aminohexanoic | In vitro evaluation | Very preliminary data | [56] |
| 68Ga-T2P | Prostate cancer | TJ12P2 | Glypican-3 (GPC3) | 2-PMPA | PSMA | NOTA | PEG | Pre-Clinical trial | Notable uptake in liver and kidneys | [35] |
| [68Ga]Ga-LNC1015 | Different types of cancer | RM26 | GRPR | RGD | Integrin αvβ3 | NOTA | n.d.a. | Clinical trial | Rapid tumor wash out and high kidney uptake | [57] |
| 64Cu | ||||||||||
| BRL | Pathology | Peptide 1 | Target 1 | Peptide 2 | Target 2 | Chelator | Linker | Development phase | Limitations | Ref. |
| 64Cu-FB-PEG3-RGD-BBN | Breast cancer | BBN | GRPR | RGD | Integrin αvβ4 | -SFB | PEG3 | Pre-Clinical trial | High and prolonged liver uptake | [31] |
| 64Cu-NOTA-RGD-bombesin | Different types of cancer | BBN | GRPR | RGD | Integrin αvβ8 | NOTA | glutamic acid. | Pre-Clinical trial | Significant renal and abdominal uptake | [58] |
| 64Cu-NO2A-RGD-Glu-6-Ahx-BBN(7-14)NH2 | Prostate cancer | BBN(7-14)NH(2) | GRPR | RGD | Integrin αvβ9 | NO2A | Glu-6-Ahx | Pre-Clinical trial | High pancreatic uptake | [59] |
| E[c(RGDyK)]2-PEG3-Glu-(Pro-Gly)12-BBN(7-14)-NH2 (RGD2-PG12-BBN) | Prostate cancer | BBN | GRPR | RGD | RGD | NODAGA | [Pro-Gly]x | Pre-Clinical trial | Low tumor uptake | [60] |
| [64Cu]Cu-NODAGA-RGD-BBN | GRPR+ tumors | BBN | GRPR | RGD | Integrin αvβ3 | NODAGA | - | Pre-Clinical trial | Uptake in GRPR-expressing organs | [61] |
| [RGD-Glu-[64Cu-NO2A]-6-Ahx-RM2] | Prostate cancer | RM2 | GRPR | RGD | Integrin αvβ10 | NOTA | glutamic acid. | Pre-Clinical trial | High pancreatic and hepatic uptake | [62] |
| [64Cu]Cu-NOTA-RGD-GE11 | pancreatic cancer | GE11 | EGFR | RGD | Integrin αvβ3 | NOTA | PEG4 | Pre-Clinical trial | High renal uptake | [63] |
| 177Lu | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BRL | Pathology | Peptide 1 | Target 1 | Peptide 2 | Target 2 | Chelator | Linker | Development Phase | Limitations | Ref. |
| 177Lu-DOTA-iPSMA-Lys3-BN | Prostate cancer | Lys3-BBN | GRPR | Nal-Lys-CO-Glu-OH | PSMA | DOTA | - | In vitro evaluation | High pancreatic uptake | [64] |
| 177Lu-PSMA-617/RM2 | Prostate cancer | RM2 | GRPR | PSMA-617 | PSMA | NOTA | PEG/Aoc-Phe | In vitro evaluation | Tumor/pancreas ratio increased over time | [43] |
| 177Lu-iPSMA-RGD | Prostate cancer | iPSMA | PSMA | RGD | Integrin αvβ13 | DOTA | - | Pre-Clinical trial | Very preliminary data | [65] |
| 177Lu-DO3A-RGD-BBN | Prostate cancer | BBN | GRPR | RGD | Integrin αvβ9 | DO3A | 6-Ahx | Pre-Clinical trial | High pancreatic uptake | [66] |
| RGD-Glu-[177Lu-DO3A]-6-Ahx-RM2 | Prostate cancer | RM2 | GRPR | RGD | Integrin αvβ11 | DO3A | Glu-6Ahx | Pre-Clinical trial | Lower efficacy than monovalent RM2 antagonists | [24] |
| [DUPA-6-Ahx-([177Lu]In-DO3A)-8-Aoc-BBN ANT] | Prostate cancer | BBN | GRPR | DUPA | PSMA | DO3A | 6-Ahx-8-Aoc | Pre-Clinical trial | High uptake in lung, liver, kidney, and spleen | [22] |
| 177Lu-Folate-BN | Breast cancer | BBN | GRPR | Folate | FRα | DOTA | - | Pre-Clinical trial | High renal uptake | [67] |
| 188Re | ||||||||||
| BRL | Pathology | Peptide 1 | Target 1 | Peptide 2 | Target 2 | Chelator | Linker | Development phase | Limitations | Ref. |
| 188Re-MAG2-RGD-BBN | Prostate cancer | BBN | GRPR | RGD | Integrin αvβ14 | MAG2 | glutamate linker | Pre-Clinical trial | Low specific activity | [68] |
| 89Y/90Y | ||||||||||
| BRL | Pathology | Peptide 1 | Target 1 | Peptide 2 | Target 2 | Chelator | Linker | Development phase | Limitations | Ref. |
| 86YRGD-Glu-[DO3A]-6-Ahx-RM2] | Prostate cancer | RM2 | GRPR | RGD | Integrin αvβ11 | DO3A | - | Pre-Clinical trial | Moderate affinity for αvβ3 | [69] |
| 90Y [RGD-Glu-[DO3A]-6-Ahx-RM2] | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bentivoglio, V.; D’Ippolito, E.; Nayak, P.; Giorgio, A.; Lauri, C. Bispecific Radioligands (BRLs): Two Is Better Than One. J. Clin. Med. 2025, 14, 5628. https://doi.org/10.3390/jcm14165628
Bentivoglio V, D’Ippolito E, Nayak P, Giorgio A, Lauri C. Bispecific Radioligands (BRLs): Two Is Better Than One. Journal of Clinical Medicine. 2025; 14(16):5628. https://doi.org/10.3390/jcm14165628
Chicago/Turabian StyleBentivoglio, Valeria, Enrico D’Ippolito, Pallavi Nayak, Anna Giorgio, and Chiara Lauri. 2025. "Bispecific Radioligands (BRLs): Two Is Better Than One" Journal of Clinical Medicine 14, no. 16: 5628. https://doi.org/10.3390/jcm14165628
APA StyleBentivoglio, V., D’Ippolito, E., Nayak, P., Giorgio, A., & Lauri, C. (2025). Bispecific Radioligands (BRLs): Two Is Better Than One. Journal of Clinical Medicine, 14(16), 5628. https://doi.org/10.3390/jcm14165628





