Chronic Heart Failure Rehabilitation: Diaphragm Training Needs More Attention
Abstract
1. Introduction
2. Physical Rehabilitation in Chronic HF
2.1. Endurance Training
2.2. Resistance Training
2.3. Benefits with Physical Activity
3. Inspiratory Muscle Training (IMT)
4. Pathophysiological Rationale for IMT
4.1. Role of the Diaphragm in Cardiac Function
4.2. Diaphragm and Muscular Strength
5. Chronic HF Patients with Lower Back Pain
6. Additional Arguments for Including IMT
7. Grey Areas and Research Directions
8. Challenges for the Future
9. Conclusions
Funding
Conflicts of Interest
References
- Fang, Z.; Raza, U.; Song, J.; Lu, J.; Yao, S.; Liu, X.; Zhang, W.; Li, S. Systemic aging fuels heart failure: Molecular mechanisms and therapeutic avenues. ESC Heart Fail. 2025, 12, 1059–1080. [Google Scholar] [CrossRef] [PubMed]
- Fadhillah, F.S.; Habibah, K.; Juniarto, A.Z.; Sobirin, M.A.; Maharani, N.; Pramono, A. Diet and the gut microbiota profiles in individuals at risk of chronic heart failure—A review on the Asian population. Asia Pac. J. Clin. Nutr. 2025, 34, 141–152. [Google Scholar] [CrossRef]
- Barriault, A.; Iftikhar, U.; Stone, J.A. Cardiac Rehabilitation and Heart Failure with Reduced Ejection Fraction: Pathophysiology, Benefits, and Precautions. Can. J. Cardiol. 2025, 41, 443–455. [Google Scholar] [CrossRef]
- Cuomo, G.; Di Lorenzo, A.; Tramontano, A.; Iannone, F.P.; D’Angelo, A.; Pezzella, R.; Testa, C.; Parlato, A.; Merone, P.; Pacileo, M.; et al. Exercise Training in Patients with Heart Failure: From Pathophysiology to Exercise Prescription. Rev. Cardiovasc. Med. 2022, 23, 144. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Serrano-Cumplido, A.; Escobar-Cervantes, C.; Arranz-Martínez, E.; Turégano-Yedro, M.; Pallarés-Carratalá, V. Heart Failure Prevalence Rates and Its Association with Other Cardiovascular Diseases and Chronic Kidney Disease: SIMETAP-HF Study. J. Clin. Med. 2023, 12, 4924. [Google Scholar] [CrossRef]
- Siddiqi, A.K.; Shahzad, M.; Kumar, A.; Ahmed, M.; Sridharan, L.; Abdou, M.H.; Naeem, M. The efficacy of inspiratory muscle training in improving clinical outcomes in heart failure patients: An updated systematic review and meta-analysis. J. Cardiol. 2025, 85, 374–385. [Google Scholar] [CrossRef]
- Li, H.; Tao, L.; Huang, Y.; Li, Z.; Zhao, J. Inspiratory muscle training in patients with heart failure: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 993846. [Google Scholar] [CrossRef]
- Emmons-Bell, S.; Johnson, C.; Roth, G. Prevalence, incidence and survival of heart failure: A systematic review. Heart 2022, 108, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Năstasie, O.-C.; Radu, D.-A.; Onciul, S.; Drăgoescu, M.-B.; Popa-Fotea, N.-M. Nexilin mutations, a cause of chronic heart failure: A state-of-the-art review starting from a clinical case. World J. Cardiol. 2025, 17, 100290. [Google Scholar] [CrossRef] [PubMed]
- Shahim, B.; Kapelios, C.J.; Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure: An Updated Review. Card. Fail. Rev. 2023, 9, e11. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2024, 26, 5–17. [Google Scholar] [CrossRef]
- Ostrominski, J.W.; DeFilippis, E.M.; Bansal, K.; Riello, R.J., 3rd; Bozkurt, B.; Heidenreich, P.A.; Vaduganathan, M. Contemporary American and European Guidelines for Heart Failure Management. JACC Heart Fail. 2024, 12, 810–825. [Google Scholar] [CrossRef]
- Formiga, F.; Nuñez, J.; Moraga, M.J.C.; Marcos, M.C.; Egocheaga, M.I.; García-Prieto, C.F.; Trueba-Sáiz, A.; Gilarranz, A.M.; Rodriguez, J.M.F. Diagnosis of heart failure with preserved ejection fraction: A systematic narrative review of the evidence. Heart Fail. Rev. 2024, 29, 179–189. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Konstantinou, K.; Tsioufis, K. The Role of NT-proBNP Levels in the Diagnosis and Treatment of Heart Failure with Preserved Ejection Fraction—It Is Not Always a Hide-and-Seek Game. J. Cardiovasc. Dev. Dis. 2024, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, V.; Sharma, K.; Keteyian, S.J.; Alcain, C.F.; Desvigne-Nickens, P.; Fleg, J.L.; Florea, V.G.; Franklin, B.A.; Guglin, M.; Halle, M.; et al. Supervised Exercise Training for Chronic Heart Failure with Preserved Ejection Fraction: A Scientific Statement from the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2023, 81, 1524–1542. [Google Scholar] [CrossRef]
- Sharfo, A.; Sørensen, A.L.; Nielsen, E.E.; Raymond, I.E.; Soja, A.M.B.; Olsen, M.H. OPTIHEART: Determinants and prognostic importance of optimal medical treatment in patients with heart failure with reduced ejection fraction discharged from a heart failure clinic from 2018 to 2020. Blood Press. 2025, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P. Heart failure management guidelines: New recommendations and implementation. J. Cardiol. 2024, 83, 67–73. [Google Scholar] [CrossRef]
- Monzo, L.; Musella, F.; Girerd, N.; Rossignol, P. Sodium zirconium cyclosilicate for MRAs optimization in HFrEF: Lessons learned from the REALIZE-K trial. Heart Fail. Rev. 2025, 30, 565–574. [Google Scholar] [CrossRef]
- Beghini, A.; Sammartino, A.M.; Papp, Z.; von Haehling, S.; Biegus, J.; Ponikowski, P.; Adamo, M.; Falco, L.; Lombardi, C.M.; Pagnesi, M.; et al. 2024 update in heart failure. ESC Heart Fail. 2025, 12, 8–42. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.J.; Virani, S.A.; Zieroth, S.; Turgeon, R. Heart Failure Management in 2023: A Pharmacotherapy- and Lifestyle-Focused Comparison of Current International Guidelines. CJC Open 2023, 5, 629–640. [Google Scholar] [CrossRef]
- Chen, Q.-F.; Lu, Y.; Katsouras, C.S.; Peng, Y.; Sun, J.; Li, M.; Liu, C.; Yao, H.; Lian, L.; Feng, X.; et al. Characteristics, outcomes and the necessity of continued guideline-directed medical therapy in patients with heart failure with improved ejection fraction. Ann. Med. 2025, 57, 2442535. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Wang, L. Impact of Different Exercise Modalities on Physical Function and Quality of Life in Patients with Heart Failure. J. Multidiscip. Healthc. 2024, 17, 2551–2559. [Google Scholar] [CrossRef]
- Silva, J.M.; Camillo, C.A.; Vanderlei, L.C.M. Inspiratory Muscle Training in Cardiac Rehabilitation of Patients with Heart Failure: Optional or Fundamental? Heart Lung Circ. 2024, 33, e73–e74. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Wang, Q.; Zhu, J.; Ren, Z.; Wang, L. Effects of exercise modalities on physical function and quality of life in patients with heart failure: A systematic review and network meta-analysis. ESC Heart Fail. 2025, 12, 2427–2440, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Myers, J.; Bonikowske, A.R. Practical guidelines for exercise prescription in patients with chronic heart failure. Heart Fail. Rev. 2023, 28, 1285–1296. [Google Scholar] [CrossRef]
- Ambrosetti, M.; Abreu, A.; Corrà, U.; Davos, C.H.; Hansen, D.; Frederix, I.; Iliou, M.C.; E Pedretti, R.F.; Schmid, J.-P.; Vigorito, C.; et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2021, 28, 460–495. [Google Scholar] [CrossRef]
- Bozkurt, B.; Fonarow, G.C.; Goldberg, L.R.; Guglin, M.; Josephson, R.A.; Forman, D.E.; Lin, G.; Lindenfeld, J.; O’cOnnor, C.; Panjrath, G.; et al. Cardiac Rehabilitation for Patients With Heart Failure: JACC Expert Panel. J. Am. Coll. Cardiol. 2021, 77, 1454–1469. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, T.; Hu, X.; Wen, Z.; Lu, W.; Jiang, W. High-Intensity Interval Training Programs Versus Moderate-Intensity Continuous Training for Individuals with Heart Failure: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2025, 106, 98–112. [Google Scholar] [CrossRef]
- Eleyan, L.; Gonnah, A.R.; Farhad, I.; Labib, A.; Varia, A.; Eleyan, A.; Almehandi, A.; Al-Naseem, A.O.; Roberts, D.H. Exercise Training in Heart Failure: Current Evidence and Future Directions. J. Clin. Med. 2025, 14, 359. [Google Scholar] [CrossRef]
- Scarà, A.; Palamà, Z.; Robles, A.G.; Dei, L.-L.; Borrelli, A.; Zanin, F.; Pignalosa, L.; Romano, S.; Sciarra, L. Non-Pharmacological Treatment of Heart Failure—From Physical Activity to Electrical Therapies: A Literature Review. J. Cardiovasc. Dev. Dis. 2024, 11, 122. [Google Scholar] [CrossRef]
- Paluch, A.E.; Boyer, W.R.; Franklin, B.A.; Laddu, D.; Lobelo, F.; Lee, D.-C.; McDermott, M.M.; Swift, D.L.; Webel, A.R.; Lane, A. Resistance Exercise Training in Individuals with and Without Cardiovascular Disease: 2023 Update: A Scientific Statement from the American Heart Association. Circulation 2024, 149, E217–E231. [Google Scholar] [CrossRef]
- Gevaert, A.B.; Winzer, E.B.; Mueller, S.; De Schutter, S.; Beckers, P.J.; Hommel, J.; Linke, A.; Wisløff, U.; Adams, V.; Pieske, B.; et al. Training-induced change of diastolic function in heart failure with preserved ejection fraction. ESC Heart Fail. 2025, 12, 1652–1662, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Laoutaris, I.D. The ‘aerobic/resistance/inspiratory muscle training hypothesis in heart failure’. Eur. J. Prev. Cardiol. 2018, 25, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, A.d.C.M.; de Oliveira, L.Z.; Sbruzzi, G. Inspiratory Muscle Training in Patients with Heart Failure: What Is New? Systematic Review and Meta-Analysis. Phys. Ther. 2020, 100, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Cahalin, L.P.; Arena, R.A. Breathing exercises and inspiratory muscle training in heart failure. Heart Fail. Clin. 2015, 11, 149–172. [Google Scholar] [CrossRef]
- Laoutaris, I.D.; Piotrowicz, E.; Kallistratos, M.S.; Dritsas, A.; Dimaki, N.; Miliopoulos, D.; Andriopoulou, M.; Manolis, A.J.; Volterrani, M.; Piepoli, M.F.; et al. Combined aerobic/resistance/inspiratory muscle training as the ‘optimum’ exercise programme for patients with chronic heart failure: ARISTOS-HF randomized clinical trial. Eur. J. Prev. Cardiol. 2021, 28, 1626–1635. [Google Scholar] [CrossRef]
- Andrade, C.C.F.; Silva, R.T.; Brunherotti, M.A.d.A. Effects of Inspiratory Muscle Training in Patients with Class III and IV Heart Failure. Curr. Probl. Cardiol. 2022, 47, 101307. [Google Scholar] [CrossRef]
- Ahmad, A.M.; Hassan, M.H. Effects of Addition of Inspiratory Muscle Training to Exercise-Based Cardiac Rehabilitation on Inspiratory Muscle Strength, Peak Oxygen Consumption, and Selected Hemodynamics in Chronic Heart Failure. Acta Cardiol. Sin. 2022, 38, 485–494. [Google Scholar] [CrossRef]
- Yokota, J.; Takahashi, R.; Matsushima, K.; Suzuki, T.; Matsukawa, Y. Efficacy of Inspiratory Muscle Training in Patients with Acute Decompensated Heart Failure. Circ. Rep. 2024, 6, 430–440. [Google Scholar] [CrossRef]
- Tanriverdi, A.; Savci, S.; Kahraman, B.O.; Odaman, H.; Ozpelit, E.; Senturk, B.; Ozsoy, I.; Baran, A.; Akdeniz, B.; Acar, S.; et al. Effects of high intensity interval-based inspiratory muscle training in patients with heart failure: A single-blind randomized controlled trial. Heart Lung 2023, 62, 1–8. [Google Scholar] [CrossRef]
- Paneroni, M.; Pasini, E.; Comini, L.; Vitacca, M.; Schena, F.; Scalvini, S.; Venturelli, M. Skeletal Muscle Myopathy in Heart Failure: The Role of Ejection Fraction. Curr. Cardiol. Rep. 2018, 20, 116. [Google Scholar] [CrossRef]
- Kelley, R.C.; Ferreira, L.F. Diaphragm abnormalities in heart failure and aging: Mechanisms and integration of cardiovascular and respiratory pathophysiology. Heart Fail. Rev. 2017, 22, 191–207. [Google Scholar] [CrossRef]
- Bordoni, B.; Escher, A.R.; Escher, A.R. The Importance of Diaphragmatic Function in Neuromuscular Expression in Patients with Chronic Heart Failure. Cureus 2023, 15, e34629. [Google Scholar] [CrossRef] [PubMed]
- Mangner, N.; Garbade, J.; Heyne, E.; Berg, M.v.D.; Winzer, E.B.; Hommel, J.; Sandri, M.; Jozwiak-Nozdrzykowska, J.; Meyer, A.L.; Lehmann, S.; et al. Molecular Mechanisms of Diaphragm Myopathy in Humans with Severe Heart Failure. Circ. Res. 2021, 128, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Luiso, D.; Villanueva, J.A.; Belarte-Tornero, L.C.; Fort, A.; Blázquez-Bermejo, Z.; Ruiz, S.; Farré, R.; Rigau, J.; Martí-Almor, J.; Farré, N.; et al. Surface respiratory electromyography and dyspnea in acute heart failure patients. PLoS ONE 2020, 15, e0232225. [Google Scholar] [CrossRef] [PubMed]
- Scarlata, S.; Di Matteo, E.; Finamore, P.; Perri, G.; Mancini, D.; Sogaro, L.; Grandi, T.; Brando, E.; Travaglino, F.; Sambuco, F.; et al. Diaphragmatic ultrasound evaluation in acute heart failure: Clinical and functional associations. Intern. Emerg. Med. 2024, 19, 705–711. [Google Scholar] [CrossRef]
- Empinado, H.M.; Deevska, G.M.; Nikolova-Karakashian, M.; Yoo, J.; Christou, D.D.; Ferreira, L.F. Diaphragm dysfunction in heart failure is accompanied by increases in neutral sphingomyelinase activity and ceramide content. Eur. J. Heart Fail. 2014, 16, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, P.M.C.; Ribeiro, M.L.; Lourenco, B.N.; Villacorta, H.; Jorge, A.J.L.; Rocha, N.d.N.; Martins, W.d.A. Dyspnea and Heart Failure: The Role of the Diaphragm. Curr. Cardiol. Rev. 2025, 21, 1–8, Online ahead of print. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Yabe, H.; Sawano, K.; Tawara, Y.; Ohgi, S. Effects of exertional dyspnea on early mobilization of patients with acute decompensated heart failure. J. Phys. Ther. Sci. 2022, 34, 547–553. [Google Scholar] [CrossRef]
- Kagiyama, N.; Kamiya, K.; Toki, M.; Saito, H.; Iwata, K.; Matsue, Y.; Yoshioka, K.; Saito, K.; Kitai, T.; Maekawa, E. Prognostic Impact of Diaphragm Thickness in Geriatric Patients with Heart Failure: The SONIC-HF Multicenter Registry. JACC Cardiovasc. Imaging 2025, 18, 389–391. [Google Scholar] [CrossRef]
- Bordoni, B.; Escher, A.R. Functional evaluation of the diaphragm with a noninvasive test. J. Osteopath. Med. 2021, 121, 835–842. [Google Scholar] [CrossRef]
- Bordoni, B.; Marelli, F.; Morabito, B.; Sacconi, B.; Caiazzo, P.; Castagna, R. Low back pain and gastroesophageal reflux in patients with COPD: The disease in the breath. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 325–334. [Google Scholar] [CrossRef]
- Salah, H.M.; Goldberg, L.R.; Molinger, J.; Felker, G.M.; Applefeld, W.; Rassaf, T.; Tedford, R.J.; Mirro, M.; Cleland, J.G.; Fudim, M. Diaphragmatic Function in Cardiovascular Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 80, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, B.; Marelli, F.; Morabito, B.; Sacconi, B. Depression and anxiety in patients with chronic heart failure. Future Cardiol. 2018, 14, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, B.; Marelli, F.; Morabito, B.; Sacconi, B. Depression, anxiety and chronic pain in patients with chronic obstructive pulmonary disease: The influence of breath. Monaldi Arch. Chest Dis. 2017, 87, 811. [Google Scholar] [CrossRef][Green Version]
- Teloudi, A.; Anifanti, M.; Chatzinikolaou, K.; Grouios, G.; Hatzitaki, V.; Chouvarda, I.; Kouidi, E. Assessing Static Balance, Balance Confidence, and Fall Rate in Patients with Heart Failure and Preserved Ejection Fraction: A Comprehensive Analysis. Sensors 2024, 24, 6423. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Nahid, M.; Musse, M.; Chen, L.; Hilmer, S.N.; Zullo, A.; Kwak, M.J.; Lachs, M.; Levitan, E.B.; Safford, M.M.; et al. Prescribing patterns of fall risk-increasing drugs in older adults hospitalized for heart failure. BMC Cardiovasc. Disord. 2023, 23, 372. [Google Scholar] [CrossRef]
- McDonagh, J.; Ferguson, C.; Hilmer, S.N.; Hubbard, R.E.; Lindley, R.I.; Driscoll, A.; Maiorana, A.; Wu, L.; Atherton, J.J.; Bajorek, B.V.; et al. An Expert Opinion on the Management of Frailty in Heart Failure from the Australian Cardiovascular Alliance National Taskforce. Heart Lung Circ. 2025, 34, 693–703. [Google Scholar] [CrossRef]
- Bordoni, B.; Escher, A.R. Motor Dysfunctions in Fibromyalgia Patients: The Importance of Breathing. Open Access Rheumatol. Res. Rev. 2024, 16, 55–66. [Google Scholar] [CrossRef]
- Bordoni, B.; Kotha, R.; Escher, A.R. Symptoms Arising from the Diaphragm Muscle: Function and Dysfunction. Cureus 2024, 16, e53143. [Google Scholar] [CrossRef]
- Bordoni, B.; Escher, A.; Compalati, E.; Mapelli, L.; Toccafondi, A. The Importance of the Diaphragm in Neuromotor Function in the Patient with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 837–848. [Google Scholar] [CrossRef]
- Bordoni, B.; Zanier, E. Anatomic connections of the diaphragm influence of respiration on the body system. J. Multidiscip. Healthc. 2013, 6, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; O Suwarno, N.; A Dempsey, J. Influence of lung volume on sympathetic nerve discharge in normal humans. Circ. Res. 1990, 67, 130–141. [Google Scholar] [CrossRef]
- Leahy, M.G.; Busch, S.A.; Thrall, S.F.; Hillen, S.J.; Sheel, A.W.; Foster, G.E. Reflex sympathetic activation to inspiratory muscle loading is attenuated in females relative to males. Am. J. Physiol. Circ. Physiol. 2024, 327, H28–H37. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, L.M.; Edgett, B.A.; Gray, S.; Al-Mufty, S.; Huber, J.S.; Brunt, K.R.; Simpson, J.A. A new approach to improve the hemodynamic assessment of cardiac function independent of respiratory influence. Sci. Rep. 2021, 11, 17223. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, F.V.; Gavin, J.P.; Wainwright, T.; McConnell, A. The effects of 8 weeks of inspiratory muscle training on the balance of healthy older adults: A randomized, double-blind, placebo-controlled study. Physiol. Rep. 2019, 7, e14076. [Google Scholar] [CrossRef]
- Mello, E.S.d.F.; Oliveira, A.L.M.B.; Santanna, T.D.C.; Soares, P.P.d.S.; Rodrigues, G.D. Updates in inspiratory muscle training for older adults: A systematic review. Arch. Gerontol. Geriatr. 2024, 127, 105579. [Google Scholar] [CrossRef]
- Sheraz, S.; Ferraro, F.V.; Siddiqui, F.A.; Tariq, H.; Faghy, M.A.; Malik, A.N. The effects of inspiratory muscle training on balance and functional mobility: A systematic review. Postgrad. Med. 2023, 135, 690–700. [Google Scholar] [CrossRef]
- Bosnak-Guclu, M.; Arikan, H.; Savci, S.; Inal-Ince, D.; Tulumen, E.; Aytemir, K.; Tokgözoglu, L. Effects of inspiratory muscle training in patients with heart failure. Respir. Med. 2011, 105, 1671–1681. [Google Scholar] [CrossRef]
- Denfeld, Q.E.; Goodlin, S.; Abedalweli, R.; Davis, M.R.; Hiatt, S.O.; Lee, C.S.; Winters-Stone, K. Frequency and Predictors of Falls Among Adults with Heart Failure: A Prospective Study. J. Card. Fail. 2022, 29, 414–418. [Google Scholar] [CrossRef]
- Friend, T.H.; Thomas, H.M.; Ordoobadi, A.J.; Bain, P.A.; Jarman, M.P. Community emergency medical services approaches to fall prevention: A systematic review. Inj. Prev. 2024, 30, 446–453. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Simonsick, E.; Starkweather, A.; Chen, M.-H.; McCauley, P.; Chyun, D.; Cong, X. Back Pain and Heart Failure in Community-Dwelling Older Adults: Findings from the Health ABC Study. Geriatr. Nurs. 2021, 42, 643–649. [Google Scholar] [CrossRef]
- Marshall, L.M.; Litwack-Harrison, S.; Cawthon, P.M.; Kado, D.M.; Deyo, R.A.; Makris, U.E.; Carlson, H.L.; Nevitt, M.C. A Prospective Study of Back Pain and Risk of Falls Among Older Community-dwelling Women. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Bunphrom, W.; Chatprem, T.; Puntumetakul, R.; Siritaratiwat, W.; Phimphasak, C.; Leungbootnak, A.; Boucaut, R. Diaphragm excursion and thickness in patients with chronic low back pain with and without lumbar instability. Sci. Rep. 2025, 15, 9353. [Google Scholar] [CrossRef]
- Ahmadnezhad, L.; Yalfani, A.; Borujeni, B.G. Inspiratory Muscle Training in Rehabilitation of Low Back Pain: A Randomized Controlled Trial. J. Sport Rehabil. 2020, 29, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Fabero-Garrido, R.; Rodríguez-Marcos, I.; del Corral, T.; Plaza-Manzano, G.; López-De-Uralde-Villanueva, I. Effects of Respiratory Muscle Training on Functional Ability, Pain-Related Outcomes, and Respiratory Function in Individuals with Low Back Pain: Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3053. [Google Scholar] [CrossRef] [PubMed]
- Janssens, L.; Mcconnell, A.K.; Pijnenburg, M.; Claeys, K.; Goossens, N.; Lysens, R.; Troosters, T.; Brumagne, S. Inspiratory muscle training affects proprioceptive use and low back pain. Med. Sci. Sports Exerc. 2015, 47, 12–19. [Google Scholar] [CrossRef]
- Farghaly, A.; Fitzsimons, D.; Bradley, J.; Sedhom, M.; Atef, H. The Need for Breathing Training Techniques: The Elephant in the Heart Failure Cardiac Rehabilitation Room: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 14694. [Google Scholar] [CrossRef]
- Alemzadeh-Ansari, M.J.; Ansari-Ramandi, M.M.; Naderi, N. Chronic Pain in Chronic Heart Failure: A Review Article. J. Tehran Heart Cent. 2017, 12, 49–56. [Google Scholar]
- Godfrey, C.; Harrison, M.B.; Medves, J.; Tranmer, J.E. The symptom of pain with heart failure: A systematic review. J. Card. Fail. 2006, 12, 307–313. [Google Scholar] [CrossRef]
- Smith, A.B.; Jung, M.; Pressler, S.J.; Mocci, E.; Dorsey, S.G. Differential Gene Expression Among Patients with Heart Failure Experiencing Pain. Nurs. Res. 2023, 72, 175–184. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Y.; Li, Y.; Zheng, S.; Yang, S. Diaphragm dysfunction is found in patients with chronic painful temporomandibular disorder: A case-control study. Heliyon 2024, 10, e32872. [Google Scholar] [CrossRef]
- Colak, G.Y.; Ozyurek, S.; Sengul, Y.S.; Kalemci, O. Differences of diaphragmatic muscle contraction between female patients with chronic neck pain and asymptomatic controls: A case-control study based on ultrasonography. Musculoskelet. Sci. Pract. 2024, 69, 102894. [Google Scholar] [CrossRef] [PubMed]
- Melo, T.M.; Cunha, F.L.L.; Bezerra, L.M.R.; Salemi, M.; de Albuquerque, V.A.; de Alencar, G.G.; de Siqueira, G.R. Abdominal and Diaphragmatic Mobility in Adults with Chronic Gastritis: A Cross-Sectional Study. J. Chiropr. Med. 2023, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Busch, V.; Magerl, W.; Kern, U.; Haas, J.; Hajak, G.; Eichhammer, P. The effect of deep and slow breathing on pain perception, autonomic activity, and mood processing--an experimental study. Pain Med. 2012, 13, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Zunhammer, M.; Eichhammer, P.; Busch, V. Do cardiorespiratory variables predict the antinociceptive effects of deep and slow breathing? Pain Med. 2013, 14, 843–854. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Liu, X.-L.; Wang, T.; Tan, J.-Y.; Huang, H. Breathing Exercises for Pain Management in Cancer Survivors: A Systematic Review. Pain Manag. Nurs. 2022, 24, 299–310. [Google Scholar] [CrossRef]
- Jarrah, M.I.; Hweidi, I.M.; Al-Dolat, S.A.; Alhawatmeh, H.N.; Al-Obeisat, S.M.; Hweidi, L.I.; Hweidi, A.I.; Alkouri, O.A. The effect of slow deep breathing relaxation exercise on pain levels during and post chest tube removal after coronary artery bypass graft surgery. Int. J. Nurs. Sci. 2022, 9, 155–161. [Google Scholar] [CrossRef]
- Venezia, A.; Fawsitt-Jones, H.; Hohenschurz-Schmidt, D.; Mancini, M.; Howard, M.; Makovac, E. Investigating the effects of artificial baroreflex stimulation on pain perception: A comparative study in no-pain and chronic low back pain individuals. J. Physiol. 2024, 602, 6941–6957. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, L.; Porta, C.; Spicuzza, L.; Bellwon, J.; Spadacini, G.; Frey, A.W.; Yeung, L.Y.; Sanderson, J.E.; Pedretti, R.; Tramarin, R. Slow Breathing Increases Arterial Baroreflex Sensitivity in Patients with Chronic Heart Failure. Circulation 2002, 105, 143–145. [Google Scholar] [CrossRef]
- Steffen, P.R.; Austin, T.; DeBarros, A.; Brown, T. The Impact of Resonance Frequency Breathing on Measures of Heart Rate Variability, Blood Pressure, and Mood. Front. Public Health 2017, 5, 222. [Google Scholar] [CrossRef] [PubMed]
- Craighead, D.H.; Freeberg, K.A.; Heinbockel, T.C.; Rossman, M.J.; Jackman, R.A.; Mccarty, N.P.; Jankowski, L.R.; Nemkov, T.; Reisz, J.A.; D’aLessandro, A.; et al. Time-Efficient, High-Resistance Inspiratory Muscle Strength Training Increases Exercise Tolerance in Midlife and Older Adults. Med. Sci. Sports Exerc. 2024, 56, 266–276. [Google Scholar] [CrossRef]
- Ladriñán-Maestro, A.; Sánchez-Infante, J.; Martín-Vera, D.; Sánchez-Sierra, A. Influence of an inspiratory muscle fatigue protocol on older adults on respiratory muscle strength, muscle oxygen saturation, and functional capacity: A randomized controlled trial. BMC Geriatr. 2024, 24, 1015. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Maruyama, H.; Kato, M.; Uchida, M.; Kubo, A. Characteristics of respiratory muscle fatigue upon inhalation resistance with a maximal inspiratory mouth pressure of 50%. J. Phys. Ther. Sci. 2019, 31, 318–325. [Google Scholar] [CrossRef][Green Version]
- Peters, C.M.; Welch, J.F.; Dominelli, P.B.; Molgat-Seon, Y.; Romer, L.M.; McKenzie, D.C.; Sheel, A.W. Influence of inspiratory resistive loading on expiratory muscle fatigue in healthy humans. Exp. Physiol. 2017, 102, 1221–1233. [Google Scholar] [CrossRef]
- Welch, J.F.; Archiza, B.; Guenette, J.A.; West, C.R.; Sheel, A.W. Effect of diaphragm fatigue on subsequent exercise tolerance in healthy men and women. J. Appl. Physiol. (1985) 2018, 125, 1987–1996. [Google Scholar] [CrossRef]
- Ratamess, N.A.; Alvar, B.A.; Evetoch, T.E.; Housh, T.J.; Ben Kibler, W.; Kraemer, W.J.; Triplett, N.T. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Taylor, R.S.; Dalal, H.M.; McDonagh, S.T.J. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat. Rev. Cardiol. 2022, 19, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Mirzai, S.; Sandesara, U.; Haykowsky, M.J.; Brubaker, P.H.; Kitzman, D.W.; Peters, A.E. Aerobic, resistance, and specialized exercise training in heart failure with preserved ejection fraction: A state-of-the-art review. Heart Fail. Rev. 2025, 30, 1015–1034, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Sagris, M.; Koufakis, T.; Vlachakis, P.K.; Rangraze, I.R.; El Tanani, M.; Tsioufis, K.; et al. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists in the Management of Obesity-Related Heart Failure with Preserved Ejection Fraction: Benefits beyond What Scales Can Measure? Biomedicines 2024, 12, 2112. [Google Scholar] [CrossRef] [PubMed]
| Benefits of Physical Activity in Patients with Heart Failure |
|---|
| Combining ET and RT improves VO2max by about 4–16% and chronotropic incompetence; a 6% increase in VO2max reduces all-cause mortality and hospitalization in patients with HF by 5–8% [6,25,27,32]. |
| Constant training reduces the activity of the sympathetic system and improves the antioxidant capacity of the organism; reduces systemic inflammatory values; improves endothelial structure and function; improves the oxygen extraction capacity of the periphery (skeletal muscles) at rest and under stress; leads to greater cardiac electrical stability; and stimulates the coronary collateral network [3,6,24,32]. |
| Glycemic levels improve and are better controlled [24]. |
| Some cardiac contractility parameters improve, mainly in patients with HFpEF [32]. |
| Organizations and Guidelines | Indications on Diaphragm Training (IMT) | Reference |
|---|---|---|
| American guidelines (AHA/ACC/HFSA) | No recommended procedure to improve the functional capacity of the respiratory muscles | Ref. [21] |
| ESC guidelines | Indications are specific to HFrEF. A resistance of 30% of the patient’s PImax, reaching up to 60% of the patient’s subjective PImax. The resistance must be recalibrated approximately every week, with 3–5 sessions per week and for 20–30 min per session. | Ref. [28] |
| HF Patient Adaptations When IMT is Present in Rehabilitation | References |
|---|---|
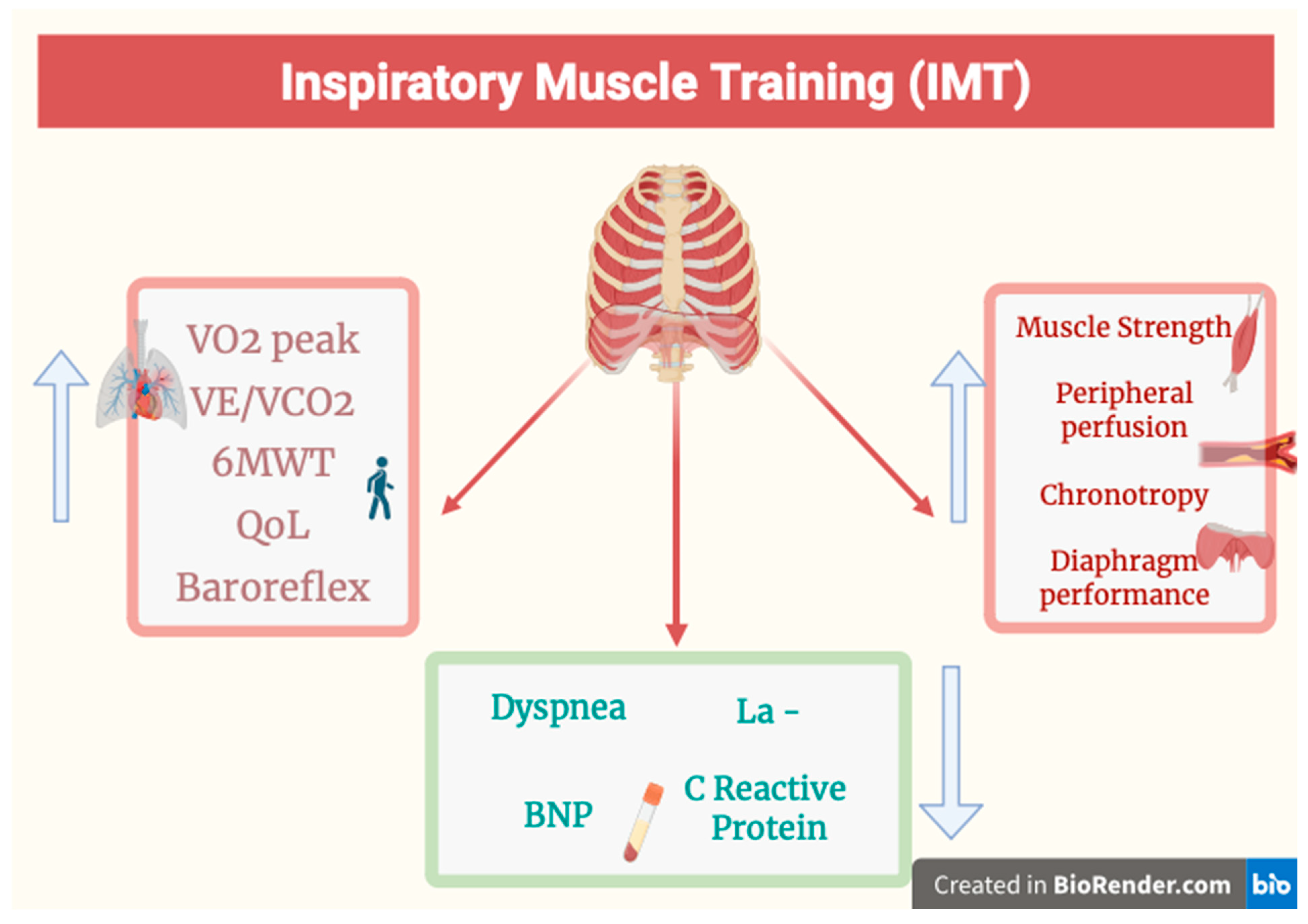
| IMT increases PImax, VO2peak, and the minute ventilation/carbon dioxide production (VE/VCO2) slope; reduces the sense of dyspnea; increases the distance covered in the 6-min walk test (in patients with HFpEF); improves their quality of life; reduces lactate levels; improves ventilation efficiency, especially when combined with HIIT; improves the baroreceptor reflex, with an increase in peripheral perfusion phenomena when exerting effort, consequently reducing peripheral chemoreceptor responses; and increases lower limb muscle strength. IMT combined with ET reduces the values of NT-pro-BNP and C-reactive protein | [3,5,7,24,26,27,36] |
| Improvements in chronotropic, diastolic, and systolic blood pressure and saturation; increased walking distance; and increased inspiratory force, probably due to a more optimal modulation of the autonomic system (in patients with HFrEF) | [39] |
| Improves quality of life (in patients with HFrEF) | [40] |
| In acute hospitalized patients (HFrEF), IMT increases functionality, measured in terms of the distance covered in the 2-min walking test in meters | [41] |
| IMT for HFrEF induces several functional improvements, such as a more balanced autonomic expression, less dyspnea, increased diaphragm thickness, and better arterial response (such as tone and vasodilation) | [42] |
| Rationale for the Use of IMT: Counteracting Pathological Changes |
|---|
| The respiratory muscles are hypotrophic with a metabolic/phenotypic change towards a greater number of anaerobic (poorly functioning) fibers and reduced capillarization, which leads to a more anaerobic environment [43]. |
| Peripheral information from skeletal musculature will be recorded by the central nervous system as constant fatigue, with an overexcitation of the sympathetic system, vasoconstriction, a decreased VO2peak, an increased heart rate, and intolerance to effort [43]. |
| There seems to be a degeneration of the synaptic plate with partial denervation and a reduction in the force expression of 15–30%; in animal models, the contraction speed is reduced by 20–30%, which leads to a decrease in peak force (for example, when coughing or under stress) of 35–50% [44]. |
| There is a decline in the number of cross-bridges, titin (shock absorber protein), myosin heavy chain (MHC), and the movement capacity of the same protein, with a decrease in myosin ATPase activity [44]. |
| There seems to be an increase in red fibers compared to anaerobic fibers (with the opposite tendency in the limbs) [46]. |
| There is an increase in connective and adipose tissue within the diaphragm, which is a sign of atrophy, with a decline in MIP (maximal inspiratory pressure) of about 30% [45]. |
| The diaphragm moves with a reduced excursion and with an inverse relationship between the severity of symptoms and contractile capacity. The diaphragm presents with a decreased thickness [48]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordoni, B.; Morabito, B.; Myftari, V.; D’Amato, A.; Severino, P. Chronic Heart Failure Rehabilitation: Diaphragm Training Needs More Attention. J. Clin. Med. 2025, 14, 5624. https://doi.org/10.3390/jcm14165624
Bordoni B, Morabito B, Myftari V, D’Amato A, Severino P. Chronic Heart Failure Rehabilitation: Diaphragm Training Needs More Attention. Journal of Clinical Medicine. 2025; 14(16):5624. https://doi.org/10.3390/jcm14165624
Chicago/Turabian StyleBordoni, Bruno, Bruno Morabito, Vincenzo Myftari, Andrea D’Amato, and Paolo Severino. 2025. "Chronic Heart Failure Rehabilitation: Diaphragm Training Needs More Attention" Journal of Clinical Medicine 14, no. 16: 5624. https://doi.org/10.3390/jcm14165624
APA StyleBordoni, B., Morabito, B., Myftari, V., D’Amato, A., & Severino, P. (2025). Chronic Heart Failure Rehabilitation: Diaphragm Training Needs More Attention. Journal of Clinical Medicine, 14(16), 5624. https://doi.org/10.3390/jcm14165624






