Evaluation of Suburethral Tissue Elasticity Using Strain Elastography in Women with Stress Urinary Incontinence
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Ultrasound Examination
2.3. Introital Sonography
2.4. Strain Elastography Protocol
2.5. Regions of Interest (ROIs) Placement
2.5.1. Yellow ROI: Adipose Layer (AL)
2.5.2. Blue ROI: Internal Urethral Orifice (IUO) Level
2.5.3. Purple ROI: Midurethral (MU) Level
2.5.4. Green ROI: External Urethral Orifice (EUO) Level
2.6. Statistical Evaluation
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Strain Elastography Measurements
3.3. Reproducibility of Strain Elastography Measurements
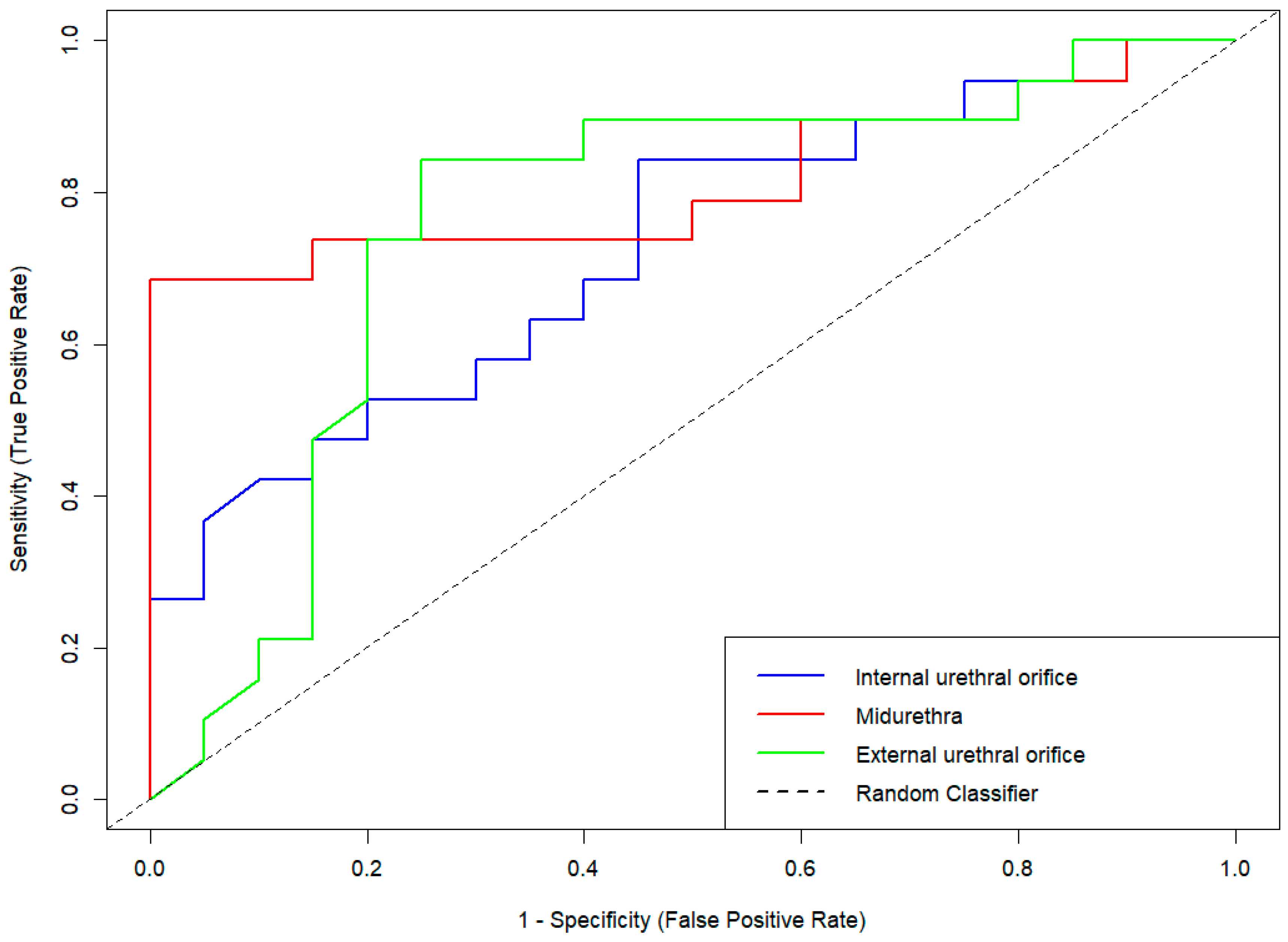
3.4. Diagnostic Performance of Strain Elastography in Stress Urinary Incontinence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| AL | adipose layer |
| AUC | area under the curve |
| BMI | body mass index |
| EUO | external urethral orifice |
| FN | false negative |
| FP | false positive |
| GE | General Electric |
| ICC | intraclass correlation coefficient |
| IUS | internal urethral sphincter |
| IUO | internal urethral orifice |
| LA | levator ani |
| MANOVA | multivariate analysis of variance |
| Meas | measurement |
| MRI | magnetic resonance imaging |
| MU | midurethra |
| N | number of participants |
| NPV | negative predictive values |
| NS | not significant |
| PB | perineal body |
| PPV | positive predictive values |
| ROI | region of interest |
| ROC | receiver operating characteristic |
| SD | standard deviation |
| SE | strain elastography |
| SUI | stress urinary incontinence |
| TN | true negative |
| TP | true positive |
| UI | urinary incontinence |
| US | ultrasound |
References
- DeLancey, J.O.L. Structural Support of the Urethra as It Relates to Stress Urinary Incontinence: The Hammock Hypothesis. Am. J. Obstet. Gynecol. 1994, 170, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Haylen, B.T.; De Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Floor Dysfunction. Int. Urogynecol. J. 2010, 21, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.J.; Godecker, A.L.; Giles, D.L.; Brown, H.W. Updated Prevalence of Urinary Incontinence in Women: 2015–2018 National Population-Based Survey Data. Female Pelvic. Med. Reconstr. Surg. 2022, 28, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, L.; Liu, M.; Shen, H.; Luo, D. Diagnostic Value of Transperineal Ultrasound in Patients with Stress Urinary Incontinence (SUI): A Systematic Review and Meta-Analysis. World J. Urol. 2023, 41, 687–693. [Google Scholar] [CrossRef]
- Falconer, C.; Ekman-Ordeberg, G.; Blomgren, B.; Johansson, O.; Ulmsten, U.; Westergren-Thorsson, G.; Malmström, A. Paraurethral Connective Tissue in Stress-Incontinent Women after Menopause. Acta Obstet. Gynecol. Scand. 1998, 77, 95–100. [Google Scholar] [CrossRef]
- Hannestad, Y.S.; Rortveit, G.; Sandvik, H.; Hunskaar, S. A Community-Based Epidemiological Survey of Female Urinary Incontinence: The Norwegian EPINCONT Study. J. Clin. Epidemiol. 2000, 53, 1150–1157. [Google Scholar] [CrossRef]
- Zhong, C.; Hu, P.; Ran, S.; Tang, J.; Xiao, C.; Lin, Y.; Zhang, X.; Rong, Y.; Liu, M. Association Between Urinary Stress Incontinence and Levator Avulsion Detected by 3D Transperineal Ultrasound. Ultraschall Med. 2023, 44, e39–e46. [Google Scholar] [CrossRef]
- Hinata, N.; Murakami, G.; Abe, S.; Honda, M.; Isoyama, T.; Sejima, T.; Takenaka, A. Detailed Histological Investigation of the Female Urethra: Application to Radical Cystectomy. J. Urol. 2012, 187, 451–456. [Google Scholar] [CrossRef]
- Chen, Y.; DeSautel, M.; Anderson, A.; Badlani, G.; Kushner, L. Collagen Synthesis Is Not Altered in Women with Stress Urinary Incontinence. Neurourol. Urodyn. 2004, 23, 367–373. [Google Scholar] [CrossRef]
- Song, Y.; Hong, X.; Yu, Y.; Lin, Y. Changes of Collagen Type III and Decorin in Paraurethral Connective Tissue from Women with Stress Urinary Incontinence and Prolapse. Int. Urogynecol. J. 2007, 18, 1459–1463. [Google Scholar] [CrossRef]
- Trabucco, E.; Soderberg, M.; Cobellis, L.; Torella, M.; Bystrom, B.; Ekman-Ordeberg, G.; Petraglia, F.; Colacurci, N. Role of Proteoglycans in the Organization of Periurethral Connective Tissue in Women with Stress Urinary Incontinence. Maturitas 2007, 58, 395–405. [Google Scholar] [CrossRef]
- Bamber, J.; Cosgrove, D.; Dietrich, C.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.-M.; D’Onofrio, M.; Drakonaki, E.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography. Part 1: Basic Principles and Technology. Ultraschall Med. 2013, 34, 169–184. [Google Scholar] [CrossRef]
- Tunn, R.; Petri, E. Introital and Transvaginal Ultrasound as the Main Tool in the Assessment of Urogenital and Pelvic Floor Dysfunction: An Imaging Panel and Practical Approach. Ultrasound Obs. Gynecol. 2003, 22, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Macura, K.J. Magnetic Resonance Imaging in Assessment of Stress Urinary Incontinence in Women: Parameters Differentiating Urethral Hypermobility and Intrinsic Sphincter Deficiency. World J. Radiol. 2015, 7, 394. [Google Scholar] [CrossRef]
- DeLancey, J.O.L.; Masteling, M.; Pipitone, F.; LaCross, J.; Mastrovito, S.; Ashton-Miller, J.A. Pelvic Floor Injury during Vaginal Birth Is Life-Altering and Preventable: What Can We Do about It? Am. J. Obstet. Gynecol. 2024, 230, 279–294.e2. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.; Barr, R.; Farrokh, A.; Dighe, M.; Hocke, M.; Jenssen, C.; Dong, Y.; Saftoiu, A.; Havre, R. Strain Elastography—How To Do It? Ultrasound Int. Open 2017, 3, E137–E149. [Google Scholar] [CrossRef] [PubMed]
- Kreutzkamp, J.M.; Schäfer, S.D.; Amler, S.; Strube, F.; Kiesel, L.; Schmitz, R. Strain Elastography as a New Method for Assessing Pelvic Floor Biomechanics. Ultrasound Med. Biol. 2017, 43, 868–872. [Google Scholar] [CrossRef]
- Ciraci, S.; Tan, S.; Ozcan, A.S.; Aslan, A.; Keskin, H.L.; Ates, O.F.; Akcay, Y.; Arslan, H. Contribution of Real-Time Elastography in Diagnosis of Polycystic Ovary Syndrome. Diagn. Interv. Radiol. 2015, 21, 118–122. [Google Scholar] [CrossRef]
- Szabó, G.; Madár, I.; Rigó, J.R.; Dobó, N.; Ács, N.; Bokor, A. A Novel Complementary Method for Ultrasonographic Screening of Deep Endometriosis: A Case Series of 5 Patients Diagnosed with Transvaginal Strain Elastography. Clin. Exp. Obstet. Gynecol. 2022, 49, 1. [Google Scholar] [CrossRef]
- Dewilde, K.; Vanthienen, M.; Van Schoubroeck, D.; Froyman, W.; Timmerman, D.; Van Den Bosch, T. Elastography in Ultrasound Assessment of the Uterus. J. Endometr. Uterine Disord. 2023, 1, 100014. [Google Scholar] [CrossRef]
- D’Ancona, C.; Haylen, B.; Oelke, M.; Abranches-Monteiro, L.; Arnold, E.; Goldman, H.; Hamid, R.; Homma, Y.; Marcelissen, T.; Rademakers, K.; et al. The International Continence Society (ICS) Report on the Terminology for Adult Male Lower Urinary Tract and Pelvic Floor Symptoms and Dysfunction. Neurourol. Urodyn. 2019, 38, 433–477. [Google Scholar] [CrossRef]
- Kang, H. Sample Size Determination and Power Analysis Using the G*Power Software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, A.K.; Arlandis, S.; Bø, K.; Cobussen-Boekhorst, H.; Costantini, E.; De Heide, M.; Farag, F.; Groen, J.; Karavitakis, M.; Lapitan, M.C.; et al. European Association of Urology Guidelines on the Diagnosis and Management of Female Non-Neurogenic Lower Urinary Tract Symptoms. Part 1: Diagnostics, Overactive Bladder, Stress Urinary Incontinence, and Mixed Urinary Incontinence. Eur. Urol. 2022, 82, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.P.; Pandya, P.R.; Nguyen, M.-L.; Fashokun, T.; Macura, K.J. Use of Dynamic MRI of the Pelvic Floor in the Assessment of Anterior Compartment Disorders. Curr. Urol. Rep. 2018, 19, 112. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Ramos, J.G.L.; Martins-Costa, S. Translabial Ultrasonography in the Assessment of Urethral Diameter and Intrinsic Urethral Sphincter Deficiency. J. Ultrasound Med. 2006, 25, 1153–1158. [Google Scholar] [CrossRef]
- Ophir, J.; Céspedes, I.; Ponnekanti, H.; Yazdi, Y.; Li, X. Elastography: A Quantitative Method for Imaging the Elasticity of Biological Tissues. Ultrason. Imaging 1991, 13, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Stoelinga, B.; Hehenkamp, W.J.K.; Brölmann, H.A.M.; Huirne, J.A.F. Real-time Elastography for Assessment of Uterine Disorders. Ultrasound Obs. Gynecol. 2014, 43, 218–226. [Google Scholar] [CrossRef]
- De Landsheere, L.; Munaut, C.; Nusgens, B.; Maillard, C.; Rubod, C.; Nisolle, M.; Cosson, M.; Foidart, J.-M. Histology of the Vaginal Wall in Women with Pelvic Organ Prolapse: A Literature Review. Int. Urogynecol. J. 2013, 24, 2011–2020. [Google Scholar] [CrossRef]
- Mazloomdoost, D.; Westermann, L.B.; Mutema, G.; Crisp, C.C.; Kleeman, S.D.; Pauls, R.N. Histologic Anatomy of the Anterior Vagina and Urethra. Female Pelvic Med. Reconstr. Surg. 2017, 23, 329–335. [Google Scholar] [CrossRef]
- Jung, J.; Ahn, H.K.; Huh, Y. Clinical and Functional Anatomy of the Urethral Sphincter. Int. Neurourol. J. 2012, 16, 102. [Google Scholar] [CrossRef]
- Ashton-Miller, J.A.; De Lancey, J.O.L. Functional Anatomy of the Female Pelvic Floor. Ann. N. Y. Acad. Sci. 2007, 1101, 266–296. [Google Scholar] [CrossRef]
- Pomian, A.; Majkusiak, W.; Kociszewski, J.; Tomasik, P.; Horosz, E.; Zwierzchowska, A.; Lisik, W.; Barcz, E. Demographic Features of Female Urethra Length. Neurourol. Urodyn. 2018, 37, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Tierney, L. The R Statistical Computing Environment. In Statistical Challenges in Modern Astronomy V; Lecture Notes in Statistics; Springer: New York, NY, USA, 2012; Volume 209, pp. 435–447. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.-J.; Zhou, J.-Q.; Jia, X.-H.; Li, S.; Zhan, W.-W. Similar Reproducibility for Strain and Shear Wave Elastography in Breast Mass Evaluation: A Prospective Study Using the Same Ultrasound System. Ultrasound Med. Biol. 2020, 46, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Madarász, B.; Jakab, G.; Szalai, Z.; Juhos, K.; Kotroczó, Z.; Tóth, A.; Ladányi, M. Long-Term Effects of Conservation Tillage on Soil Erosion in Central Europe: A Random Forest-Based Approach. Soil Tillage Res. 2021, 209, 104959. [Google Scholar] [CrossRef]
- De Vicari, D.; Barba, M.; Costa, C.; Cola, A.; Frigerio, M. Assessment of Urethral Elasticity by Shear Wave Elastography: A Novel Parameter Bridging a Gap Between Hypermobility and ISD in Female Stress Urinary Incontinence. Bioengineering 2025, 12, 373. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Li, Y.; Jiang, Y.; Zhao, C.; Fang, S.; Yang, Z.; Sun, L. Assessment of Perineal Body Properties in Women with Stress Urinary Incontinence Using Transperineal Shear Wave Elastography. Sci. Rep. 2024, 14, 21647. [Google Scholar] [CrossRef]


| Variable | SUI ** (n = 20) | Continent Controls (n = 20) | p-Value | ||
|---|---|---|---|---|---|
| Age (years) | 57.50 ± 12.60 | 54.15 ± 12.88 | NS | ||
| Weight (kg) | 82.95 ± 17.46 | 71.40 ± 16.72 | 0.03 | ||
| BMI (kg/m2) | 30.30 ± 5.02 | 26.56 ± 6.16 | 0.02 | ||
| Number of pregnancies | 2.30 ± 1.08 | 2.05 ± 1.36 | NS | ||
| Number of deliveries | 2.05 ± 0.94 | 1.60 ± 0.88 | NS | ||
| Urethral length (mm) | 3.17 ± 0.60 | 3.00 ± 0.46 | NS | ||
| Postmenopausal status, n (%) | 15 (no) | 75.0 (yes) | 11 (no) | 55.0 (yes) | NS |
| Group | Internal Urethral Orifice (IUO) | Midurethra (MU) | External Urethral Orifice (EUO) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ICC | 95% CI | p-Value | ICC | 95% CI | p-Value | ICC | 95% CI | p-Value | |
| Continent controls | 0.96 | 0.94–0.98 | <0.001 | 0.98 | 0.96–0.99 | <0.001 | 0.97 | 0.95–0.99 | <0.001 |
| SUI ** | 0.96 | 0.92–0.98 | <0.002 | 0.95 | 0.913–0.98 | <0.002 | 0.99 | 0.98–0.99 | <0.002 |
| Categories | IUO | MU | EUO |
|---|---|---|---|
| Sensitivity or Recall | 0.58 | 0.65 | 0.60 |
| Specificity | 0.70 | 0.85 | 0.80 |
| Positive Predictive Value | 0.65 | 0.81 | 0.75 |
| Negative Predictive Value | 0.64 | 0.71 | 0.67 |
| Prevalence | 0.49 | 0.50 | 0.50 |
| Detection Rate | 0.28 | 0.33 | 0.30 |
| Detection Prevalence | 0.44 | 0.40 | 0.40 |
| Balanced Accuracy | 0.64 | 0.75 | 0.70 |
| Area Under the ROC Curve | 0.72 | 0.81 | 0.76 |
| F1 score | 0.61 | 0.72 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csákány, L.; Kozinszky, Z.; Kovács, F.; Krajczár, S.K.; Várbíró, S.; Keresztúri, A.; Németh, G.; Surányi, A.; Pásztor, N. Evaluation of Suburethral Tissue Elasticity Using Strain Elastography in Women with Stress Urinary Incontinence. J. Clin. Med. 2025, 14, 5617. https://doi.org/10.3390/jcm14165617
Csákány L, Kozinszky Z, Kovács F, Krajczár SK, Várbíró S, Keresztúri A, Németh G, Surányi A, Pásztor N. Evaluation of Suburethral Tissue Elasticity Using Strain Elastography in Women with Stress Urinary Incontinence. Journal of Clinical Medicine. 2025; 14(16):5617. https://doi.org/10.3390/jcm14165617
Chicago/Turabian StyleCsákány, Lóránt, Zoltan Kozinszky, Flórián Kovács, Seron Kíra Krajczár, Szabolcs Várbíró, Attila Keresztúri, Gábor Németh, Andrea Surányi, and Norbert Pásztor. 2025. "Evaluation of Suburethral Tissue Elasticity Using Strain Elastography in Women with Stress Urinary Incontinence" Journal of Clinical Medicine 14, no. 16: 5617. https://doi.org/10.3390/jcm14165617
APA StyleCsákány, L., Kozinszky, Z., Kovács, F., Krajczár, S. K., Várbíró, S., Keresztúri, A., Németh, G., Surányi, A., & Pásztor, N. (2025). Evaluation of Suburethral Tissue Elasticity Using Strain Elastography in Women with Stress Urinary Incontinence. Journal of Clinical Medicine, 14(16), 5617. https://doi.org/10.3390/jcm14165617






