10-Year Long-Term Outcomes of Robotic-Assisted Segmentectomy for Early-Stage Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Preoperative Assessment
2.2. Surgical Procedures
2.3. Postoperative Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Short-Term Outcomes
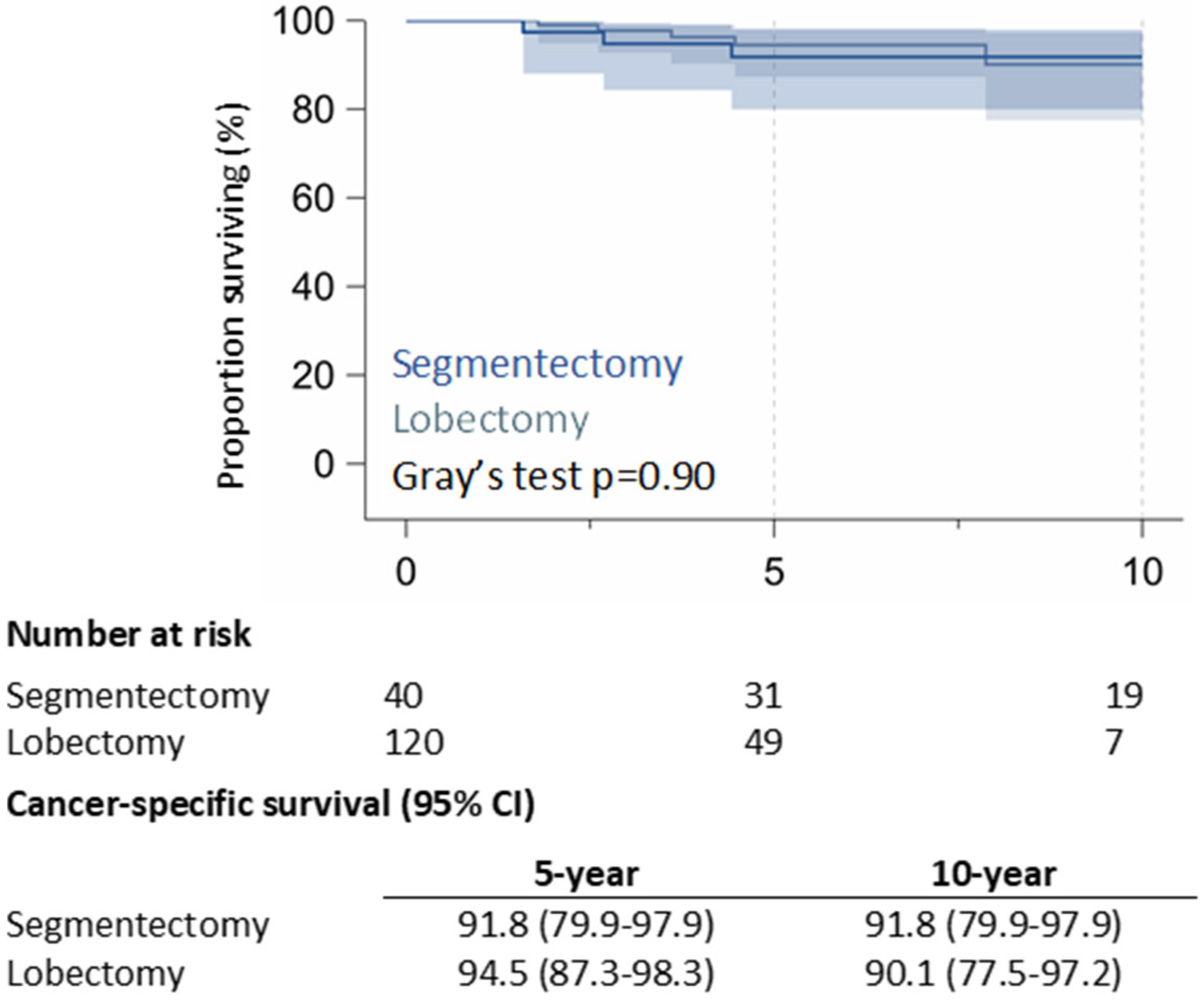
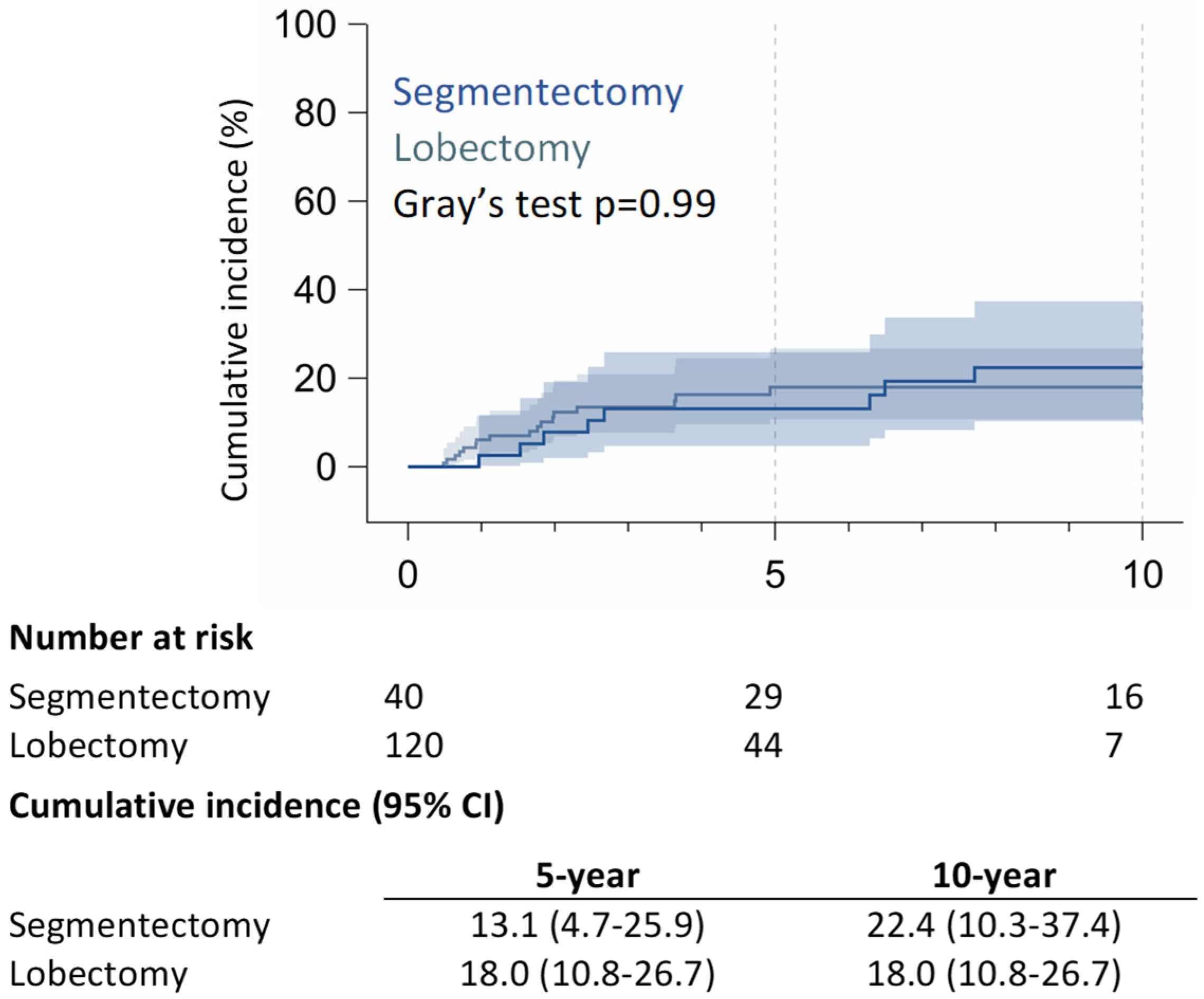
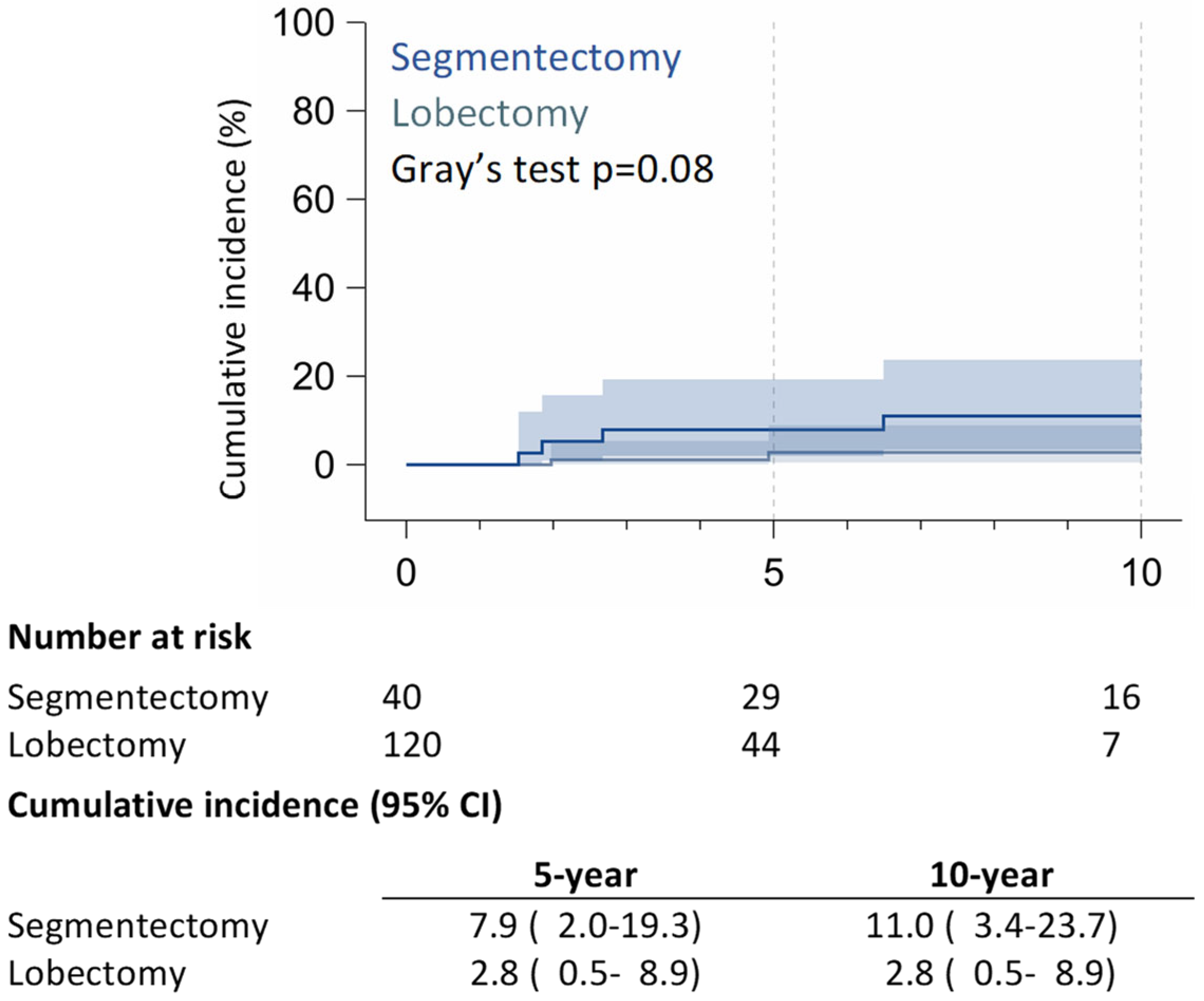
3.2. Long-Term Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSS | Cancer-specific survival |
| RR | Rate of relapse |
| ICU | Intensive care unit |
| NSCLC | Non-small-cell lung cancer |
| RAL | Robotic-assisted lobectomy |
| RAS | Robotic-assisted segmentectomy |
| CI | Confidence interval |
| SD | Standard deviation |
| LR | Local recurrence |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Non-Small Cell Lung Cancer, Version 9.2024. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 5 August 2025).
- Ginsberg, R.J.; Rubinstein, L.V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann. Thorac. Surg. 1995, 60, 615–622; discussion 622–623. [Google Scholar] [CrossRef]
- Saji, H.; Okada, M.; Tsuboi, M.; Nakajima, R.; Suzuki, K.; Aokage, K.; Aoki, T.; Okami, J.; Yoshino, I.; Ito, H.; et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022, 399, 1607–1617. [Google Scholar] [CrossRef]
- Altorki, N.; Wang, X.; Kozono, D.; Watt, C.; Landrenau, R.; Wigle, D.; Port, J.; Jones, D.R.; Conti, M.; Ashrafi, A.S.; et al. Lobar or Sub-lobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 388, 489–498. [Google Scholar] [CrossRef]
- Park, B.J.; Flores, R.M.; Rusch, V.W. Robotic assistance for video-assisted thoracic surgical lobectomy: Technique and initial results. J. Thorac. Cardiovasc. Surg. 2006, 131, 54–59. [Google Scholar] [CrossRef]
- Mattioni, G.; Palleschi, A.; Mendogni, P.; Tosi, D. Approaches and outcomes of Robotic-Assisted Thoracic Surgery (RATS) for lung cancer: A narrative review. J. Robot. Surg. 2023, 17, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, Q.; Huang, Y.; Ouyang, L.; Luo, F. Updated Evaluation of Robotic- and Video-Assisted Thoracoscopic Lobectomy or Segmentectomy for Lung Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 853530. [Google Scholar] [CrossRef] [PubMed]
- Handa, Y.; Tsutani, Y.; Mimae, T.; Tasaki, T.; Miyata, Y.; Okada, M. Surgical Outcomes of Complex Versus Simple Segmentectomy for Stage I Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2019, 107, 1032–1039. [Google Scholar] [CrossRef]
- Casiraghi, M.; Cara, A.; Mazzella, A.; Girelli, L.; Lo Iacono, G.; Uslenghi, C.; Caffarena, G.; Orlandi, R.; Bertolaccini, L.; Maisonneuve, P.; et al. 1000 Robotic-assisted lobectomies for primary lung cancer: 16 years single center experience. Lung Cancer. 2024, 195, 107903. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, J.; Al-Hussaini, A.; Al-Shehab, D.; Threader, J.; Villeneuve, P.J.; Ramsay, T.; Maziak, D.E.; Gilbert, S.; Shamji, F.M.; Sundaresan, R.S.; et al. Evaluating the reliability and reproducibility of the Ottawa Thoracic Morbidity and Mortality classification system. Ann. Thorac. Surg. 2011, 91, 387–393. [Google Scholar] [CrossRef]
- Xia, F.; Ning, J.; Huang, X. Empirical Comparison of the Breslow Estimator and the Kalbfleisch Prentice Estimator for Survival Functions. J. Biom. Biostat. 2018, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Dignam, J.J.; Kocherginsky, M.N. Choice and interpretation of statistical tests used when competing risks are present. J. Clin. Oncol. 2008, 26, 4027–4034. [Google Scholar] [CrossRef]
- Dylewski, M.R.; Ohaeto, A.C.; Pereira, J.F. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin. Thorac. Cardiovasc. Surg. 2011, 23, 36–42. [Google Scholar] [CrossRef]
- Pardolesi, A.; Park, B.; Petrella, F.; Borri, A.; Gasparri, R.; Veronesi, G. Robotic anatomic segmentectomy of the lung: Technical aspects and initial results. Ann. Thorac. Surg. 2012, 94, 929–934. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, J.; Pan, F.; Li, J.; Liu, Y.; Hou, Y.; Song, W.; Luo, Q. Operative outcomes and long-term survival of robotic-assisted segmentectomy for stage IA lung cancer compared with video-assisted thoracoscopic segmentectomy. Transl. Lung Cancer Res. 2020, 9, 306–315. [Google Scholar] [CrossRef]
- Perroni, G.; Veronesi, G. Robotic segmentectomy: Indication and technique. J. Thorac. Dis. 2020, 12, 3404–3410. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Watson, C.; Minnich, D.J.; Calloway, S.; Wei, B. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann. Thorac. Surg. 2016, 101, 1089–1095; discussion 1095–1096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.; Hu, J.; Han, Y.; Huang, M.; Xiang, J.; Li, H. Early outcomes of robotic versus thoracoscopic segmentectomy for early-stage lung cancer: A multi-institutional propensity score-matched analysis. J. Thorac. Cardiovasc. Surg. 2020, 160, 1363–1372. [Google Scholar] [CrossRef]
- Haruki, T.; Kubouchi, Y.; Kidokoro, Y.; Matsui, S.; Ohno, T.; Kojima, S.; Nakamura, H. A comparative study of robot-assisted thoracoscopic surgery and conventional approaches for short-term outcomes of anatomical segmentectomy. Gen. Thorac. Cardiovasc. Surg. 2024, 72, 338–345. [Google Scholar] [CrossRef]
- Leung, A.; Akhmerov, A.; Justo, M.; Fong, A.; Mahfoozi, A.; Soukiasian, H.J.; Imai, T.A. Trends in segmentectomy for the treatment of stage 1A non-small cell lung cancers: Does the robot have an impact? Am. J. Surg. 2023, 225, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Kneuertz, P.J.; Zhao, J.; D’Souza, D.M.; Abdel-Rasoul, M.; Merritt, R.E. National Trends and Outcomes of Segmentectomy in the Society of Thoracic Surgeons Database. Ann. Thorac. Surg. 2022, 113, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Z.; Tan, Z.H.; Li, J.B.; Xie, C.L.; Sun, T.Y.; Long, H.; Fu, J.H.; Zhang, L.J.; Lin, P.; Yang, H.X. Comparison of Short-Term Outcomes Between Robot-Assisted and Video-Assisted Segmentectomy for Small Pulmonary Nodules: A Propensity Score-Matching Study. Ann. Surg. Oncol. 2023, 30, 2757–2764. [Google Scholar] [CrossRef]
- Mao, J.; Tang, Z.; Mi, Y.; Xu, H.; Li, K.; Liang, Y.; Wang, N.; Wang, L. Robotic and video-assisted lobectomy/segmentectomy for non-small cell lung cancer have similar perioperative outcomes: A systematic review and meta-analysis. Transl. Cancer Res. 2021, 10, 3883–3893. [Google Scholar] [CrossRef]
- Echavarria, M.F.; Cheng, A.M.; Velez-Cubian, F.O.; Ng, E.P.; Moodie, C.C.; Garrett, J.R.; Fontaine, J.P.; Robinson, L.A.; Toloza, E.M. Comparison of pulmonary function tests and perioperative outcomes after robotic-assisted pulmonary lobectomy vs segmentectomy. Am. J. Surg. 2016, 212, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Gharagozloo, F.; Tempesta, B.; Meyer, M.; Gruessner, A. Long-term results of robotic anatomical segmentectomy for early-stage non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2019, 55, 427–433. [Google Scholar] [CrossRef] [PubMed]
| Segmentectomy * | Lobectomy | p-Value | |
|---|---|---|---|
| N = 40 | N = 120 | ||
| Age | 0.69 | ||
| Median [range] IQR | 64.5 [50–85] 62–72 | 66 [43–83] 62–70 | |
| <60 | 6 (15.0) | 14 (11.7) | |
| 60–69 | 22 (55.0) | 75 (62.5) | |
| 70+ | 12 (30.0) | 31 (25.8) | |
| Sex | 0.23 | ||
| Men [43–83] | 22 (55.0) | 53 (44.2) | |
| Women | 18 (45.0) | 67 (55.8) | |
| Conversion | 1.00 | ||
| No | 39 (97.2) | 117 (97.5) | |
| Yes | 1 (2.5) | 3 (2.5) | |
| Side | 0.46 | ||
| Right | 14 (35.0) | 50 (41.7) | |
| Left | 26 (65.0) | 70 (58.3) | |
| Site | 0.31 | ||
| Upper | 24 (60.0) | 61 (50.8) | |
| Lower | 16 (40.0) | 59 (49.2) | |
| Histology | 1.00 | ||
| Adenocarcinoma | 35 (87.5) | 105 (87.5) | |
| Squamous | 2 (5.0) | 6 (5.0) | |
| Other | 3 (7.5) | 9 (7.5) | |
| Tumor grade | 0.63 | ||
| G1 | 11 (27.5) | 26 (21.7) | |
| G2 | 24 (60.0) | 82 (68.3) | |
| G3 | 5 (12.5) | 12 (10.0) | |
| Tumor size | 0.64 | ||
| Median [range] IQR | 12.5 [6–34] 8–17 | 15 [6–32] 11–18 | |
| ≤15 mm | 28 (70.0) | 81 (67.5) | |
| >15–30 mm | 10 (25.0) | 36 (30.0) | |
| >30–40 mm | 2 (5.0) | 3 (2.5) | |
| Clinical stage | 0.007 | ||
| 1a | 26 (65.0) | 44 (36.7) | |
| 1b | 10 (25.0) | 50 (41.7) | |
| 1c | 1 (2.5) | 19 (15.8) | |
| 2a | 3 (7.5) | 7 (5.8) | |
| Pathological stage | 0.17 | ||
| Stage I | 36 (90.0) | 116 (96.7) | |
| Stage II | 3 (7.5) | 2 (1.7) | |
| Stage III | 1 (2.5) | 2 (1.7) | |
| Adjuvant therapy | 1.00 | ||
| No | 39 (97.5) | 115 (95.8) | |
| Yes | 1 (2.5) | 5 (4.2) |
| Segmentectomy | Lobectomy | p-Value | |
|---|---|---|---|
| N = 40 | N = 120 | ||
| Operative time | |||
| Median [range] IQR | 159 [95–224] 150–182 | 167 [70–348] 138–193 | 0.27 |
| N1 resected | |||
| Median [range] IQR | 4 [0–17] 3–7 | 9 [0–27] 6–13 | <0.0001 |
| N2 resected | |||
| Median [range] IQR | 3 [0–15] 2–5 | 5 [1–32] 3–7 | 0.06 |
| Total N1 + N2 resected | |||
| Median [range] IQR | 9 [1–20] 6–13 | 15 [4–44] 11–19 | 0.0004 |
| N1 stations | |||
| Median [range] IQR | 2 [0–4] 2–3 | 2 [0–5] 2–3 | 0.18 |
| N2 Stations | |||
| Median [range] IQR | 2 [0–5] 1–3 | 3 [1–5] 2–3 | 0.0001 |
| Total N1 + N2 stations | |||
| Median [range] IQR | 4 [1–7] 3–5 | 5 [2–8] 4–6 | 0.0004 |
| Upstaging | |||
| pN0 | 39 (97.5) | 138 (98.3) | |
| pN+ | 1 (2.5) | 2 (1.6) | 1.00 |
| Length of stay | |||
| Median [range] IQR | 4 [3–21] 3–5.5 | 5 [3–35] 4–6 | 0.10 |
| ICU | |||
| No | 40 (100.0) | 105 (87.5) | |
| Yes | 0 (0.0) | 15 (12.5) | 0.02 |
| Postoperative complications | |||
| No | 30 (75.0) | 92 (76.7) | |
| Yes | 10 (25.0) | 28 (23.3) | 0.83 |
| Minor | 8 (20.0) | 25 (20.8) | |
| Major | 2 (5.0) | 3 (2.5) | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casiraghi, M.; Orlandi, R.; Mazzella, A.; Girelli, L.; Caffarena, G.; Chiari, M.; Bertolaccini, L.; Lo Iacono, G.; Diotti, C.; Bardoni, C.; et al. 10-Year Long-Term Outcomes of Robotic-Assisted Segmentectomy for Early-Stage Non-Small-Cell Lung Cancer. J. Clin. Med. 2025, 14, 5608. https://doi.org/10.3390/jcm14165608
Casiraghi M, Orlandi R, Mazzella A, Girelli L, Caffarena G, Chiari M, Bertolaccini L, Lo Iacono G, Diotti C, Bardoni C, et al. 10-Year Long-Term Outcomes of Robotic-Assisted Segmentectomy for Early-Stage Non-Small-Cell Lung Cancer. Journal of Clinical Medicine. 2025; 14(16):5608. https://doi.org/10.3390/jcm14165608
Chicago/Turabian StyleCasiraghi, Monica, Riccardo Orlandi, Antonio Mazzella, Lara Girelli, Giovanni Caffarena, Matteo Chiari, Luca Bertolaccini, Giorgio Lo Iacono, Cristina Diotti, Claudia Bardoni, and et al. 2025. "10-Year Long-Term Outcomes of Robotic-Assisted Segmentectomy for Early-Stage Non-Small-Cell Lung Cancer" Journal of Clinical Medicine 14, no. 16: 5608. https://doi.org/10.3390/jcm14165608
APA StyleCasiraghi, M., Orlandi, R., Mazzella, A., Girelli, L., Caffarena, G., Chiari, M., Bertolaccini, L., Lo Iacono, G., Diotti, C., Bardoni, C., Maisonneuve, P., & Spaggiari, L. (2025). 10-Year Long-Term Outcomes of Robotic-Assisted Segmentectomy for Early-Stage Non-Small-Cell Lung Cancer. Journal of Clinical Medicine, 14(16), 5608. https://doi.org/10.3390/jcm14165608









