Breast Augmentation in Body Contouring Using Autologous Stem Cell-Enriched Fat Grafting: Fifteen-Year Clinical Experience
Abstract
1. Introduction
2. Methods
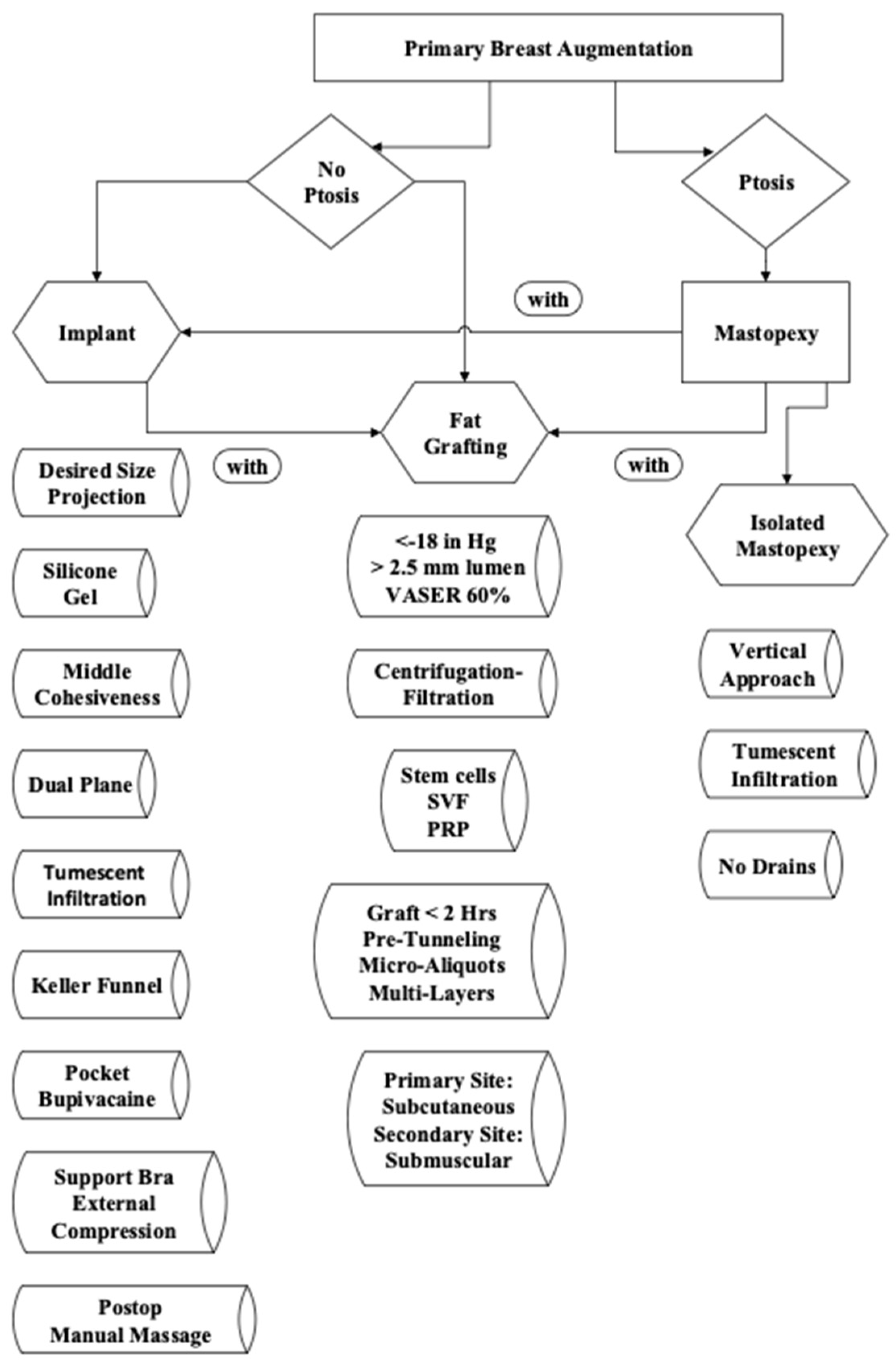
2.1. Patient Selection and Decision-Making Process
2.2. Harvesting
2.3. Ultrasound-Assisted (VASER) Energy Delivery
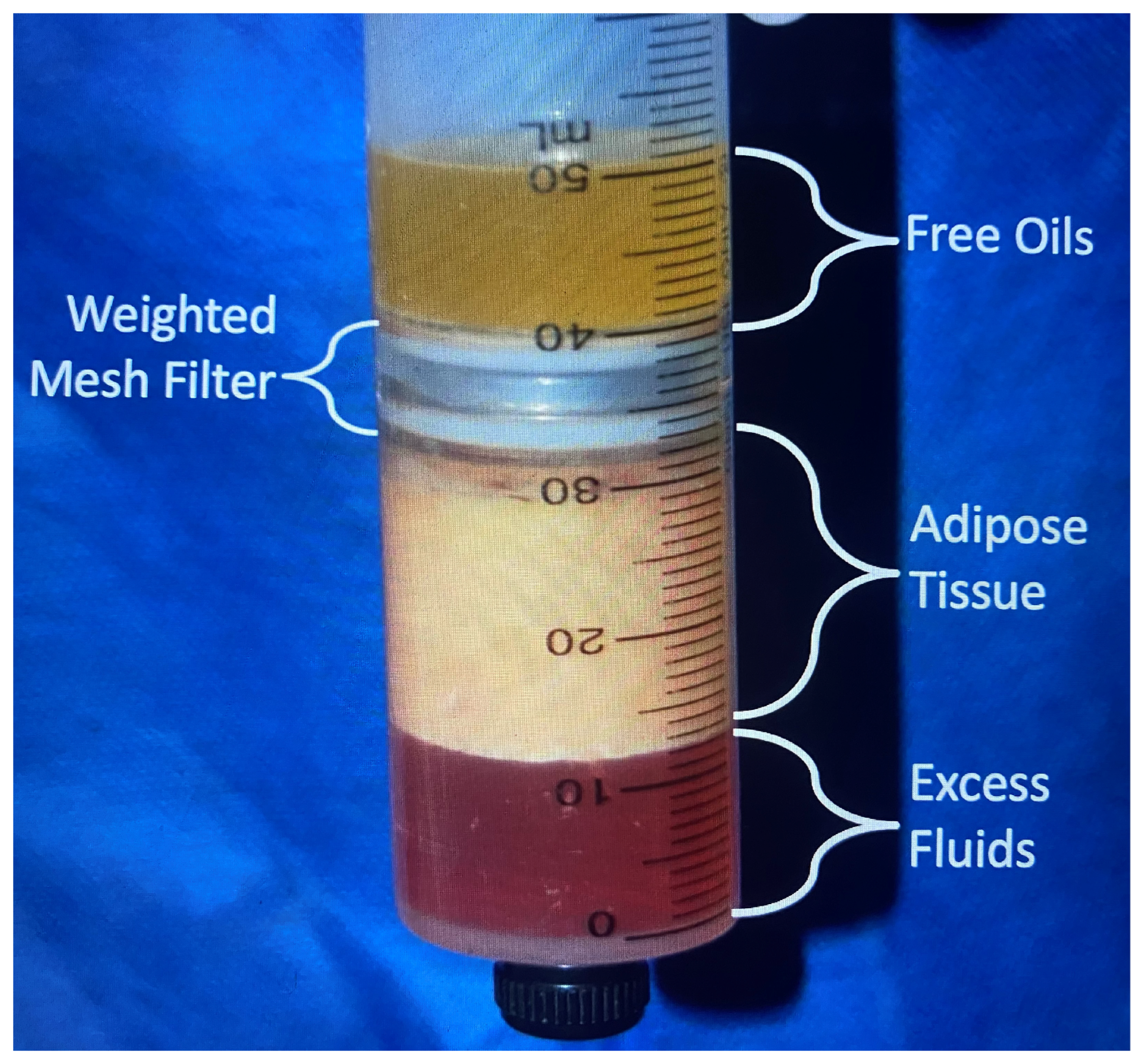
2.4. Processing
2.5. Enrichment
2.5.1. Background
2.5.2. Platelet-Rich Plasma (PRP)
2.5.3. Autologous Adipose-Derived Stem Cells
Discovery
Method of Action
Clinical Studies Verifying Stem Cell Benefits
Stem Cell Policy Statement
2.6. Storage
2.7. Administration
2.7.1. Breast Capsular Contracture Necessitating Implant Removal
2.7.2. Breast Asymmetry Patients
2.7.3. Breast Implant Removal Patients
2.7.4. Breast Mastopexy and Reduction Mammoplasty Patients
2.8. High-Definition VASER Liposuction and Renuvion Helium-Based Plasma
2.9. Patient Follow-Up
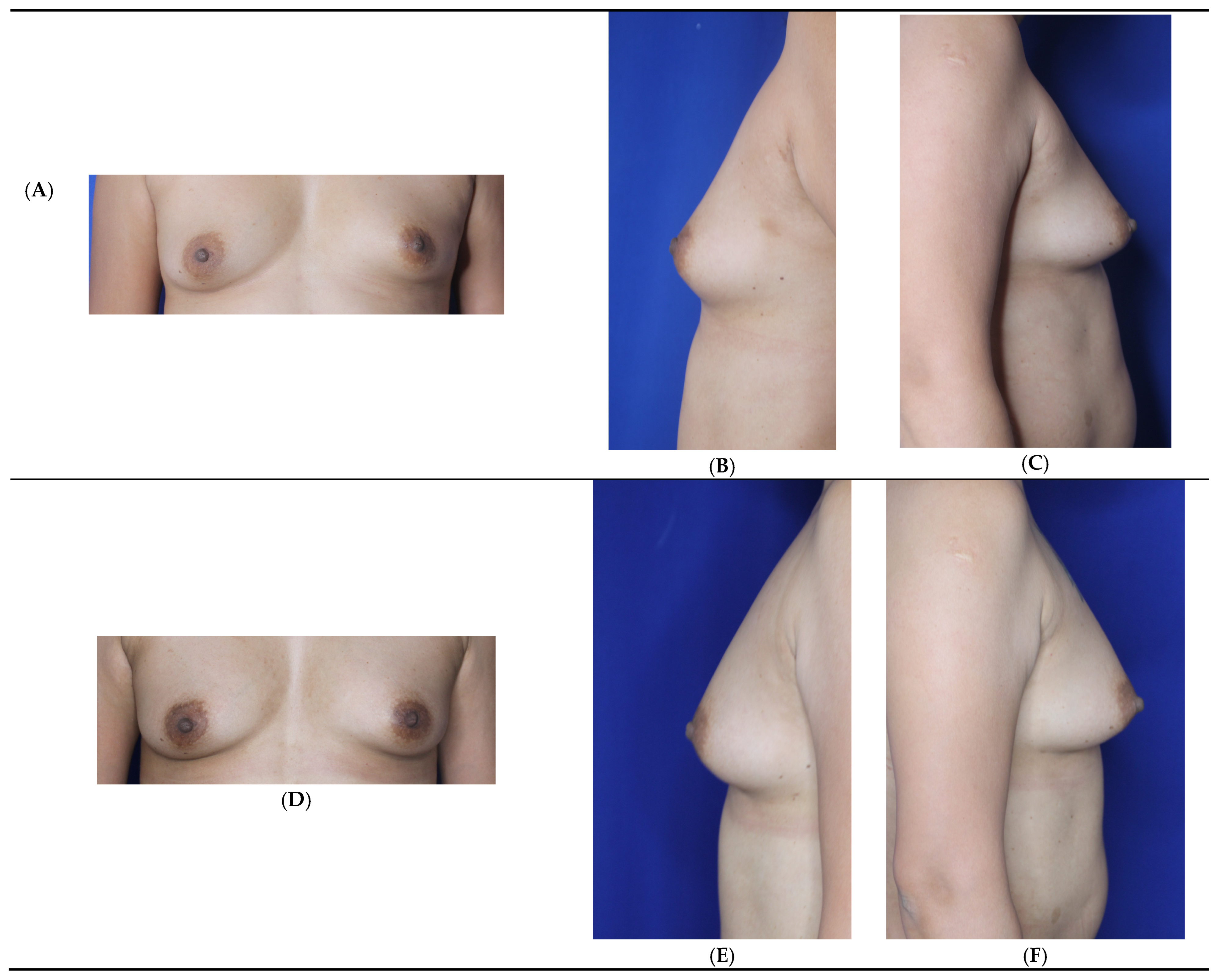
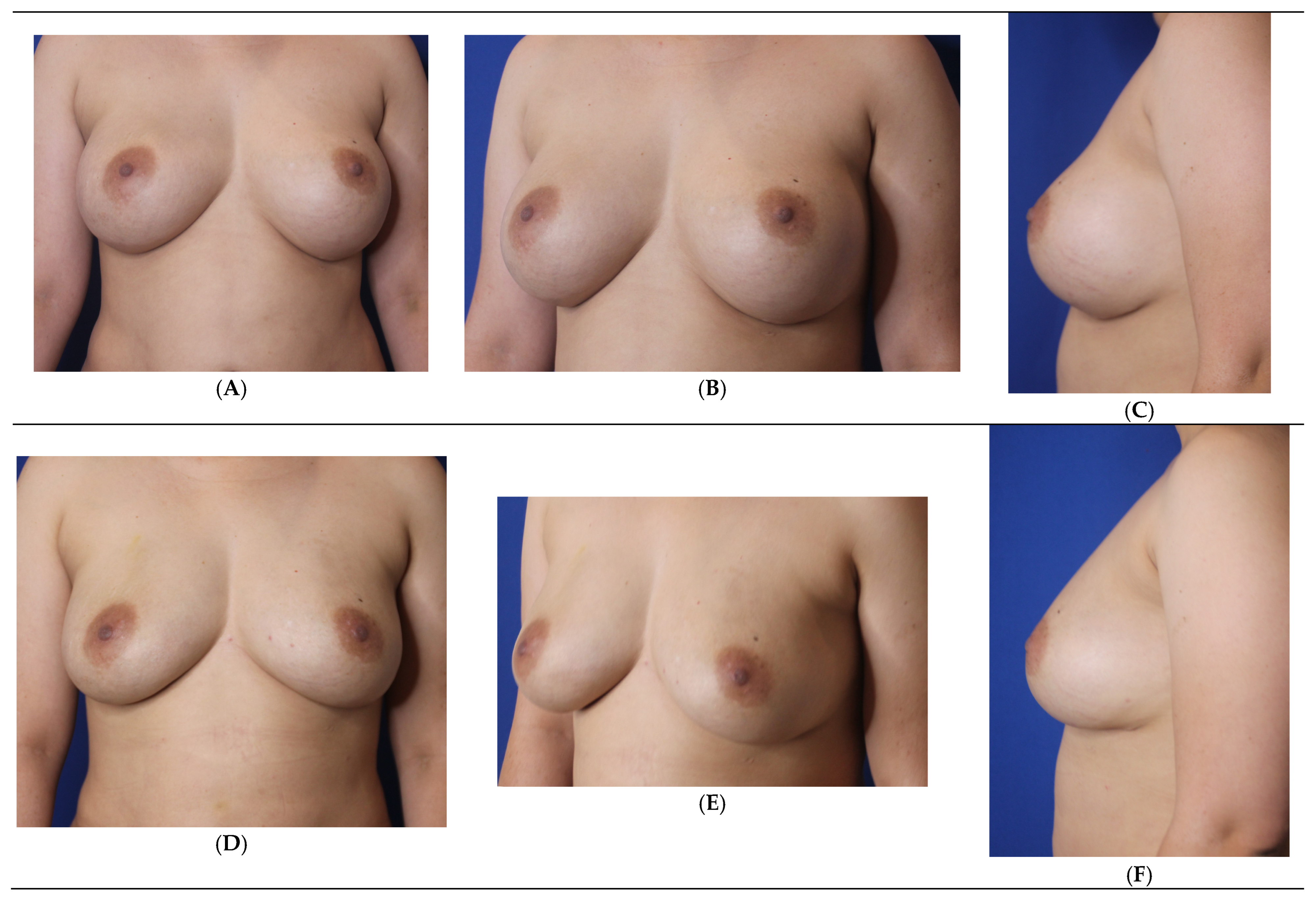
3. Results
3.1. Patient Satisfaction Assessment
3.2. Revisions (7.6%, n = 9)
4. Discussion
Study Limitations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Society for Aesthetic Plastic Surgery. Statistical Survey; American Society for Aesthetic Plastic Surgery: Garden Grove, CA, USA, 2023. [Google Scholar]
- Gabriel, S.E.; Woods, J.E.; O’Fallon, W.M.; Beard, C.M.; Kurland, L.T.; Melton, L.J. Complications leading to surgery after breast implantation. N. Engl. J. Med. 1997, 336, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Marotta, J.S.; Widenhouse, C.W.; Habal, M.B.; Goldberg, E.P. Silicone gel breast implant failure and frequency of additional surgeries: Analysis of 35 studies reporting examination of more than 8000cc explants. J. Biomed. Mater. Res. 1999, 48, 354–364. [Google Scholar] [CrossRef]
- Brown, S.L.; Pennello, G. Replacement surgery and silicone gel breast implant rupture: Self-report by women after mammoplasty. J. Women’s Health Gend. Based Med. 2002, 11, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Kjøller, K.; Hölmich, L.R.; Jacobsen, P.H.; Friis, S.; Fryzek, J.; McLaughlin, J.K.; Lipworth, L.; Henriksen, T.F.; Jørgensen, S.; Bittmann, S.; et al. Epidemiological investigation of local complications after cosmetic breast implant surgery in Denmark. Ann. Plast. Surg. 2002, 48, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Troell, R. Breast Fat Grafting: Ten-Year Learned Clinical Experience. Am. J. Cosmet. Surg. 2024, 41, 196–213. [Google Scholar] [CrossRef]
- Silkiss, R.Z.; Baylis, H.I. Autogenous fat grafting by injection. Ophthal. Plast. Reconstr. Surg. 1987, 3, 71–75. [Google Scholar] [CrossRef]
- Bircoll, M. Cosmetic breast augmentation utilizing autologous fat and liposuction techniques. Plast. Reconstr. Surg. 1987, 79, 267–271. [Google Scholar] [CrossRef]
- Bircoll, M.; Novack, B.H. Autologous fat transplantation employing liposuction techniques. Ann. Plast. Surg. 1987, 18, 327–329. [Google Scholar] [CrossRef]
- Rubin, J.P.; Coon, D.; Zuley, M.; Toy, J.; Asano, Y.; Kurita, M.; Aoi, N.; Harii, K.; Yoshimura, K. Mammographic changes after fat transfer to the breast compared with changes after breast reduction: A blinded study. Plast. Reconstr. Surg. 2012, 129, 1029–1038. [Google Scholar] [CrossRef]
- Troell, R. Primary & Secondary Aesthetic Breast Augmentation: Proposed Algorithm for Optimal Cosmetic Outcomes While Minimizing Complications. Am. J. Cosmet. Surg. 2025, 1–28. [Google Scholar] [CrossRef]
- International Society of Plastic Aesthetic Surgery. International Survey on Aesthetic Cosmetic Procedures Performed in 2009; International Society of Plastic Aesthetic Surgery: West Lebanon, NH, USA, 2009. [Google Scholar]
- US Food & Drug Administration (FDA). Regulatory Considerations for Human Cells, Tissues and Cellular & Tissue-Based Products: Minimal Manipulation and Homologous Use Definitions; US Food & Drug Administration (FDA): Silver Spring, MD, USA, 2020.
- Troell, R. Breast fat grafting: Comparing filtration-centrifugation to filtration-washing fat processing. Am. J. Cosmet. Surg. 2024, 41, 100–112. [Google Scholar] [CrossRef]
- Moore, J.H., Jr.; Kolaczynski, J.W.; Morales, L.M.; Considine, R.V.; Pietrzkowski, Z.; Noto, P.F.; Caro, J.F. Viability of fat obtained by syringe suction lipectomy: Effects of local anesthesia with lidocaine. Aesthetic Plast. Surg. 1995, 19, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Shoshani, O.; Berger, J.; Fodor, L.; Ramon, Y.; Shupak, A.; Kehat, I.; Gilhar, A.; Ullmann, Y. The effect of lidocaine and adrenaline on the viability of injected adipose tissue: An experimental study in nude mice. J. Drugs Dermatol. 2005, 4, 311–316. [Google Scholar] [PubMed]
- Rohrich, R.J.; Sorokin, E.S.; Brown, S.A. In search of improved fat transfer viability: A quantitative analysis of the role of centrifugation and harvest site. Plast. Reconstr. Surg. 2004, 113, 391–395; discussion 396–397. [Google Scholar] [CrossRef]
- Ozsoy, Z.; Kul, Z.; Bilir, A. The role of cannula diameter in improved adipocyte viability: A quantitative analysis. Aesthetic Surg. J. 2006, 26, 287–289. [Google Scholar] [CrossRef]
- Ferguson, R.E.; Cui, X.; Fink, B.F.; Vasconez, H.C.; Pu, L.L. The viability of autologous fat grafts harvested with the LipiVage system: A comparative study. Ann. Plast. Surg. 2008, 60, 594–597. [Google Scholar] [CrossRef]
- Erdim, M.; Tezel, E.; Numanoglu, A.; Sav, A. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: An experimental study. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 1210–1214. [Google Scholar] [CrossRef]
- Troell, R. Liposuction 20-Year Learned Experience Liposuction 20-Year Learned Experience: Optimizing Cosmetic Outcomes While Minimizing Complications (Part Two). Am. J. Cosmet. Surg. Online 2025, 1–18. [Google Scholar] [CrossRef]
- Hoyos, A.E.; Prendergast, P.M. (Eds.) High-Definition Body Sculpting: Art and Advanced Lipoplasty Techniques, 1st ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2014. [Google Scholar]
- Schafer, M.; Hicok, K.; Mills, D.; Cohen, S.R.; Chao, J.J. Acute Adipocyte Viability After Third- Generation Ultrasound-Assisted Liposuction. Aesthetic Surg. J. 2013, 33, 698–704. [Google Scholar] [CrossRef]
- Fisher, C.; Grahovac, T.L.; Schafer, M.E.; Shippert, R.D.; Marra, K.G.; Rubin, J.P. Comparison of harvest and processing techniques for fat grafting and adipose cell isolation. Plast. Reconstr. Surg. 2013, 132, 351–361. [Google Scholar] [CrossRef]
- Panetta, N.J.; Gupta, D.M.; Kwan, M.D.; Wan, D.C.; Commons, G.W.; Longaker, M.T. Tissue harvest by means of suction-assisted or third-generation ultrasound-assisted lipoaspiration has no effect on osteogenic potential of human adipose-derived stromal cells. Plast. Reconstr. Surg. 2009, 124, 65–73. [Google Scholar] [CrossRef]
- Nagy, M.W.; Vanek, P.F. A multicenter, prospective, randomized, single-blind, controlled clinical trial comparing VASER-assisted lipoplasty and suction-assisted lipoplasty. Plast. Reconstr. Surg. 2012, 129, 681e–689e. [Google Scholar] [CrossRef]
- Garcia, O.; Nathan, N. Comparative analysis of blood loss in suction-assisted lipoplasty and third-generation internal ultrasound-assisted lipoplasty. Aesthetic Surg. J. 2008, 28, 430–435. [Google Scholar] [CrossRef]
- Kurita, M.; Matsumoto, D.; Shigeura, T.; Sato, K.; Gonda, K.; Harii, K.; Yoshimura, K. Influences of centrifugation on cells and tissues in liposuction aspirates: Optimized centrifugation for lipotransfer and cell isolation. Plast. Reconstr. Surg. 2008, 121, 1033–1041; discussion 1042–1043. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R. Structural Fat Grafting; Quality Medical Pub: St. Louis, MO, USA, 2004. [Google Scholar]
- Coleman, S.R.; Saboeiro, A.P. Fat grafting to the breast revisited: Safety and efficacy. Plast. Reconstr. Surg. 2007, 119, 775–785; discussion 786–787. [Google Scholar] [CrossRef] [PubMed]
- Troell, R.J. Adipose Derived Stem Cells: Harvesting, Processing, Storage and Administration. In Stem Cells with Fat Transfer in Aesthetic Procedures: Science, Art, and Clinical Techniques; Shiffman, M.A., Di Giuseppe, A., Bassetto, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Cervelli, V.; Gentile, P.; Scioli, M.G.; Grimaldi, M.; Casciani, C.U.; Spagnoli, L.G.; Orlandi, A. Application of Platelet-Rich Plasma in Plastic Surgery: Clinical and In Vitro Evaluation. Tissue Eng. Part C 2009, 15, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multi-lineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Eto, H.; Suga, H.; Matsumoto, D.; Inoue, K.; Aoi, N.; Kato, H.; Araki, J.; Yoshimura, K. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast. Reconstr. Surg. 2009, 124, 1087–1097. [Google Scholar] [CrossRef]
- Kitamura, K.; Kajitani, K.; Hedrick, A.T.; Sugimachi, K. Stem cell augmented reconstruction: A new hope for reconstruction after breast conservative therapy. Breast Cancer Res. Treat. 2007, 106, S190. [Google Scholar]
- Pérez-Cano, R.; Vranckx, J.J.; Lasso, J.M.; Calabrese, C.; Merck, B.; Milstein, A.; Sassoon, E.; Delay, E.; Weiler-Mithoff, E. Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: The RESTORE-2 trial. Eur. J. Surg. Oncol. 2012, 38, 382–389. [Google Scholar] [CrossRef]
- Fraser, J.; Wulur, I.; Alfonso, Z.; Hedrick, M. Fat tissue: An under-appreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Eaves, F.F., III; Haeck, P.C.; Rohrich, R.J. ASAPS/ASPS Position Statement on Stem Cells and Fat Grafting. Plast. Reconstr. Surg. 2012, 129, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-Assisted lipotransfer for cosmetic breast augmentation supportive use of adipose-derived stem/stromal cells. Aesthetic Plast. Surg. 2008, 22, 48–55. [Google Scholar] [CrossRef]
- Moscatello, D.K.; Dougherty, M.; Narins, R.S.; Lawrence, N. Cryopreservation of human fat for soft tissue augmentation: Viability requires use of cryoprotectant and controlled freezing and storage. Dermatol. Surg. 2005, 31, 1506–1510. [Google Scholar] [CrossRef]
- Wolter, T.P.; von Heimburg, D.; Stoffels, I.; Groeger, A.; Pallua, N. Groeger A, Cryopreservation of mature human adipocytes: In vitro measurement of viability. Ann. Plast. Surg. 2005, 55, 408–413. [Google Scholar] [CrossRef]
- Matsumoto, D.; Shigeura, T.; Sato, T.; Inoue, K.; Suga, H.; Kato, H.; Aoi, N.; Murase, S.; Gonda, K.; Yoshimura, K. Influences of Preservation at Various Temperatures on Liposuction Aspirates. Plast. Reconstr. Surg. 2007, 120, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Oh, J.; Choi, T.; Kim, J.; Han, K.; Ha, S.; Lee, K. Viability of fat cells over time after syringe suction lipectomy: The effects of cryopreservation. Ann. Plast. Surg. 2010, 65, 354–360. [Google Scholar] [CrossRef]
- Bae, Y.C.; Song, J.S.; Song, J.S.; Bae, S.H.; Kim, J.H. Effects of human adipose-derived stem cells and stromal vascular fraction on cryopreserved fat transfer. Dermatol. Surg. 2015, 41, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, D.; Sato, K.; Gonda, K.; Takaki, Y.; Shigeura, T.; Sato, T.; Aiba-Kojima, E.; Iizuka, F.; Inoue, K.; Suga, H.; et al. Cell-assisted lipotransfer: Supportive use of human adipose derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006, 12, 3375–3382. [Google Scholar] [CrossRef]
- Ersek, R.A.; Chang, P.; Salisbury, M.A. Lipo-layering of autologous fat: An improved technique with promising results. Plast. Reconstr. Surg. 1998, 101, 820–826. [Google Scholar] [CrossRef]
- Carpaneda, C.A.; Ribeiro, M.T. Percentage of graft viability versus injected volume in adipose autotransplants. Aesthetic Plast. Surg. 1994, 18, 17–19. [Google Scholar] [CrossRef] [PubMed]
- di Summa, P.G.; Osinga, R.; Sapino, G.; Glen, K.; Higgins, G.; Tay, S.; Weiler-Mithoff, E. Fat grafting versus implant-based treatment of breast asymmetry, a single surgeon experience over 13 years: A paradigm shift. Gland Surg. 2021, 10, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, D.A. “SIEF” simultaneous implant exchange with fat: A new option in revision breast implant surgery. Plast. Reconstr. Surg. 2012, 130, 1187–1196. [Google Scholar] [CrossRef]
- Auclair, E.; Anavekar, N. Combined use of implant and fat grafting for breast augmentation. Clin. Plast. Surg. 2015, 42, 307–314. [Google Scholar] [CrossRef]
- Paul, M.; Blugerman, G.; Kreindel, M.; Muholland, R.S. Three-dimensional radiofrequency tissue tightening: A proposed mechanism and applications for body contouring. Aesthetic Plast. Surg. 2011, 35, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.I. Improving outcomes in upper arm liposuction: Adding radiofrequency-assisted liposuction to induce skin contraction. Aesthetic Surg. J. 2012, 32, 84–95. [Google Scholar] [CrossRef]
- Hurwitz, D.; Smith, D. Treatment of overweight patients by radiofrequency-assisted liposuction (RFAL) for aesthetic reshaping and skin tightening. Aesthetic Plast. Surg. 2012, 36, 62–71. [Google Scholar] [CrossRef]
- Chia, C.T.; Theodorou, S.J.; Hoyos, A.E.; Pitman, G.H. Radiofrequency-assisted liposuction compared with aggressive superficial, subdermal liposuction of the arms: A bilateral quantitative comparison. Plast. Reconstr. Surg.-Glob. Open 2015, 3, e459. [Google Scholar] [CrossRef]
- Coleman, S.R. Long-term survival of fat transplants: Controlled demonstrations. Aesthetic Plast. Surg. 1995, 19, 421–425. [Google Scholar] [CrossRef]
- Delay, E.; Garson, S.; Tousson, G.; Sinna, R. Fat injection to the breast: Technique, results, and indications based on 880 procedures over 10 years. Aesthetic Surg. J. 2009, 29, 360–376. [Google Scholar] [CrossRef]
- Ohashi, M.; Yamakawa, M.; Chiba, A.; Nagano, H.; Nakai, H. Our experience with 131 cases of simultaneous breast implant exchange with fat (SIEF). Plast. Reconstr. Surg.-Glob. Open 2016, 4, e691. [Google Scholar] [PubMed]
- Valente, D.S.; Ely, P.B.; Kieling, L.; Konzen, A.T.; Steffen, L.P.; Lazzaretti, G.S.; Zanella, R.K. Breast fat grafting: A systemic review of the science behind enhancements and concerns. Transl. Breast Cancer Rev. 2024, 5, 14. [Google Scholar] [CrossRef]
- Tukiama, R.; Vierira, R.A.C.; Moura, E.C.R.; Oliveira, A.G.; Facina, G.; Zucca-Matthes, G.; Neto, J.N.; de Oliveira, C.M.; Leal, P.d.C. Oncologic safety of breast reconstruction with autologous fat grafting: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2022, 48, 727–735. [Google Scholar] [CrossRef]
- Kempa, S.; Brix, E.; Heine, N.; Hösl, V.; Strauss, C.; Eigenberger, A.; Brébant, V.; Seitz, S.; Prantl, L. Autologous fat grafting for breast reconstruction after breast cancer: A 12-experience. Arch. Gynecol. Obstet. 2022, 305, 921–927. [Google Scholar] [CrossRef]
- Nguyen, L.; Afshari, A.; Grotting, J.C.; Perdikis, G.; Higdon, K.K. Preoperative risk factors and complication rates of breast augmentation with fat grafting. Aesthetic Surg. J. 2022, 42, 749–757. [Google Scholar] [CrossRef]
- Sterodimas, A.; Moutafis, A.; Nicaretta, B.; Champsas, G. A prospective study on helium-based plasma radiofrequency for minimally invasive breast lift scarless mastopexy. Aesthetic Surg. J. Open Forum 2025, 7, ojaf004. [Google Scholar] [CrossRef]
- Troell, R.; Javaheri, S. Safety and efficacy combining VASER ultrasound liposuction with Renuvion helium plasma for body contouring. Aesthetic Surg. J. 2025, submitted.
- Duncan, D.I. Nonexcisional tissue tightening creating skin surface area reduction during abdominal liposuction by adding radiofrequency heating. Aesthetic Surg. J. 2013, 33, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Murillo, W.L. Buttock augmentation: Case studies of fat injection monitored by magnetic resonance imaging. Plast. Reconstr. Surg. 2004, 114, 1606–1614; discussion 1615–1616. [Google Scholar] [CrossRef]
- Pierrefeu-Lagrange, A.C.; Delay, E.; Guerin, N.; Chekaroua, K.; Delaporte, T. Radiological evaluation of breasts reconstructed with lipomodeling. Ann. Chir. Plast. Esthet. 2006, 51, 18–28. (In French) [Google Scholar] [CrossRef] [PubMed]
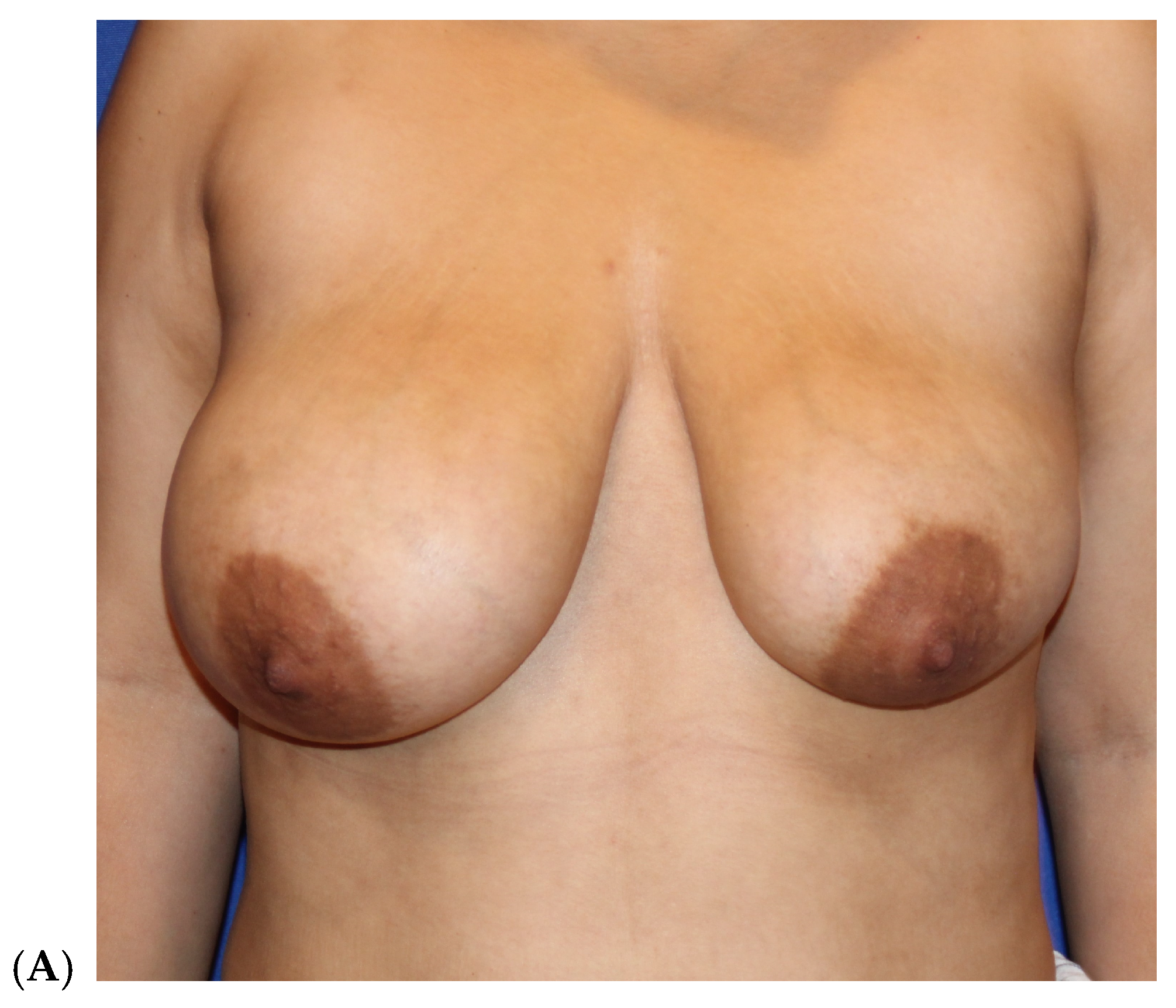
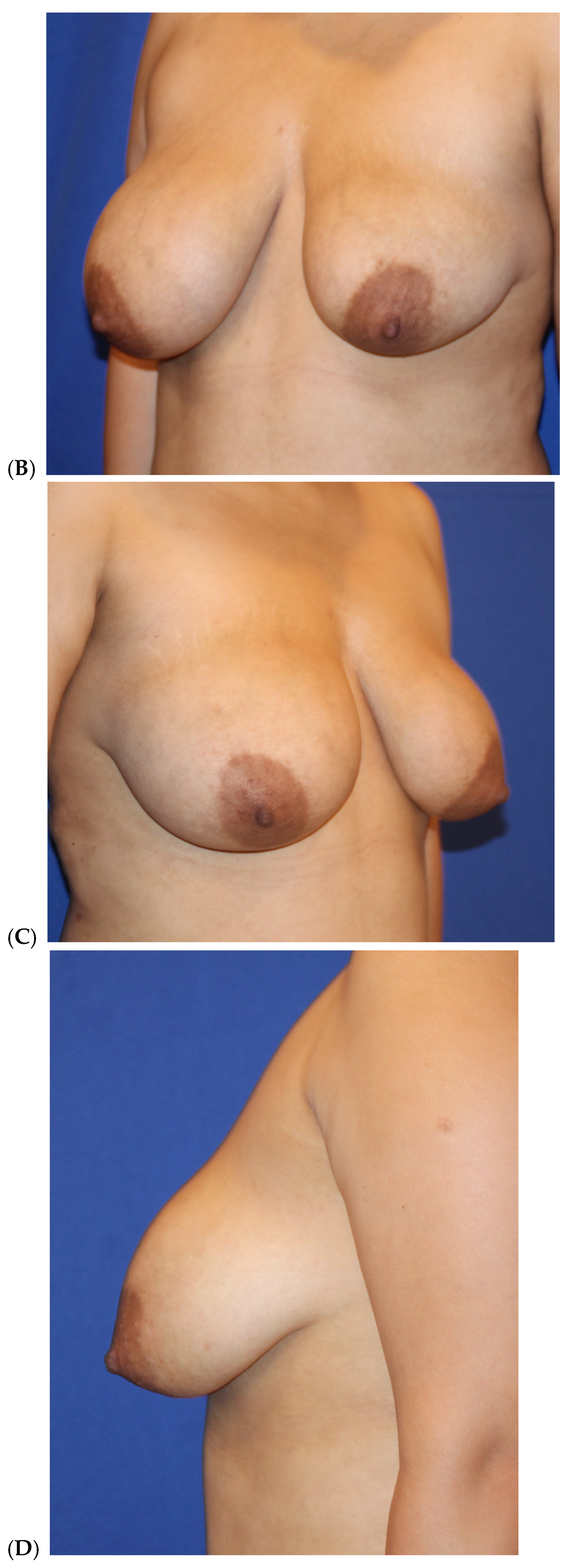
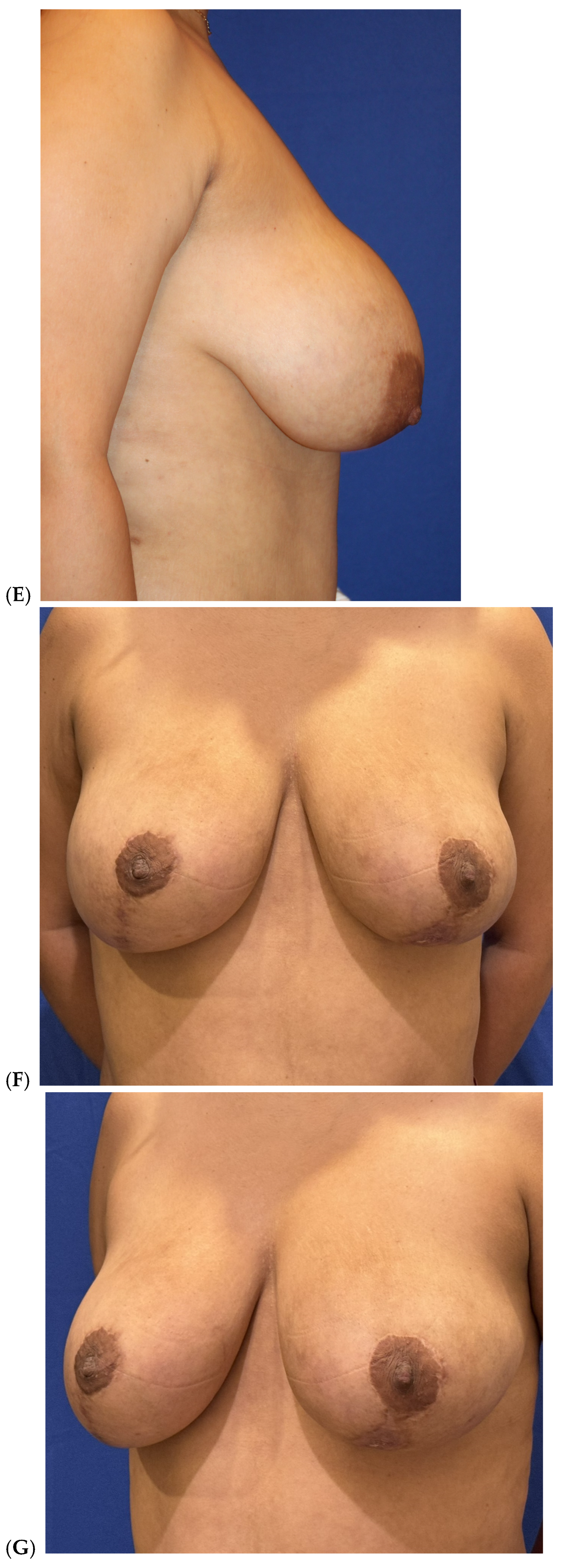
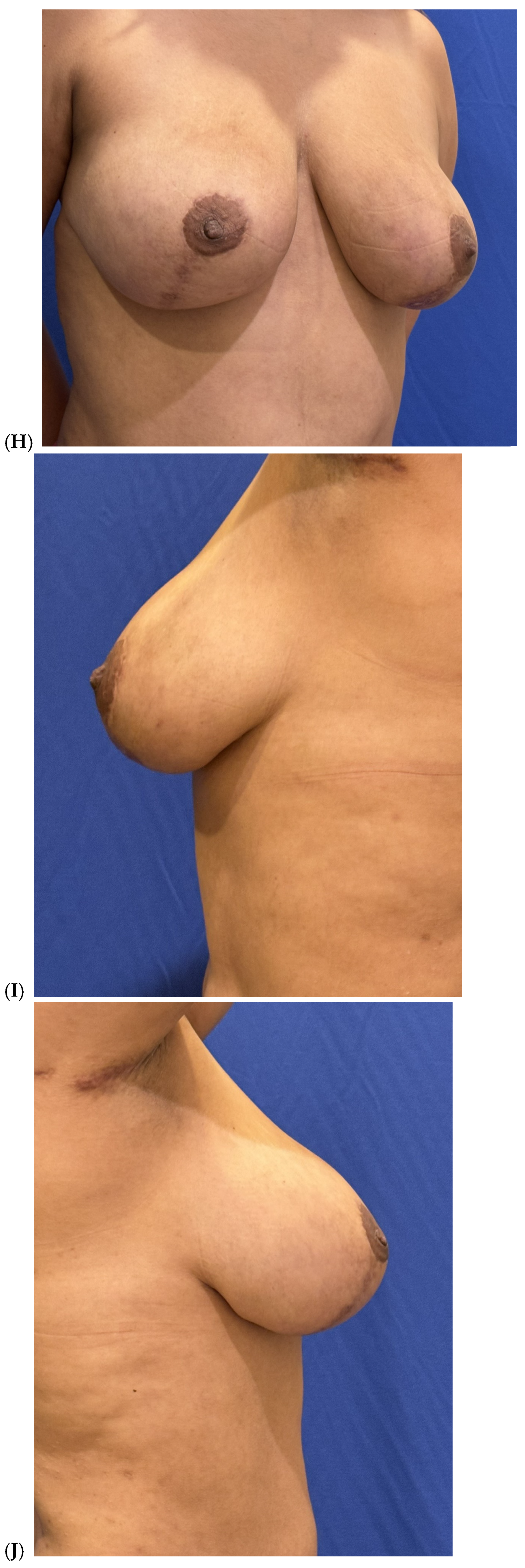
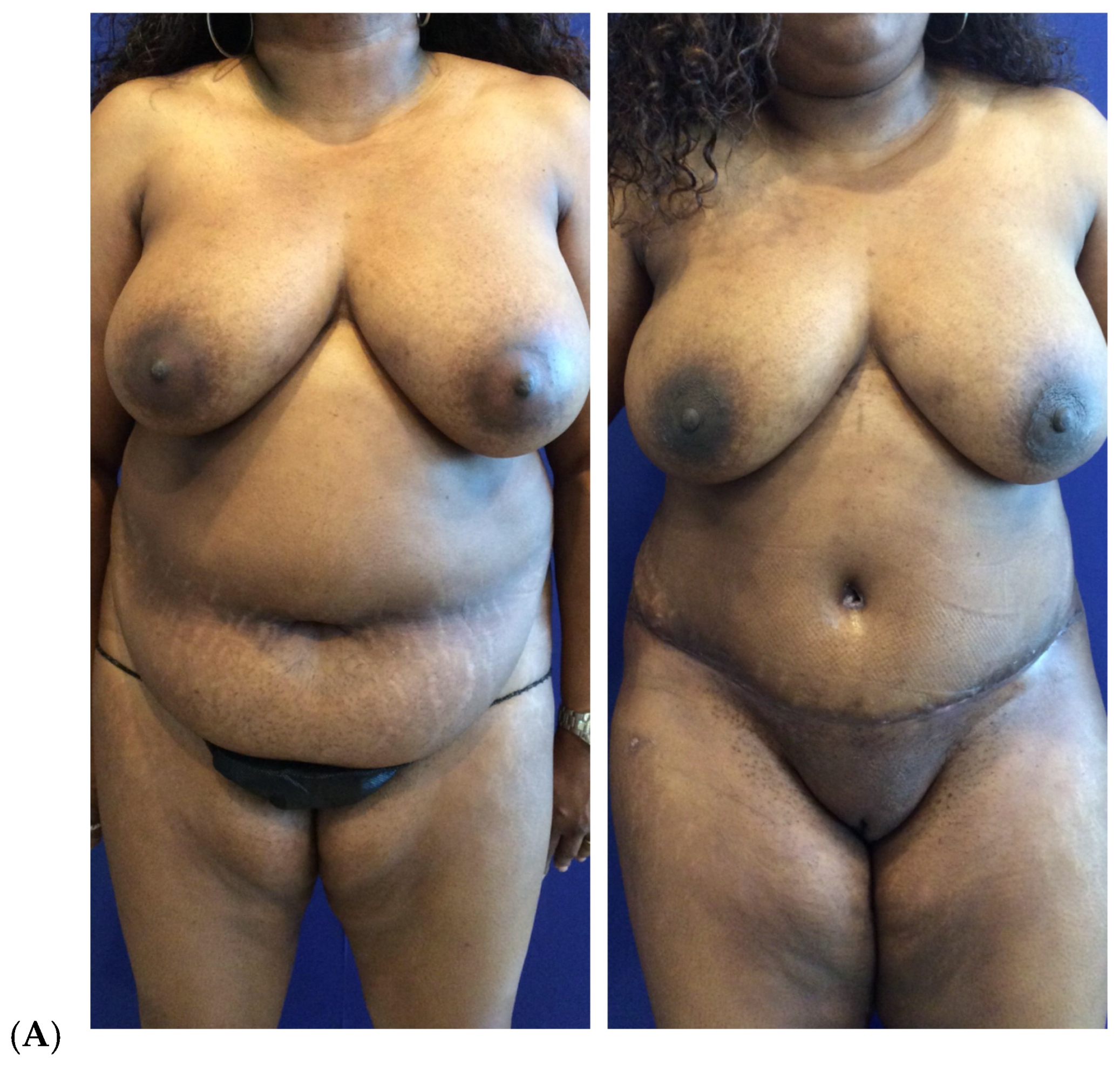
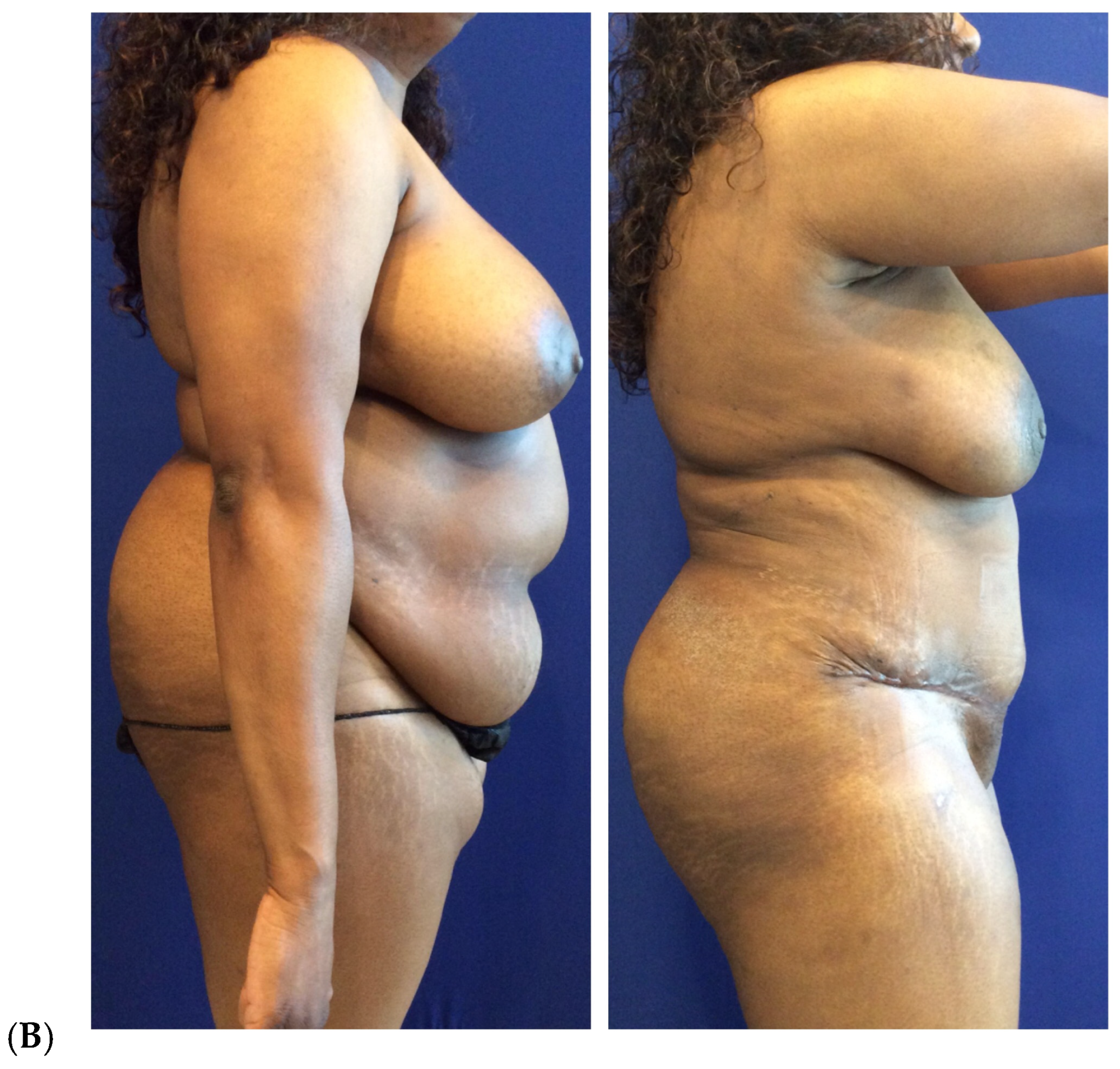
| Primary breast augmentation Simultaneous implant exchange with fat (SIEF) Improve shape and/or volume post-mastopexy Improve shape or volume post-reduction mammoplasty Improve shape and/or volume post-implant placement Complete breast augmentation after mastectomy Treatment of implant-related capsular contracture Fill isolated volume breast defects Asymmetric breasts (natural or iatrogenic) Tuberous breasts Repair chest wall defects |
| HIV disease Collagen vascular disease Autoimmune diseases, such as rheumatoid arthritis Previous and (more impactfully) current smoking history Diabetes mellitus (especially if hemoglobinA1C > 7.0) Underlying cardiovascular disease Chronic steroid use Increased patient age (>60 years of age) Previous breast radiation therapy Breast implant capsule presence |
| Liposuction technique: Suction at ½ atm (<−18 in Hg) Use ≥ 3.0 mm sized harvesting cannulas Vented harvesting cannulas preferred Closed fat container (avoid fat exposure to air)
|
| Proper identification of recipient area for fat grafting Pre-tunneling of recipient area soft tissues Minimal washing of fat (removes less growth factors) Centrifugation–filtration extraction of maximum fluid, blood, and oil (3000 rpms for 3 min, 100 μ filter Enrichment with platelet-rich plasma (PRP) Enrichment with autologous stem cells and SVF Micro-aliquot injection Multiple tissue plane depth placement Administration within two hours of harvesting Administration cannula > 3.0 mm diameter best Less volume grafted, greater adipocyte survival rate Subcutaneously preferred, then submuscular placement |
| Sex: All women Age: 22–75 years Height: 5′1″–5′10″ Weight: 46–106 kg BMI: 19.4–33.3 Fat volume (mean): 192.3 cc (first 83 patients) Fat volume (mean): 206.2 cc (last 35 patients) |
| Procedure Indications | Number of Cases |
|---|---|
| Primary aesthetic breast augmentation | 67 |
| Fat grafting after implant removal (staged) | 16 |
| Simultaneous implant exchange with fat (SIEF) | 15 |
| During or after breast lift/reduction | 16 |
| Breast cancer reconstruction | 4 |
| Complication | Number |
|---|---|
| Seroma τ | 6 |
| Hematoma | 0 |
| Palpable fibrotic areas Ω | 4 |
| Breast oil cysts | 0 |
| Bacterial infection ¶ | 1 |
| Nipple–areolar vascular compromise € | 1 |
| Scar revision of mammoplasty incision | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troell, R.J. Breast Augmentation in Body Contouring Using Autologous Stem Cell-Enriched Fat Grafting: Fifteen-Year Clinical Experience. J. Clin. Med. 2025, 14, 5607. https://doi.org/10.3390/jcm14165607
Troell RJ. Breast Augmentation in Body Contouring Using Autologous Stem Cell-Enriched Fat Grafting: Fifteen-Year Clinical Experience. Journal of Clinical Medicine. 2025; 14(16):5607. https://doi.org/10.3390/jcm14165607
Chicago/Turabian StyleTroell, Robert J. 2025. "Breast Augmentation in Body Contouring Using Autologous Stem Cell-Enriched Fat Grafting: Fifteen-Year Clinical Experience" Journal of Clinical Medicine 14, no. 16: 5607. https://doi.org/10.3390/jcm14165607
APA StyleTroell, R. J. (2025). Breast Augmentation in Body Contouring Using Autologous Stem Cell-Enriched Fat Grafting: Fifteen-Year Clinical Experience. Journal of Clinical Medicine, 14(16), 5607. https://doi.org/10.3390/jcm14165607






