Impact of Transcatheter Edge-to-Edge Repair on Tricuspid Annular Remodeling in Patients with Tricuspid Regurgitation
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Echocardiographic Evaluation
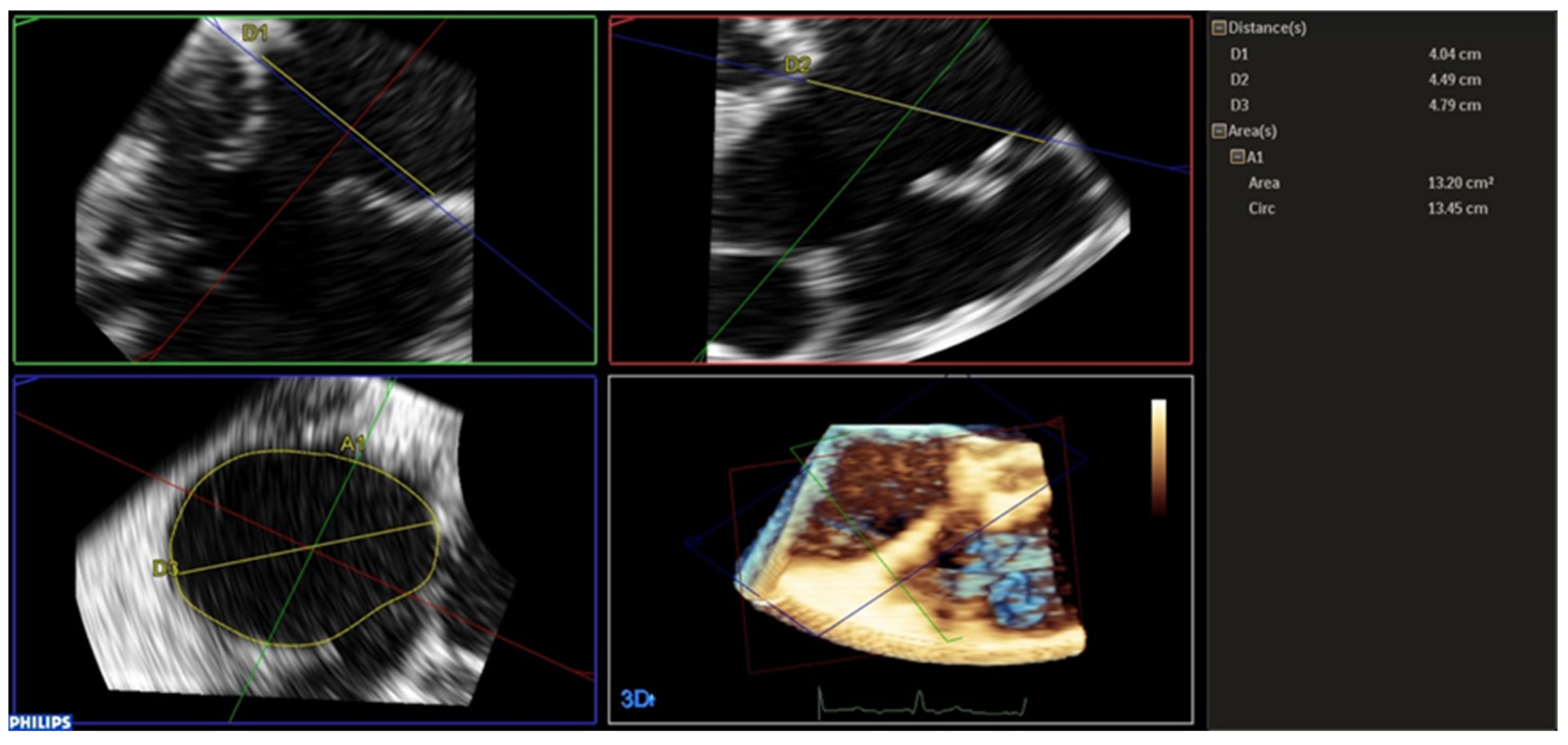
2.3. Tricuspid Annulus Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical and Echocardiographic Characteristics
3.2. Operative Outcomes
3.3. Echocardiographic Outcomes
3.4. Acute Tricuspid Annulus Remodelling
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TR | Tricuspid Regurgitation |
| TA | Tricuspid Annulus |
| RV | Right Ventricle |
| TV | Tricuspid Valve |
| 3D | Three Dimensional |
| CMR | Cardiac Magnetic Resonance |
| TEE | Transesophageal Echocardiogram |
| AF | Atrial Fibrillation |
References
- Prihadi, E.A.; Delgado, V.; Leon, M.B.; Enriquez-Sarano, M.; Topilsky, Y.; Bax, J.J. Morphologic Types of Tricuspid Regurgitation: Characteristics and Prognostic Implications. JACC Cardiovasc. Imaging 2019, 12, 491–499. [Google Scholar] [CrossRef]
- Nath, J.; Foster, E.; Heidenreich, P.A. Impact of Tricuspid Regurgitation on Long-Term Survival. J. Am. Coll. Cardiol. 2004, 43, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Chorin, E.; Rozenbaum, Z.; Topilsky, Y.; Konigstein, M.; Ziv-Baran, T.; Richert, E.; Keren, G.; Banai, S. Tricuspid regurgitation and long-term clinical outcomes. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-B.; Lee, S.-P.; Lee, J.-H.; Yoon, Y.E.; Park, E.-A.; Kim, H.-K.; Lee, W.; Kim, Y.-J.; Cho, G.-Y.; Sohn, D.-W. Quantification of Right Ventricular Volume and Function Using Single-Beat Three-Dimensional Echocardiography: A Validation Study with Cardiac Magnetic Resonance. J. Am. Soc. Echocardiogr. 2016, 29, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Zamorano, J.L. The need for a new tricuspid regurgitation grading scheme. Eur. Heart J.-Cardiovasc. Imaging 2017, 18, 1342–1343. [Google Scholar] [CrossRef]
- Hahn, R.T.; Meduri, C.U.; Davidson, C.J.; Lim, S.; Nazif, T.M.; Ricciardi, M.J.; Rajagopal, V.; Ailawadi, G.; Vannan, M.A.; Thomas, J.D.; et al. Early Feasibility Study of a Transcatheter Tricuspid Valve Annuloplasty: SCOUT Trial 30-Day Results. J. Am. Coll. Cardiol. 2017, 69, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Makkar, R.M.; Whisenant, B.K.; Hamid, N.; Naik, H.; Tadros, P.; Price, M.J.; Singh, G.; Schwartz, J.G.; Kapadia, S.; et al. Two-Year Outcomes of Transcatheter Edge-to-Edge Repair for Severe Tricuspid Regurgitation: The TRILUMINATE Pivotal Randomized Controlled Trial. Circulation 2025, 151, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Saracino, G.; Matsumura, Y.; Daimon, M.; Tran, H.; Greenberg, N.L.; Hozumi, T.; Yoshikawa, J.; Thomas, J.D.; Shiota, T. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: A real-time, 3-dimensional echocardiographic study. Circulation 2006, 114 (Suppl. S1), I492–I498. [Google Scholar] [CrossRef] [PubMed]
- Bocchino, P.P.; Angelini, F.; Vairo, A.; Andreis, A.; Fortuni, F.; Franchin, L.; Frea, S.; Raineri, C.; Pidello, S.; Conrotto, F.; et al. Clinical Outcomes Following Isolated Transcatheter Tricuspid Valve Repair: A Meta-Analysis and Meta-Regression Study. JACC Cardiovasc. Interv. 2021, 14, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Weber, M.; Lurz, P.; von Bardeleben, R.S.; Sitges, M.; Sorajja, P.; Hausleiter, J.; Denti, P.; Trochu, J.-N.; Näbauer, M.; et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet 2020, 394, 2002–2011, Erratum in Lancet 2020, 395, 870. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, M.; Benfari, G.; van der Bijl, P.; Alessandrini, H.; Attinger-Toller, A.; Biasco, L.; Lurz, P.; Braun, D.; Brochet, E.; Connelly, K.A.; et al. Transcatheter Versus Medical Treatment of Patients with Symptomatic Severe Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 2998–3008. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, A.; Vogelhuber, J.; Öztürk, C.; Schwaibold, Z.; Reckers, D.; Goto, T.; Kavsur, R.; Becher, M.U.; Zimmer, S.; Nickenig, G.; et al. PASCAL versus MitraClip-XTR edge-to-edge device for the treatment of tricuspid regurgitation: A propensity-matched analysis. Clin. Res. Cardiol. 2021, 110, 451–459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castriota, F.; Gobbi, G.; Squeri, A.; Nerla, R. Effective 3 Leaflet Grasping During TriClip Procedure: The “3-Point” Buzzer Beater. J. Invasive Cardiol. 2022, 34, E884. [Google Scholar] [CrossRef] [PubMed]
- Mangieri, A.; Sticchi, A.; Gohar, A.; Regazzoli, D.; Fazzari, F.; Pini, D.; Pellegrino, M.; Pagliaro, B.; Loiacono, F.; Chiarito, M.; et al. Percutaneous Tricuspid Valve Repair. Rev. Cardiovasc. Med. 2022, 23, 220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donal, E.; Shueler, R.; Goebel, B.; Lapp, H.; Moellmann, H.; Nickenig, G.; Bekeredjian, R.; Estevez, R.; Atmowihardjo, I.; Schmeisser, A.; et al. Real-world outcomes for tricuspid edge-to-edge repair: Initial echocardiographic results from the TriClip bRIGHT study. Eur. Heart J.-Cardiovasc. Imaging 2022, 23 (Suppl. S1), jeab289-203. [Google Scholar] [CrossRef]
- Dannenberg, V.; Koschutnik, M.; Donà, C.; Nitsche, C.; Mascherbauer, K.; Heitzinger, G.; Halavina, K.; Kammerlander, A.A.; Spinka, G.; Winter, M.-P.; et al. Invasive Hemodynamic Assessment and Procedural Success of Transcatheter Tricuspid Valve Repair-Important Factors for Right Ventricular Remodeling and Outcome. Front. Cardiovasc. Med. 2022, 9, 891468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albertini, A.; Nerla, R.; Castriota, F.; Squeri, A. Right ventricle remodeling after transcatheter tricuspid leaflet repair in patients with functional tricuspid regurgitation: Lessons from the surgical experience. Front. Cardiovasc. Med. 2022, 9, 977142. [Google Scholar] [CrossRef]
- Rommel, K.-P.; Besler, C.; Noack, T.; Blazek, S.; von Roeder, M.; Fengler, K.; Ender, J.; Gutberlet, M.; Desch, S.; Borger, M.A.; et al. Physiological and Clinical Consequences of Right Ventricular Volume Overload Reduction After Transcatheter Treatment for Tricuspid Regurgitation. JACC Cardiovasc. Interv. 2019, 12, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Lawlor, M.K.; Davidson, C.J.; Badhwar, V.; Sannino, A.; Spitzer, E.; Lurz, P.; Lindman, B.R.; Topilsky, Y.; Baron, S.J.; et al. Tricuspid valve academic research consortium definitions for tricuspid regurgitation and trial endpoints. Eur. Heart J. 2023, 44, 4508–4532. [Google Scholar] [CrossRef]
- Kodali, S.; Hahn, R.T.; Eleid, M.F.; Kipperman, R.; Smith, R.; Lim, D.S.; Gray, W.A.; Narang, A.; Pislaru, S.V.; Koulogiannis, K.; et al. CLASP TR EFS Investigators. Feasibility Study of the Transcatheter Valve Repair System for Severe Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2021, 77, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.K.; Hahn, R.T.; Davidson, C.J.; Narang, A.; Greenbaum, A.; Gleason, P.; Kapadia, S.; Miyasaka, R.; Zahr, F.; Chadderdon, S.; et al. 1-Year outcomes of transcatheter tricuspid valve repair. J. Am. Coll. Cardiol. 2023, 81, 1766–1776. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Whisenant, B.; Hamid, N.; Naik, H.; Makkar, R.; Tadros, P.; Price, M.J.; Singh, G.; Fam, N.; Kar, S.; et al. Transcatheter repair for patients with tricuspid regurgitation. N. Engl. J. Med. 2023, 388, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Badano, L.P.; Adamo, M.; Alessandrini, H.; Andreas, M.; Braun, D.; Connelly, K.A.; Denti, P.; Estevez-Loureiro, R.; Fam, N.; et al. Characteristics and outcomes of patients with atrial versus ventricular secondary tricuspid regurgitation undergoing tricuspid transcatheter edge-to-edge repair-Results from the TriValve registry. Eur. J. Heart Fail. 2023, 25, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, V.; Antonelli, G.; De Luca, V.M.; Piscione, M.; Carpenito, M.; Gaudio, D.; Nusca, A.; Cocco, N.; Mega, S.; Grigioni, F.; et al. 3D transoesophageal echocardiographic assessment of acute reverse remodelling of the tricuspid annulus after transcatheter edge-to-edge repair. Eur. Heart J.-Cardiovasc. Imaging 2025, 26, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Lurz, P.; von Bardeleben, R.S.; Weber, M.; Sitges, M.; Sorajja, P.; Hausleiter, J.; Denti, P.; Trochu, J.-N.; Nabauer, M.; Tang, G.H.; et al. Triluminate Investigators. Transcatheter Edge-to-Edge Repair for Treatment of Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2021, 77, 229–239. [Google Scholar] [CrossRef] [PubMed]
- De Bonis, M.; Lapenna, E.; La Canna, G.; Grimaldi, A.; Maisano, F.; Torracca, L.; Caldarola, A.; Alfieri, O. A novel technique for correction of severe tricuspid valve regurgitation due to complex lesions. Eur. J. Cardio-Thorac. Surg. 2004, 25, 760–765. [Google Scholar] [CrossRef] [PubMed]
| Parameters | All Patients (n = 26) |
|---|---|
| Age (years) | 79.3 (64–88) |
| Female Sex | 23 (88.5%) |
| Body mass index (kg/m2) | 25.2 (16.7–32.9) |
| Arterial Hypertension | 18 (69.2%) |
| Diabetes | 2 (7.7%) |
| Dyslipidemia | 14 (53.8%) |
| Smoke | 1 (3.8%) |
| NYHA Class | |
| I | 0 (0%) |
| II | 8 (30.8%) |
| III | 17 (65.4%) |
| IV | 1 (3.8%) |
| Coronary Artery Disease | 8 (30.8%) |
| PCI | 5 (19.2%) |
| CABG | 1 (3.8%) |
| Valvular Intervention | 8 (30.8%) |
| Aortic Valve Replacement | 2 |
| Mitral Valve Replacement | 4 |
| Mitral Valve Repair | 2 |
| Tricuspid Valve Repair | 1 |
| Mitraclip | 1 |
| Atrial Fibrillation | 24 (92.3%) |
| PMK/ICD | 4 (15.4%) |
| COPD | 3 (11.5%) |
| HF Hospitalization | 8 (30.8%) |
| Peripheral Edema | 11 (42.3%) |
| Diuretics | 26 (100.0%) |
| Hemoglobin (g/dL) | 12.51 ± 1.49 |
| CKD (eGFR < 60 mL/min) | 15 (57.7%) |
| Creatinine (mg/dL) | 1.08 ± 0.40 |
| NTproBNP (pg/mL) | 3234 ± 2948 |
| Parameters | All Patients (n = 26) |
|---|---|
| Sinus rhythm | 3 (11.5%) |
| Atrial Fibrillation/Atrial Flutter | 22 (84.7%) |
| Pacemaker rhythm | 1 (3.8%) |
| EDA (cmq) | 21.15 ± 6.06 |
| FAC (%) | 41.43 ± 5.69 |
| TAPSE (mm) | 18.08 ± 2.07 |
| PAPS (mmHg) | 49.65 ± 16.59 |
| EF (%) | 58.67 ± 7.14 |
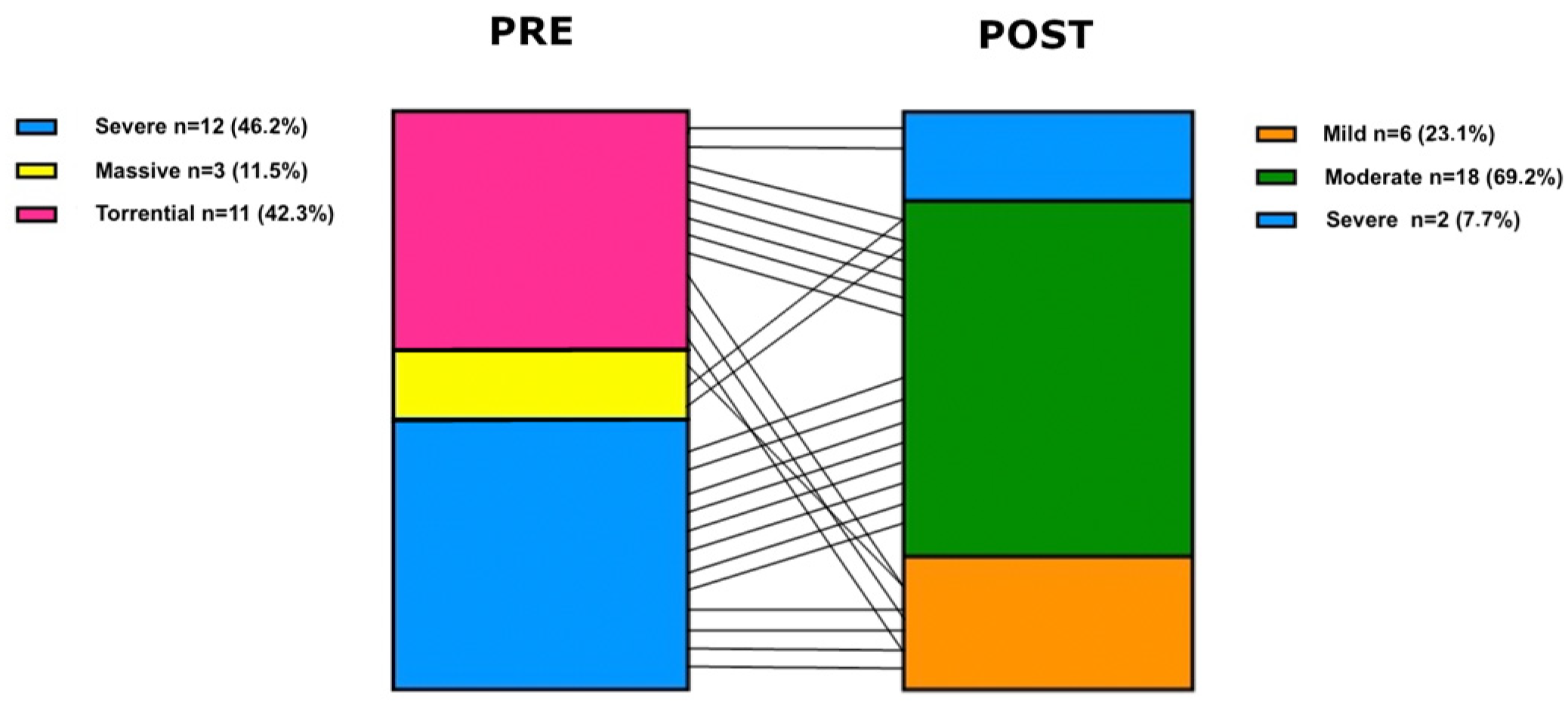
| Tricuspid Regurgitation | |
| Mild | 0 (0%) |
| Moderate | 0 (0%) |
| Severe | 12 (46.2%) |
| Massive | 3 (11.5%) |
| Torrential | 11 (42.3%) |
| Primary Mechanism | |
| Atrial Functional | 21 (80.8%) |
| Ventricular Functional | 2 (7.7%) |
| Primary | 1 (3.8%) |
| Lead associated | 2 (7.7%) |
| Mitral Regurgitation | |
| Mild | 11 (42.3%) |
| Moderate | 7 (26.9%) |
| Moderate-Severe | 3 (11.5%) |
| Aortic Regurgitation | |
| Mild | 13 (50.0%) |
| Moderate | 4 (15.4%) |
| Severe | 0 (0%) |
| Parameters | All Patients (n = 26) |
|---|---|
| Clip (n) | |
| 1 | 2 (7.7%) |
| 2 | 22 (84.6%) |
| 3 | 2 (7.69%) |
| A-S | 76.9% |
| P-S | 15.4% |
| A-P-S | 7.7% |
| A-P | 0% |
| Success | 26 (100%) |
| Complications | 2 (7.7%) |
| Death | 0 |
| Hospitalization Length (days) | 5 |
| Parameters | All Patients (n = 26) |
|---|---|
| Tricuspid regurgitation | |
| Mild | 6 (23.1%) |
| Moderate | 18 (69.2%) |
| Severe | 2 (7.7%) |
| TAPSE (mm) | 17.29 ± 3.58 |
| PAPS (mmHg) | 44.04 ± 12.85 |
| EF (%) | 59.96 ± 8.83 |
| Parameters | Before | After | p Value |
|---|---|---|---|
| SL diameter (cm) | 4.09 ± 0.44 | 3.54 ± 0.53 | <0.0001 |
| AP diameter (cm) | 4.29 ± 0.79 | 4.01 ± 0.62 | 0.0626 |
| Major diameter (cm) | 4.65 ± 0.63 | 4.28 ± 0.65 | 0.0002 |
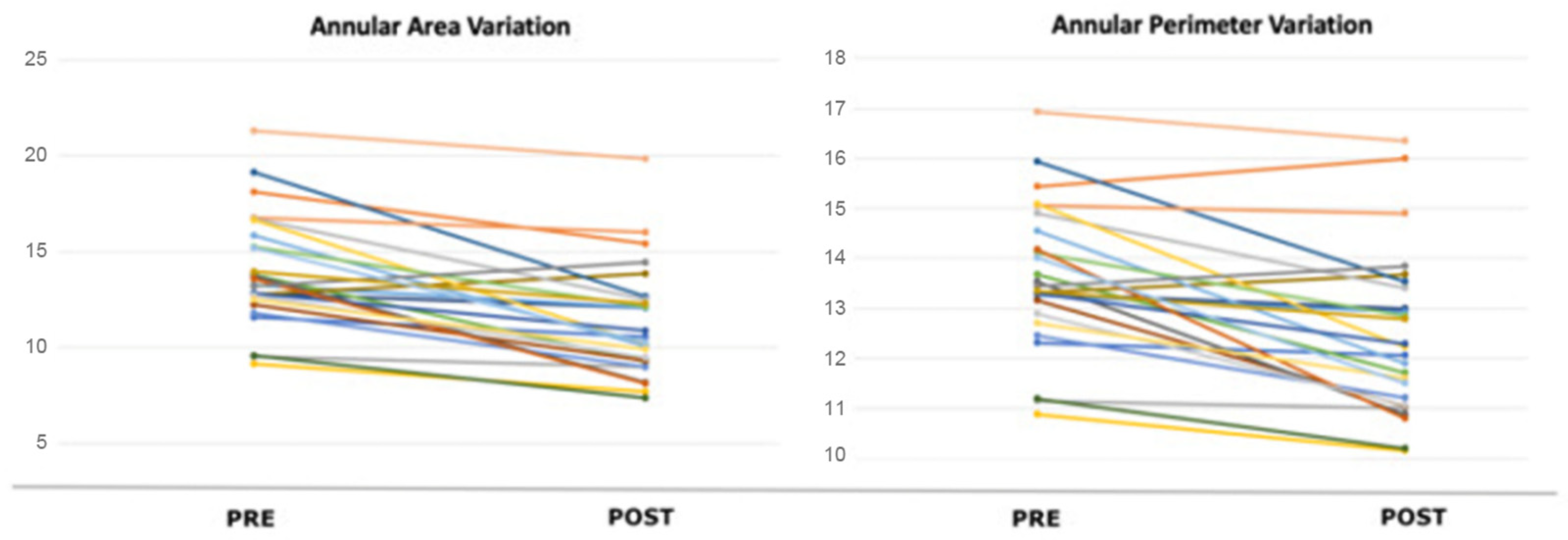
| Area (cmq) | 14.00 ± 2.91 | 11.25 ± 2.91 | <0.0001 |
| Perimeter (cm) | 13.62 ± 1.43 | 12.42 ± 1.62 | <0.0001 |
| Eccentricity Index | 0.98 ± 0.17 | 0.90 ± 0.17 | 0.0286 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widmann, M.; Nerla, R.; Castriota, F.; Fisicaro, A.; De Luca, V.M.; Pesarini, G.; Ribichini, F.L.; Squeri, A. Impact of Transcatheter Edge-to-Edge Repair on Tricuspid Annular Remodeling in Patients with Tricuspid Regurgitation. J. Clin. Med. 2025, 14, 5606. https://doi.org/10.3390/jcm14155606
Widmann M, Nerla R, Castriota F, Fisicaro A, De Luca VM, Pesarini G, Ribichini FL, Squeri A. Impact of Transcatheter Edge-to-Edge Repair on Tricuspid Annular Remodeling in Patients with Tricuspid Regurgitation. Journal of Clinical Medicine. 2025; 14(15):5606. https://doi.org/10.3390/jcm14155606
Chicago/Turabian StyleWidmann, Maddalena, Roberto Nerla, Fausto Castriota, Andrea Fisicaro, Valeria Maria De Luca, Gabriele Pesarini, Flavio Luciano Ribichini, and Angelo Squeri. 2025. "Impact of Transcatheter Edge-to-Edge Repair on Tricuspid Annular Remodeling in Patients with Tricuspid Regurgitation" Journal of Clinical Medicine 14, no. 15: 5606. https://doi.org/10.3390/jcm14155606
APA StyleWidmann, M., Nerla, R., Castriota, F., Fisicaro, A., De Luca, V. M., Pesarini, G., Ribichini, F. L., & Squeri, A. (2025). Impact of Transcatheter Edge-to-Edge Repair on Tricuspid Annular Remodeling in Patients with Tricuspid Regurgitation. Journal of Clinical Medicine, 14(15), 5606. https://doi.org/10.3390/jcm14155606






