Endoscopic Verification of Transpapillary Access in Supine Percutaneous Nephrolithotomy: A Prospective Pilot Study Comparing Freehand Ultrasound and Fluoroscopy Guidance
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Patient Consent
2.2. Study Design and Patients
- -
- Patients aged 18 years or older;
- -
- Patients with solitary renal pelvis or lower calyceal stone.
- -
- Patients aged under 18 years;
- -
- Patients with multiple or/and complex stones (partial staghorn, staghorn, etc.);
- -
- Patients with renal anomalies (horseshoe kidney, ectopic kidney, rotation anomaly, etc.);
- -
- Patients with uncontrolled coagulopathy;
- -
- Patients requiring multiple renal accesses (multi-tract PNL);
- -
- Patients in whom flexible ureteroscope could not be advanced to the access site due to stone burden.
2.3. Study Outcomes
2.4. Preoperative Evaluation
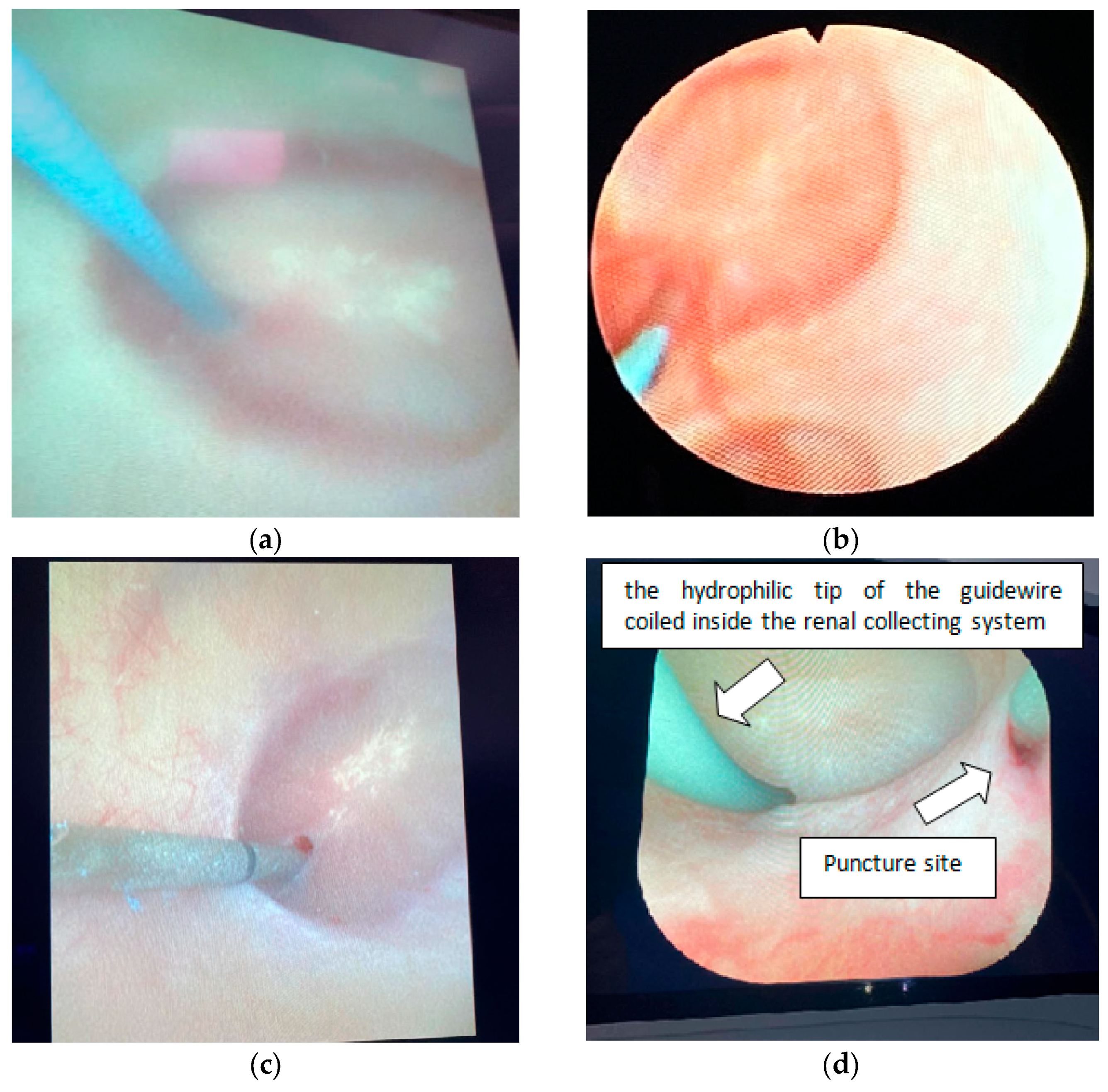
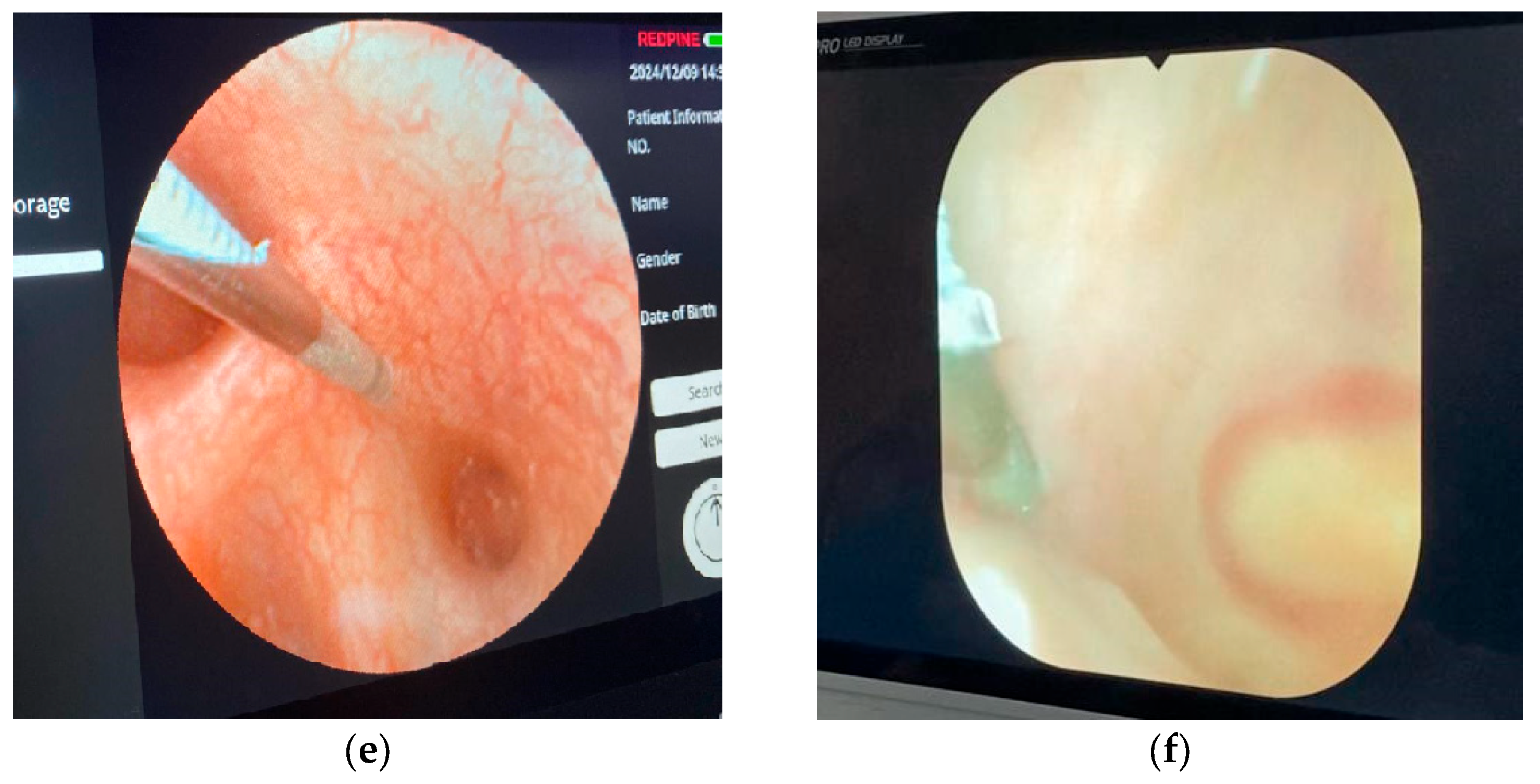
2.5. Surgical Technique
2.6. Clinical and Perioperative Data Collection and Follow-Up
2.7. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Stone Characteristics
3.3. Perioperative and Postoperative Outcomes
- -
- The targeted calyx for access differed significantly between groups (p: 0.043), with more inferior calyx access in the FG group.
- -
- No fluoroscopy was used for renal access in the F-UG group.
- -
- The average puncture time was significantly shorter in the FG group (p < 0.001).
- -
- The renal access type showed a statistically significant difference, with the FG group having more transpapillary access (p: 0.003).
- -
- The total fluoroscopy time was significantly lower in the F-UG group (p < 0.001).
- -
- Complication rates, classified using the Clavien–Dindo system, showed no significant difference (p: 0.092). The FG group had no complications. The F-UG group had two Clavien Grade 2 (bleeding required blood transfusion) and one Clavien Grade 4a (admission to the intensive care unit due to urosepsis and administration of appropriate intravenous antibiotic therapy) complications.
| FG Access (n: 23) | F-UG Access (n: 20) | p-Value | ||||
|---|---|---|---|---|---|---|
| Targeted calyx for access | Inferior calyx | 14 (60.9%) | 6 (30.0%) | 0.043 ** | ||
| Middle calyx | 9 (39.1%) | 14 (70.0%) | ||||
| Lithotripsy method | Pneumatic | 2 (8.7%) | 4 (20.0%) | 0.446 **** | ||
| Laser | 18 (78.3%) | 15 (75.0%) | ||||
| Pneumatic + laser | 3 (13.0%) | 1 (5.0%) | ||||
| D/J stent | Yes | 23 (100.0%) | 19 (95.0%) | 0.465 **** | ||
| No | 0 (0.0%) | 1 (5.0%) | ||||
| Nephrostomy tube | Yes | 1 (4.3%) | 3 (15.0%) | 0.323 **** | ||
| No | 22 (95.7%) | 17 (85.0%) | ||||
| Number of puncture attempts for access (mean ± st. deviation/median/min-max) | 2.5 ± 1.7 | 1.8 ± 0.8 | 0.177 *** | |||
| 2.0 | 2.0 | |||||
| 1.0–8.0 | 1.0–4.0 | |||||
| Fluoroscopy time for percutaneous renal access (second) (mean ± st. deviation/min-max) | 15.3 ± 12.7 | 0.0 | ||||
| 5.0–60.0 | ||||||
| Average puncture time (minute) (mean ± st. deviation/median/min-max) | 2.51 ± 2.34 | 5.68 ± 3.17 | <0.001 *** | |||
| 2.00 | 5.50 | |||||
| 0.50–10.00 | 0.75–14.00 | |||||
| Renal access | Transpapillary | 22 (95.7%) | 11 (55.0%) | 0.003 **** | ||
| Nonpapillary | 1 (4.3%) | 9 (45.0%) | ||||
| Total fluoroscopy time (second) (mean ± st. deviation/median/min-max) | 23.3 ± 12.7 | 7.8 ± 4.8 | <0.001 *** | |||
| 20.0 | 7.0 | |||||
| 10.0–66.0 | 2.0–20.0 | |||||
| Operation time (minute) (mean ± st. deviation/median/min-max) | 79.6 ± 28.0 | 72.2 ± 13.9 | 0.546 *** | |||
| 70.0 | 70.0 | |||||
| 45.0–160.0 | 50.0–95.0 | |||||
| Complications (Clavien–Dindo classification) | No | 23 (100.0%) | 17 (85.0%) | 0.092 **** | ||
| Grade 2 | 0 (0.0%) | 2 (10.0%) | ||||
| Grade 4a | 0 (0.0%) | 1 (5.0%) | ||||
| Hematocrit drop (mean ± st. deviation/median/min-max) | 2.92 ± 1.44 | 4.46 ± 3.20 | 0.428 *** | |||
| 2.90 | 3.20 | |||||
| 0.00–6.20 | 0.00–14.50 | |||||
| Blood transfusion | Yes | 0 (0.0%) | 2 (10.0%) | 0.210 **** | ||
| No | 23 (100.0%) | 18 (90.0%) | ||||
| Hospitalization time (day) (mean ± st. deviation/median/min-max) | 1.5 ± 0.5 | 2.1 ± 1.4 | 0.258 *** | |||
| 2.0 | 2.0 | |||||
| 1.0–2.0 | 1.0–7.0 | |||||
| 1st-month stone-free rate | Stone-free | 21 (91.4%) | 17 (85.0%) | 0.790 **** | ||
| CIRFs | 1 (4.3%) | 2 (10.0%) | ||||
| Failure | 1 (4.3%) | 1 (5.0%) | ||||
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PNL | Percutaneous nephrolithotomy |
| F-UG | Freehand ultrasound-guided |
| FG | Fluoroscopy-guided |
| NCCT | Non-Contrast-Enhanced Computed Tomography |
| BMI | Body mass index |
| HU | Hounsfield unit |
| SF | Stone-free |
| CIRFs | Clinically insignificant residual fragments |
References
- Fernström, I.; Johannsson, B. Percutaneous pyelolithotomy: A new extraction technique. Scand. J. Urol. Nephrol. 1976, 10, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Skolarikos, A.; Jung, H.; Neisius, A.; Petřík, A.; Somani, B.; Tailly, T.; Gambaro, G. European Association of Urology Guidelines on Urolithiasis. In EAU Guidelines; European Association of Urology: Arnhem, The Netherlands, 2024. [Google Scholar]
- Assimos, D.; Krambeck, A.; Miller, N.L.; Monga, M.; Murad, M.H.; Nelson, C.P.; Pace, K.T.; Pais, V.M.; Pearle, M.S.; Preminger, G.M.; et al. Surgical management of stones: American Urological Association/Endourological Society Guideline part, I.I. J. Urol. 2016, 196, 1161. [Google Scholar] [CrossRef]
- Sampaio, F.J.; Aragao, A.H. Anatomical relationship between the intrarenal arteries and the kidney collecting system. J. Urol. 1990, 143, 679–681. [Google Scholar] [CrossRef]
- Sampaio, F.J.; Zanier, J.F.; Aragão, A.H.; Favorito, L.A. Intrarenal access: 3-dimensional anatomical study. J. Urol. 1992, 148, 1769–1773. [Google Scholar] [CrossRef]
- Kyriazis, I.; Kallidonis, P.; Vasilas, M.; Panagopoulos, V.; Kamal, W.; Liatsikos, E. Challenging the wisdom of puncture at the calyceal fornix in percutaneous nephrolithotripsy: Feasibility and safety study with 137 patients operated via a non-calyceal percutaneous track. World J. Urol. 2017, 35, 795–801. [Google Scholar] [CrossRef]
- Kallidonis, P.; Kyriazis, I.; Kotsiris, D.; Koutava, A.; Kamal, W.; Liatsikos, E. Papillary vs Nonpapillary Puncture in Percutaneous Nephrolithotomy: A Prospective Randomized Trial. J. Endourol. 2017, 31, S4–S9. [Google Scholar] [CrossRef]
- Alken, P. Percutaneous nephrolithotomy—The puncture. BJU Int. 2022, 129, 17–24. [Google Scholar] [CrossRef]
- Pearle, M.S. Puncture for percutaneous surgery: Is the papillary puncture a dogma? No! Curr. Opin. Urol. 2019, 29, 472–473. [Google Scholar] [CrossRef]
- Kalogeropoulou, C.; Kallidonis, P.; Liatsikos, E.N. Imaging in percutaneous nephrolithotomy. J. Endourol. 2009, 23, 1571–1577. [Google Scholar] [CrossRef]
- Basiri, A.; Kashi, A.H.; Zeinali, M.; Nasiri, M.; Sarhangnejad, R.; Valipour, R. Ultrasound—Guided access during percutaneous nephrolithotomy: Entering desired calyx with appropriate entry site and angle. Int. Braz. J. Urol. 2016, 42, 1160–1167. [Google Scholar] [CrossRef]
- Tahra, A.; Sobay, R.; Bindayi, A.; Suceken, F.Y.; Kucuk, E.V. Papillary vs non-papillary access during percutaneous nephrolithotomy: Retrospective, match-paired case-control study. Arch. Ital. Urol. Androl. 2020, 92, 50–52. [Google Scholar] [CrossRef]
- Bach, C.; Goyal, A.; Kumar, P.; Kachrilas, S.; Papatsoris, A.G.; Buchholz, N.; Masood, J. The Barts ‘flank-free’ modified supine position for percutaneous nephrolithotomy. Urol. Int. 2012, 89, 365–368. [Google Scholar] [CrossRef]
- Desai, M. Ultrasonography-guided punctures-with and without puncture guide. J. Endourol. 2009, 23, 1641–1643. [Google Scholar] [CrossRef]
- Chu, C.; Masic, S.; Usawachintachit, M.; Hu, W.; Yang, W.; Stoller, M.; Li, J.; Chi, T. Ultrasound-Guided Renal Access for Percutaneous Nephrolithotomy: A Description of Three Novel Ultrasound-Guided Needle Techniques. J. Endourol. 2016, 30, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Scoffone, C.M.; Cracco, C.M.; Manera, A.; Poggio, M.; Scarpa, R.M. Supine Percutaneous Nephrolithotomy and ECIRS; Springer: Paris, France, 2014; pp. 1–196. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.; Smith, N.C.; Hegarty, N.; Glass, J.M. The Guy’s stone score—Grading the complexity of percutaneous nephrolithotomy procedures. Urology 2011, 78, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Breda, A.; Territo, A.; Scoffone, C.; Seitz, C.; Knoll, T.; Herrmann, T.; Brehmer, M.; Osther, P.J.S.; Liatsikos, E. The evaluation of radiologic methods for access guidance in percutaneous nephrolithotomy: A systematic review of the literature. Scand. J. Urol. 2018, 52, 81–86. [Google Scholar] [CrossRef]
- Cracco, C.M.; Scoffone, C.M. Comment on: “Non-papillary percutaneous nephrolithotomy for treatment of staghorn stones”. Minerva Urol. Nephrol. 2021, 73, 691–693. [Google Scholar] [CrossRef]
- Khan, F.; Borin, J.F.; Pearle, M.S.; McDougall, E.M.; Clayman, R.V. Endoscopically guided percutaneous renal access: “seeing is believing”. J. Endourol. 2006, 20, 451–455; discussion 455. [Google Scholar] [CrossRef]
- Sugino, T.; Hamamoto, S.; Unno, R.; Taguchi, K.; Okada, A.; Yasui, T. Effectiveness of ureteroscope-assisted renal puncture for endoscopic combined intrarenal surgery. Int. J. Urol. 2019, 26, 424–425. [Google Scholar] [CrossRef]
- Jagtap, J.; Mishra, S.; Bhattu, A.; Ganpule, A.; Sabnis, R.; Desai, M.R. Which is the preferred modality of renal access for a trainee urologist: Ultrasonography or fluoroscopy? Results of a prospective randomized trial. J. Endourol. 2014, 28, 1464–1469. [Google Scholar] [CrossRef]
- Agarwal, M.; Agrawal, M.S.; Jaiswal, A.; Kumar, D.; Yadav, H.; Lavania, P. Safety and efficacy of ultrasonography as an adjunct to fluoroscopy for renal access in percutaneous nephrolithotomy (PCNL). BJU Int. 2011, 108, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Birowo, P.; Raharja, P.A.R.; Putra, H.W.K.; Rustandi, R.; Atmoko, W.; Rasyid, N. X-ray-free ultrasound-guided versus fluoroscopy-guided percutaneous nephrolithotomy: A comparative study with historical control. Int. Urol. Nephrol. 2020, 52, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Andonian, S.; Scoffone, C.M.; Louie, M.K.; Gross, A.J.; Grabe, M.; Daels, F.P.; Shah, H.N.; de la Rosette, J.J.; CROES PCNL Study Group. Does imaging modality used for percutaneous renal access make a difference? A matched case analysis. J. Endourol. 2013, 27, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Basiri, A.; Ziaee, A.M.; Kianian, H.R.; Mehrabi, S.; Karami, H.; Moghaddam, S.M.H. Ultrasonographic versus fluoroscopic access for percutaneous nephrolithotomy: A randomized clinical trial. J. Endourol. 2008, 22, 281–284. [Google Scholar] [CrossRef]
- Rao, P.N.; Faulkner, K.; Sweeney, J.K.; Asbury, D.L.; Sambrook, P.; Blacklock, N.J. Radiation dose to patient and staff during percutaneous nephrostolithotomy. Br. J. Urol. 1987, 59, 508–512. [Google Scholar] [CrossRef]
- Arabzadeh Bahri, R.; Maleki, S.; Shafiee, A.; Shobeiri, P. Ultrasound versus fluoroscopy as imaging guidance for percutaneous nephrolithotomy: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0276708. [Google Scholar] [CrossRef]
- Du, R.; Feng, W.; Yi, T. Efficacy and Safety of Ultrasound- vs. Fluoroscopy-Guided Percutaneous Nephrolithotomy in Managing Renal Calculi: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Urology 2025, 198, 170–178. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, L.; Cai, X.; Jin, T.; Wang, K. Fluoroscopy versus ultrasound for image guidance during percutaneous nephrolithotomy: A systematic review and meta-analysis. Urolithiasis 2017, 45, 481–487. [Google Scholar] [CrossRef]
- Yang, Y.H.; Wen, Y.C.; Chen, K.C.; Chen, C. Ultrasound-guided versus fluoroscopy-guided percutaneous nephrolithotomy: A systematic review and meta-analysis. World J. Urol. 2019, 37, 777–788. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, P.; Xu, X.; Fan, M. Ultrasonographic versus Fluoroscopic Access for Percutaneous Nephrolithotomy: A Meta-Analysis. Urol. Int. 2015, 95, 15–25. [Google Scholar] [CrossRef]
- Falahatkar, S.; Allahkhah, A.; Kazemzadeh, M.; Enshaei, A.; Shakiba, M.; Moghaddas, F. Complete supine PCNL: Ultrasound vs. fluoroscopic guided: A randomized clinical trial. Int. Braz. J. Urol. 2016, 42, 710–716. [Google Scholar] [CrossRef]

| FG Access (n: 23) | F-UG Access (n: 20) | p-Value | ||
|---|---|---|---|---|
| Age (mean ± st. deviation/median/min-max) | 47.6 ± 15.0 | 46.5 ± 13.3 | 0.802 * | |
| 49.0 | 45.5 | |||
| 22.0–81.0 | 24.0–79.0 | |||
| Gender | Male | 18 (78.3%) | 10 (50.0%) | 0.052 ** |
| Female | 5 (21.7%) | 10 (50.0%) | ||
| BMI (body mass index) (mean ± st. deviation/median/min-max) | 26.49 ± 4.26 | 27.19 ± 2.72 | 0.210 *** | |
| 26.10 | 27.35 | |||
| 19.50–37.90 | 22.00–33.10 | |||
| Comorbidities (diabetes, hypertension, etc.) | Yes | 12 (52.2%) | 6 (30%) | 0.142 ** |
| No | 11 (47.8%) | 14 (70%) | ||
| Previous SWL/surgery | SWL | 4 | 5 | |
| URS | 3 | 2 | ||
| RIRS | 0 | 1 | ||
| PNL | 4 | 0 | ||
| Open surgery | 1 | 0 | ||
| Lap. surgery | 1 | 0 | ||
| FG Access (n: 23) | F-UG Access (n: 20) | p-Value | ||
|---|---|---|---|---|
| Stone side | Right | 16 (69.6%) | 10 (50.0%) | 0.191 ** |
| Left | 7 (30.4%) | 10 (50.0%) | ||
| Stone opacity | Opaque | 18 (78.3%) | 17 (85.0%) | 0.704 **** |
| Semi-opaque | 5 (21.7%) | 3 (15.0%) | ||
| Non-opaque | 0 (0.0%) | 0 (0.0%) | ||
| Hydronephrosis | No | 7 (30.4%) | 3 (15.0%) | 0.361 **** |
| Grade 1 | 7 (30.4%) | 10 (50.0%) | ||
| Grade 2 | 9 (39.2%) | 7 (35.0%) | ||
| Stone size (mm) (mean ± st. deviation/median/min-max) | 23.5 ± 5.0 | 23.8 ± 5.1 | 0.841 *** | |
| 22.0 | 22.0 | |||
| 18.0–39.0 | 19.0–38.0 | |||
| Stone volume (mm3) (length × width × depth × π/6) (mean ± st. deviation/median/min-max) | 3413 ± 2308 | 3495 ± 2039 | 0.534 *** | |
| 2787 | 2966 | |||
| 1313–10128 | 1313–10160 | |||
| Hounsfield unit (mean ± st. deviation/median/min-max) | 1117 ± 251 | 1019 ± 250 | 0.208 * | |
| 1135 | 935 | |||
| 442–1505 | 465–1447 | |||
| Stone localization | Pelvis | 17 (73.9%) | 18 (90.0%) | 0.250 **** |
| Lower calyx | 6 (26.1%) | 2 (10.0%) | ||
| The Guy’s Stone Score | Grade 1 | 23 (100%) | 20 (100%) | ⸸ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bicaklioglu, F.; Eryildirim, B. Endoscopic Verification of Transpapillary Access in Supine Percutaneous Nephrolithotomy: A Prospective Pilot Study Comparing Freehand Ultrasound and Fluoroscopy Guidance. J. Clin. Med. 2025, 14, 5604. https://doi.org/10.3390/jcm14155604
Bicaklioglu F, Eryildirim B. Endoscopic Verification of Transpapillary Access in Supine Percutaneous Nephrolithotomy: A Prospective Pilot Study Comparing Freehand Ultrasound and Fluoroscopy Guidance. Journal of Clinical Medicine. 2025; 14(15):5604. https://doi.org/10.3390/jcm14155604
Chicago/Turabian StyleBicaklioglu, Fatih, and Bilal Eryildirim. 2025. "Endoscopic Verification of Transpapillary Access in Supine Percutaneous Nephrolithotomy: A Prospective Pilot Study Comparing Freehand Ultrasound and Fluoroscopy Guidance" Journal of Clinical Medicine 14, no. 15: 5604. https://doi.org/10.3390/jcm14155604
APA StyleBicaklioglu, F., & Eryildirim, B. (2025). Endoscopic Verification of Transpapillary Access in Supine Percutaneous Nephrolithotomy: A Prospective Pilot Study Comparing Freehand Ultrasound and Fluoroscopy Guidance. Journal of Clinical Medicine, 14(15), 5604. https://doi.org/10.3390/jcm14155604







