Balancing Stone Prevention and Kidney Function: A Therapeutic Dilemma
Abstract
1. Introduction
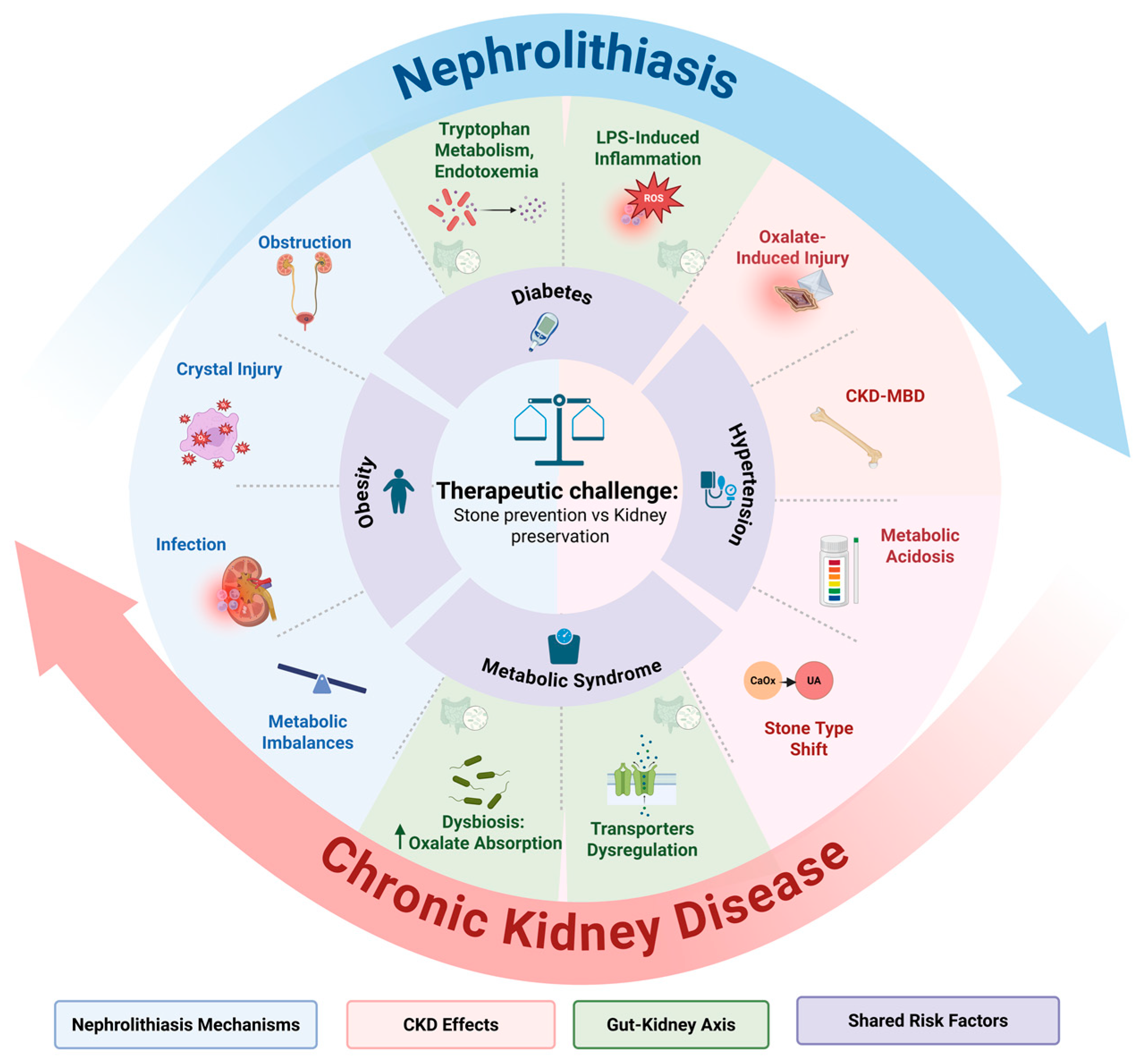
2. Pathophysiology of the Nephrolithiasis–CKD Cycle
2.1. Shared Risk Factors
2.1.1. Hypertension
2.1.2. Diabetes Mellitus
2.1.3. Obesity
2.1.4. Metabolic Syndrome
2.2. Mechanisms Linking Nephrolithiasis to Kidney Damage
2.2.1. Obstruction
2.2.2. Crystal-Induced Tubular Injury
2.2.3. Infection
2.2.4. Metabolic and Chemical Imbalance
- Hyperoxaluria (>44 mg/day), from dietary intake, enteric malabsorption, or primary hyperoxaluria, increases urinary supersaturation, fostering COM crystal formation [66,67]. Oxalates trigger NADPH oxidase-mediated ROS production, which disrupts mitochondrial membrane potential and activates caspase-9-mediated apoptosis [65,66,67]. Elevated oxalate also upregulates angiotensin II via AT1 receptors, enhancing ROS and promoting tubular injury and interstitial fibrosis through TGF-β/Smad3 signaling [37,54,67]. Clinically, hyperoxaluria correlates with a 33% higher CKD progression risk and 45% higher end-category kidney disease (ESKD) risk [68], as impaired oxalate clearance concentrates it further.
- Hypocitraturia (<320 mg/day), often secondary to metabolic acidosis (HCO3− < 22 mmol/L) or CKD-induced proximal tubular dysfunction, reduces citrate’s ability to chelate calcium and inhibit crystal growth [63,69]. Acidosis upregulates the Na+/dicarboxylate cotransporter (NaDC-1), lowering urinary citrate and increasing ROS, promoting COM and uric acid stones with IL-6/TNF-α-driven fibrosis [63,70]. In CKD, citrate depletion accelerates, creating a feedback loop where reduced GFR worsens hypocitraturia [69].
- Hypercalciuria (defined as >250 mg/day in women and >300 mg/day in men), whether idiopathic or secondary, is a significant risk factor for calcium oxalate and calcium phosphate stone formation [71,72]. Excess urinary calcium can bind to tubular epithelial cells via integrin receptors, triggering oxidative stress and activating the NLRP3 inflammasome, which leads to the release of IL-1β and amplifies inflammation [71,72,73]. Chronic hypercalciuria also contributes to the formation of Randall’s plaques, which are interstitial calcium phosphate deposits in the renal papillae [72,73]. These plaques can erode into the collecting system, serving as a nidus for stone formation. Over time, this process can cause tubulointerstitial damage and is associated with an increased risk of CKD [74].
- Hyperuricosuria (>700 mg/day), often from purine-rich diets or gout, typically acidifies urine (pH < 5.5), promoting uric acid crystal precipitation [75,76]. These crystals can obstruct tubules and activate NLRP3 inflammasome via xanthine oxidase-driven ROS [75,76]. However, in specific contexts, elevated uric acid levels may be associated with better renal outcomes [77], possibly due to its extracellular antioxidant properties, scavenging ROS, and potentially reducing oxidative stress-mediated tubular injury [78]. This protective effect is most evident in early-category CKD patients with preserved GFR, though the exact magnitude of risk reduction remains unclear. This protective effect diminishes in advanced CKD, where reduced clearance concentrates uric acid, shifting stone composition toward uric acid and amplifying damage via NLRP3 and MCP-1-driven fibrosis, reflecting a bidirectional dynamic [75,76].
2.2.5. Gut–Kidney Axis Interactions
2.3. Dual Role of CKD in Kidney Stone Formation
3. Current Therapeutic Strategies: Benefits and Risks in CKD
3.1. Iatrogenic Interventions
3.2. Fluid Intake
3.3. Dietary Modifications
3.4. Pharmacological Interventions
3.4.1. Thiazides
3.4.2. Potassium Citrate
3.4.3. Allopurinol
4. Dual-Purpose Therapeutic Strategies
4.1. Minimally Invasive Technologies
4.2. Microbiota-Based Interventions
4.3. Sodium–Glucose Cotransporter 2 Inhibitors
4.4. Omega-3 Fatty Acids
4.5. Magnesium
4.6. Noncalcium Phosphate Binders
5. Integrated Management and Future Directions for Nephrolithiasis in CKD
6. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Alelign, T.; Petros, B. Kidney Stone Disease: An Update on Current Concepts. Adv. Urol. 2018, 2018, 3068365. [Google Scholar] [CrossRef] [PubMed]
- Shastri, S.; Patel, J.; Sambandam, K.K.; Lederer, E.D. Kidney Stone Pathophysiology, Evaluation and Management: Core Curriculum 2023. Am. J. Kidney Dis. 2023, 82, 617–634. [Google Scholar] [CrossRef]
- Szymanski, J.; Chlosta, M.; Dudek, P.; Rajwa, P.; Krajewski, W.; Bryniarski, P.; Krzanowska, K.; Chlosta, P.; Przydacz, M. Prevalence, Correlates, and Treatment Behaviors for Urolithiasis and Renal Colic-like Pain Symptoms at the Population Level in Poland. Sci. Rep. 2025, 15, 10827. [Google Scholar] [CrossRef]
- Stamatelou, K.; Goldfarb, D.S. Epidemiology of Kidney Stones. Healthcare 2023, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Moftakhar, L.; Jafari, F.; Ghoddusi Johari, M.; Rezaeianzadeh, R.; Hosseini, S.V.; Rezaianzadeh, A. Prevalence and risk factors of kidney stone disease in population aged 40-70 years old in Kharameh cohort study: A cross-sectional population-based study in southern Iran. BMC Urol. 2022, 22, 205. [Google Scholar] [CrossRef]
- Awedew, A.F.; Han, H.; Berice, B.N.; Dodge, M.; Schneider, R.D.; Abbasi-Kangevari, M.; Al-Aly, Z.; Almidani, O.; Alvand, S.; Arabloo, J.; et al. The Global, Regional, and National Burden of Urolithiasis in 204 Countries and Territories, 2000–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. EClinicalMedicine 2024, 78, 102924. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of Chronic Kidney Disease: An Update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic Kidney Disease and the Global Public Health Agenda: An International Consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, C.; Jian, Z.; Wen, J.; Xiang, L.; Li, H.; Wang, K.; Jin, X. Risk Factors for Nephrolithiasis Formation: An Umbrella Review. Int. J. Surg. 2024, 110, 5733–5744. [Google Scholar] [CrossRef]
- Rule, A.D.; Bergstralh, E.J.; Melton, L.J.; Li, X.; Weaver, A.L.; Lieske, J.C. Kidney Stones and the Risk for Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 804. [Google Scholar] [CrossRef]
- Lu, G.; Tian, J.; Shi, F.; Zhang, D.-G.; Wang, D. Association of Cardiovascular-Kidney-Metabolic Syndrome Stages with Kidney Stone Prevalence: A Population-Based Analysis of NHANES 2007–2020. BMJ Open 2025, 15, e096533. [Google Scholar] [CrossRef] [PubMed]
- Keddis, M.T.; Rule, A.D. Nephrolithiasis and Loss of Kidney Function. Curr. Opin. Nephrol. Hypertens. 2013, 22, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.K.; Park, J.T.; Chang, T.I. Risk of Incident Chronic Kidney Disease among Patients with Urolithiasis: A Nationwide Longitudinal Cohort Study. Clin. Kidney J. 2024, 17, sfae030. [Google Scholar] [CrossRef]
- Kummer, A.E.; Grams, M.; Lutsey, P.; Chen, Y.; Matsushita, K.; Köttgen, A.; Folsom, A.R.; Coresh, J. Nephrolithiasis as a Risk Factor for CKD: The Atherosclerosis Risk in Communities Study. Clin. J. Am. Soc. Nephrol. 2015, 10, 2023–2229. [Google Scholar] [CrossRef]
- Chuang, T.F.; Hung, H.C.; Li, S.F.; Lee, M.W.; Pai, J.Y.; Hung, C.T. Risk of Chronic Kidney Disease in Patients with Kidney Stones—A Nationwide Cohort Study. BMC Nephrol. 2020, 21, 292. [Google Scholar] [CrossRef]
- Simmons, K.E.; Nair, H.R.; Phadke, M.; Motamedinia, P.; Singh, D.; Montgomery, T.A.; Dahl, N.K. Risk Factors for Common Kidney Stones Are Correlated with Kidney Function Independent of Stone Composition. Am. J. Nephrol. 2023, 54, 329–336. [Google Scholar] [CrossRef]
- Peerapen, P.; Thongboonkerd, V. Kidney Stone Prevention. Adv. Nutr. 2023, 14, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.T.; Garcia-Garcia, G.; Lui, S.F.; Andreoli, S.; Fung, W.W.S.; Hradsky, A.; Kumaraswami, L.; Liakopoulos, V.; Rakhimova, Z.; Saadi, G.; et al. Kidney Health for Everyone Everywhere—From Prevention to Detection and Equitable Access to Care. Can. J. Kidney Health Dis. 2020, 7, 2054358120910569. [Google Scholar] [CrossRef]
- Chew, B.H.; Miller, L.E.; Eisner, B.; Bhattacharyya, S.; Bhojani, N. Prevalence, Incidence, and Determinants of Kidney Stones in a Nationally Representative Sample of US Adults. JU Open Plus 2024, 2, e00006. [Google Scholar] [CrossRef]
- Chen, A.; Zou, M.; Young, C.A.; Zhu, W.; Chiu, H.C.; Jin, G.; Tian, L. Disease Burden of Chronic Kidney Disease Due to Hypertension From 1990 to 2019: A Global Analysis. Front. Med. 2021, 8, 690487. [Google Scholar] [CrossRef]
- Ameer, O.Z. Hypertension in Chronic Kidney Disease: What Lies behind the Scene. Front. Pharmacol. 2022, 13, 949260. [Google Scholar] [CrossRef] [PubMed]
- Muschialli, L.; Mannath, A.; Moochhala, S.H.; Shroff, R.; Ferraro, P.M. Epidemiological and Biological Associations between Cardiovascular Disease and Kidney Stone Formation: A Systematic Review and Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Is Oxidative Stress, a Link between Nephrolithiasis and Obesity, Hypertension, Diabetes, Chronic Kidney Disease, Metabolic Syndrome? Urol. Res. 2012, 40, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Shin, J.Y.; Kang, E.H. Risk of Nephrolithiasis Associated With SGLT2 Inhibitors Versus DPP4 Inhibitors Among Patients With Type 2 Diabetes: A Target Trial Emulation Study. Diabetes Care 2025, 48, 193–201. [Google Scholar] [CrossRef]
- Kumar, M.; Dev, S.; Khalid, M.U.; Siddenthi, S.M.; Noman, M.; John, C.; Akubuiro, C.; Haider, A.; Rani, R.; Kashif, M.; et al. The Bidirectional Link Between Diabetes and Kidney Disease: Mechanisms and Management. Cureus 2023, 15, e45615. [Google Scholar] [CrossRef]
- Li, H.; Klett, D.E.; Littleton, R.; Elder, J.S.; Sammon, J.D. Role of Insulin Resistance in Uric Acid Nephrolithiasis. World J. Nephrol. 2014, 3, 237–242. [Google Scholar] [CrossRef]
- Mazur, T.; Demikhova, N.; Rudenko, T.; Yurchenko, A.; Yezhova, O.; Bokova, S.; Demikhov, A. Chronic Inflammation and Progression of Chronic Kidney Disease in Patients with Type 2 Diabetes. Ukr. J. Nephrol. Dial. 2021, 4, 36–43. [Google Scholar] [CrossRef]
- Rahman, I.A.; Nusaly, I.F.; Syahrir, S.; Nusaly, H.; Mansyur, M.A. Association between Metabolic Syndrome Components and the Risk of Developing Nephrolithiasis: A Systematic Review and Bayesian Meta-Analysis. F1000Res 2021, 10, 104. [Google Scholar] [CrossRef]
- Arabi, T.; Shafqat, A.; Sabbah, B.N.; Fawzy, N.A.; Shah, H.; Abdulkader, H.; Razak, A.; Sabbah, A.N.; Arabi, Z. Obesity-Related Kidney Disease: Beyond Hypertension and Insulin-Resistance. Front. Endocrinol. 2023, 13, 1095211. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C.; World Kidney Day Steering Committee. Obesity and kidney disease: Hidden consequences of the epidemic. J. Ren. Care 2017, 43, 3–10. [Google Scholar] [CrossRef][Green Version]
- Poore, W.; Boyd, C.J.; Singh, N.P.; Wood, K.; Gower, B.; Assimos, D.G. Obesity and Its Impact on Kidney Stone Formation. Rev. Urol. 2020, 22, 17–23. [Google Scholar] [PubMed]
- Stasi, A.; Cosola, C.; Caggiano, G.; Cimmarusti, M.T.; Palieri, R.; Acquaviva, P.M.; Rana, G.; Gesualdo, L. Obesity-Related Chronic Kidney Disease: Principal Mechanisms and New Approaches in Nutritional Management. Front. Nutr. 2022, 9, 925619. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Tan, W.; Pan, X.; Tian, E.; Wu, Z.; Yang, J. Metabolic Syndrome-Related Kidney Injury: A Review and Update. Front. Endocrinol. 2022, 13, 904001. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Ke, H.L.; Lee, J.I.; Lee, Y.C.; Jhan, J.H.; Wang, H.S.; Shen, J.T.; Tsao, Y.H.; Huang, S.P.; Geng, J.H. Metabolic Syndrome Increases the Risk of Kidney Stone Disease: A Cross-Sectional and Longitudinal Cohort Study. J. Pers. Med. 2021, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.; Wood, K.; Whitaker, D.; Assimos, D.G. The Influence of Metabolic Syndrome and Its Components on the Development of Nephrolithiasis. Asian J. Urol. 2018, 5, 215–222. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Chiussi, G.; Castaldo, G.; Guerra, A.; Meschi, T. Calcium Oxalate Nephrolithiasis and Gut Microbiota: Not Just a Gut-Kidney Axis. A Nutritional Perspective. Nutrients 2020, 12, 548. [Google Scholar] [CrossRef]
- Stepanova, N. Oxalate Homeostasis in Non-Stone-Forming Chronic Kidney Disease: A Review of Key Findings and Perspectives. Biomedicines 2023, 11, 1654. [Google Scholar] [CrossRef]
- Tang, X.; Lieske, J.C. Acute and Chronic Kidney Injury in Nephrolithiasis. Curr. Opin. Nephrol. Hypertens. 2014, 23, 385. [Google Scholar] [CrossRef]
- He, L.; Wei, Q.; Liu, J.; Yi, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; et al. AKI on CKD: Heightened Injury, Suppressed Repair, and the Underlying Mechanisms. Kidney Int. 2017, 92, 1071–1083. [Google Scholar] [CrossRef]
- Nørregaard, R.; Mutsaers, H.A.M.; Frøkiær, J.; Kwon, T.H. Obstructive Nephropathy and Molecular Pathophysiology of Renal Interstitial Fibrosis. Physiol. Rev. 2023, 103, 2847. [Google Scholar] [CrossRef]
- Martínez-Klimova, E.; Aparicio-Trejo, O.E.; Tapia, E.; Pedraza-Chaverri, J. Unilateral Ureteral Obstruction as a Model to Investigate Fibrosis-Attenuating Treatments. Biomolecules 2019, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Wyczanska, M.; Lange-Sperandio, B. DAMPs in Unilateral Ureteral Obstruction. Front. Immunol. 2020, 11, 581300. [Google Scholar] [CrossRef] [PubMed]
- Rule, A.D.; Krambeck, A.E.; Lieske, J.C. Chronic Kidney Disease in Kidney Stone Formers. Clin. J. Am. Soc. Nephrol. 2011, 6, 2069–2075. [Google Scholar] [CrossRef]
- Mulay, S.R.; Evan, A.; Anders, H.J. Molecular Mechanisms of Crystal-Related Kidney Inflammation and Injury. Implications for Cholesterol Embolism, Crystalline Nephropathies and Kidney Stone Disease. Nephrol. Dial. Transpl. Transplant. 2014, 29, 507–514. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, J.; Su, X.; He, Z.; Song, Q.; Song, C.; Ke, H.; Wang, C.; Liao, W.; Yang, S. Understanding Formation Processes of Calcareous Nephrolithiasis in Renal Interstitium and Tubule Lumen. J. Cell Mol. Med. 2024, 28, e18235. [Google Scholar] [CrossRef]
- Perazella, M.A.; Herlitz, L.C. The Crystalline Nephropathies. Kidney Int. Rep. 2021, 6, 2942–2957. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gan, X.; Liu, X.; An, R. Calcium Oxalate Crystals Induces Tight Junction Disruption in Distal Renal Tubular Epithelial Cells by Activating ROS/Akt/P38 MAPK Signaling Pathway. Ren. Fail. 2017, 39, 440. [Google Scholar] [CrossRef]
- Petrović, A.; Kizivat, T.; Bilić Ćurčić, I.; Smolić, R.; Smolić, M. In Vitro Cell Culture Models of Hyperoxaluric States: Calcium Oxalate and Renal Epithelial Cell Interactions. Crystals 2021, 11, 735. [Google Scholar] [CrossRef]
- Sun, X.Y.; Ouyang, J.M.; Yu, K. Shape-Dependent Cellular Toxicity on Renal Epithelial Cells and Stone Risk of Calcium Oxalate Dihydrate Crystals. Sci. Rep. 2017, 7, 7250. [Google Scholar] [CrossRef]
- Sun, X.Y.; Xu, M.; Ouyang, J.M. Effect of Crystal Shape and Aggregation of Calcium Oxalate Monohydrate on Cellular Toxicity in Renal Epithelial Cells. ACS Omega 2017, 2, 6039–6052. [Google Scholar] [CrossRef]
- Plotnikov, E.; Palmeira, C.; Grases, F.; Sayer, J.A.; Thongboonkerd, V.; Chaiyarit, S. Mitochondrial Dysfunction and Kidney Stone Disease. Front. Physiol. 2020, 11, 566506. [Google Scholar] [CrossRef]
- Xu, Y.; Li, G.; Ge, D.; Chen, Y.; Hou, B.; Hao, Z. Mitochondrial Dysfunction in Kidney Stones and Relief of Kidney Stones after Reducing MtROS. Urolithiasis 2024, 52, 117. [Google Scholar] [CrossRef] [PubMed]
- Chaiyarit, S.; Thongboonkerd, V. Mitochondria-Derived Vesicles and Their Potential Roles in Kidney Stone Disease. J. Transl. Med. 2023, 21, 294. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Ren, Y.L.; Yao, W.; Su, Y.; He, Q. Mitochondrial Dysfunction and NLRP3 Inflammasome: Key Players in Kidney Stone Formation. BJU Int. 2024, 134, 696–713. [Google Scholar] [CrossRef]
- Halinski, A.; Bhatti, K.H.; Boeri, L.; Cloutier, J.; Davidoff, K.; Elqady, A.; Fryad, G.; Gadelmoula, M.; Hui, H.; Petkova, K.; et al. Spectrum of Bacterial Pathogens from Urinary Infections Associated with Struvite and Metabolic Stones. Diagnostics 2022, 13, 80. [Google Scholar] [CrossRef]
- Flannigan, R.K.; Battison, A.; De, S.; Humphreys, M.R.; Bader, M.; Lellig, E.; Monga, M.; Chew, B.H.; Lange, D. Evaluating Factors That Dictate Struvite Stone Composition: A Multi-Institutional Clinical Experience from the EDGE Research Consortium. Can. Urol. Assoc. J. 2017, 12, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.C.; Lee, J.J.; Hwang, D.Y.; Lim, L.M.; Lin, H.Y.H.; Hwang, S.J.; Chen, H.C.; Hung, C.C. Pyuria, Urinary Tract Infection and Renal Outcome in Patients with Chronic Kidney Disease Stage 3–5. Sci. Rep. 2020, 10, 19460. [Google Scholar] [CrossRef]
- Yang, D.C.; Chao, J.Y.; Hsiao, C.Y.; Tseng, C.T.; Lin, W.H.; Kuo, T.H.; Wang, M.C. Impact of Urinary Tract Infection Requiring Hospital Admission on Short-Term, Mid-Term and Long-Term Renal Outcomes in Adult CKD Patients—A Potentially Modifiable Factor for CKD Progression. J. Infect. Public Health 2025, 18, 102712. [Google Scholar] [CrossRef]
- Kuhn, H.W.; Hreha, T.N.; Hunstad, D.A. Immune Defenses in the Urinary Tract. Trends Immunol. 2023, 44, 701–711. [Google Scholar] [CrossRef]
- Stepanova, N. How Advanced Is Our Understanding of the Role of Intestinal Barrier Dysfunction in the Pathogenesis of Recurrent Urinary Tract Infections. Front. Pharmacol. 2022, 13, 780122. [Google Scholar] [CrossRef]
- Allam, A.M. TLR4/NF-KB Signaling Pathway Is A Key Pathogenic Event Leading To Kidney Damage In UUO Induced Renal Fibrosis. Al-Azhar J. Pharm. Sci. 2020, 61, 61–76. [Google Scholar] [CrossRef]
- Nan, Q.Y.; Piao, S.G.; Jin, J.Z.; Chung, B.H.; Yang, C.W.; Li, C. Pathogenesis and Management of Renal Fibrosis Induced by Unilateral Ureteral Obstruction. Kidney Res. Clin. Pract. 2024, 43, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J. Chronic Kidney Disease and Kidney Stones. Curr. Opin. Nephrol. Hypertens. 2020, 29, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Zhang, J.; Deng, Q.; Liang, H. Recent advances on the mechanisms of kidney stone formation (Review). Int. J. Mol. Med. 2021, 48, 149. [Google Scholar] [CrossRef]
- Xu, Z.; Yao, X.; Duan, C.; Liu, H.; Xu, H. Metabolic Changes in Kidney Stone Disease. Front. Immunol. 2023, 14, 1142207. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, N.; Aydin, S.; Gillion, V.; Morelle, J.; Jadoul, M. Pathophysiology and Management of Hyperoxaluria and Oxalate Nephropathy: A Review. Am. J. Kidney Dis. 2022, 79, 717–727. [Google Scholar] [CrossRef]
- Bao, D.; Wang, Y.; Zhao, M.H. Oxalate Nephropathy and the Mechanism of Oxalate-Induced Kidney Injury. Kidney Dis. 2023, 9, 459–468. [Google Scholar] [CrossRef]
- Waikar, S.S.; Srivastava, A.; Palsson, R.; Shafi, T.; Hsu, C.Y.; Sharma, K.; Lash, J.P.; Chen, J.; He, J.; Lieske, J.; et al. Association of Urinary Oxalate Excretion With the Risk of Chronic Kidney Disease Progression. JAMA Intern. Med. 2019, 179, 542–551. [Google Scholar] [CrossRef]
- Gianella, F.G.; Prado, V.E.; Poindexter, J.R.; Adams-Huet, B.; Li, X.; Miller, R.T.; Sakhaee, K.; Maalouf, N.M.; Moe, O.W. Spot Urinary Citrate-to-Creatinine Ratio Is a Marker for Acid-Base Status in Chronic Kidney Disease. Kidney Int. 2021, 99, 208–217. [Google Scholar] [CrossRef]
- Zuckerman, J.M.; Assimos, D.G. Hypocitraturia: Pathophysiology and Medical Management. Rev. Urol. 2009, 11, 134–144. [Google Scholar]
- Ferre, N.; Parada, E.; Balaguer, A.; Feliu, A.; Roqué-Figuls, M.; Franco, J.V.A.; Escribano, J. Pharmacological Interventions for Preventing Complications in Patients with Idiopathic Hypercalciuria: A Systematic Review. Nefrologia 2022, 42, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H. Renal Mechanisms for Hypercalciuria Induced by Metabolic Acidosis. Am. J. Nephrol. 2022, 53, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.T.; Fuster, D.G.; Dimke, H. Mechanisms Underlying Calcium Nephrolithiasis. Annu. Rev. Physiol. 2022, 84, 559–583. [Google Scholar] [CrossRef] [PubMed]
- Figueres, L.; Hourmant, M.; Lemoine, S. Understanding and Managing Hypercalciuria in Adults with Nephrolithiasis: Keys for Nephrologists. Nephrol. Dial. Transpl. Transplant. 2020, 35, 573–575. [Google Scholar] [CrossRef]
- Yang, H.; Ying, J.; Zu, T.; Meng, X.M.; Jin, J. Insights into Renal Damage in Hyperuricemia: Focus on Renal Protection (Review). Mol. Med. Rep. 2025, 31, 59. [Google Scholar] [CrossRef]
- Park, J.H.; Jo, Y.I.; Lee, J.H. Renal Effects of Uric Acid: Hyperuricemia and Hypouricemia. Korean J. Intern. Med. 2020, 35, 1291–1304. [Google Scholar] [CrossRef]
- Asahina, Y.; Sakaguchi, Y.; Oka, T.; Hattori, K.; Kawaoka, T.; Doi, Y.; Yamamoto, R.; Matsui, I.; Mizui, M.; Kaimori, J.Y.; et al. Association between Urinary Uric Acid Excretion and Kidney Outcome in Patients with CKD. Sci. Rep. 2024, 14, 5119. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Z.; An, Z.; Li, S. Association between Oxidative Balance Score and Serum Uric Acid and Hyperuricemia: A Population-Based Study from the NHANES (2011–2018). Front. Endocrinol. 2024, 15, 1414075. [Google Scholar] [CrossRef]
- Miller, A.W.; Penniston, K.L.; Fitzpatrick, K.; Agudelo, J.; Tasian, G.; Lange, D. Mechanisms of the Intestinal and Urinary Microbiome in Kidney Stone Disease. Nat. Rev. Urol. 2022, 19, 695–707. [Google Scholar] [CrossRef]
- Al, K.F.; Joris, B.R.; Daisley, B.A.; Chmiel, J.A.; Bjazevic, J.; Reid, G.; Gloor, G.B.; Denstedt, J.D.; Razvi, H.; Burton, J.P. Multi-Site Microbiota Alteration Is a Hallmark of Kidney Stone Formation. Microbiome 2023, 11, 263. [Google Scholar] [CrossRef]
- Li, J.; Huang, S.; Liu, S.; Liao, X.; Yan, S.; Liu, Q. SLC26 Family: A New Insight for Kidney Stone Disease. Front. Physiol. 2023, 14, 1118342. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, X.; Ma, Y.; Jian, Z.; Wei, Z.; Xiang, L.; Sun, Q.; Qi, S.; Wang, K.; Li, H. Short-Chain Fatty Acids Reduced Renal Calcium Oxalate Stones by Regulating the Expression of Intestinal Oxalate Transporter SLC26A6. mSystems 2021, 6, e01045-21. [Google Scholar] [CrossRef] [PubMed]
- Choy, W.H.; Adler, A.; Morgan-Lang, C.; Gough, E.K.; Hallam, S.J.; Manges, A.R.; Chew, B.H.; Penniston, K.; Miller, A.; Lange, D. Deficient Butyrate Metabolism in the Intestinal Microbiome Is a Potential Risk Factor for Recurrent Kidney Stone Disease. Urolithiasis 2024, 52, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Miao, H.; Deng, D.Q.; Vaziri, N.D.; Li, P.; Zhao, Y.Y. Gut Microbiota-Derived Tryptophan Metabolism Mediates Renal Fibrosis by Aryl Hydrocarbon Receptor Signaling Activation. Cell Mol. Life Sci. 2020, 78, 909–922. [Google Scholar] [CrossRef]
- Magliocca, G.; Mone, P.; Di Iorio, B.R.; Heidland, A.; Marzocco, S. Short-Chain Fatty Acids in Chronic Kidney Disease: Focus on Inflammation and Oxidative Stress Regulation. Int. J. Mol. Sci. 2022, 23, 5354. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhu, H.; Yao, Y.; Zeng, R. Gut Dysbiosis and Kidney Diseases. Front. Med. 2022, 9, 829349. [Google Scholar] [CrossRef]
- Tangri, N.; Mathur, V.; Reaven, N.L.; Funk, S.E.; Whitlock, R.H.; Wesson, D.E.; Bushinsky, D.A. Association of Serum Bicarbonate with the Development of Kidney Stones in Patients with Chronic Kidney Disease: A Retrospective Cohort Study. Clin. Kidney J. 2023, 16, 1113–1121. [Google Scholar] [CrossRef]
- Sui, W.; Calvert, J.K.; Kavoussi, N.L.; Gould, E.R.; Miller, N.L.; Bejan, C.A.; Hsi, R.S. Association of Chronic Kidney Disease Stage with 24-Hour Urine Values among Patients with Nephrolithiasis. J. Endourol. 2020, 34, 1263–1271. [Google Scholar] [CrossRef]
- Frassetto, L.A.; Raphael, K.L. Metabolic Acidosis in CKD: Pathogenesis, Adverse Effects, and Treatment Effects. Int. J. Mol. Sci. 2024, 25, 5187. [Google Scholar] [CrossRef]
- Hering-Smith, K.S.; Hamm, L.L. Acidosis and Citrate: Provocative Interactions. Ann. Transl. Med. 2018, 6, 374. [Google Scholar] [CrossRef]
- Ortega, L.M.; Ortega, L.M.; Arora, S. Metabolic Acidosis and Progression of Chronic Kidney Disease: Incidence, Pathogenesis, and Therapeutic Options. Nefrología 2012, 32, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Whitlock, R.H.; Ferguson, T.W.; Nour-Mohammadi, M.; Komenda, P.; Rigatto, C.; Collister, D.; Bohm, C.; Reaven, N.L.; Funk, S.E.; et al. Metabolic Acidosis Is Associated with Acute Kidney Injury in Patients With CKD. Kidney Int. Rep. 2022, 7, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Tangri, N.; Reaven, N.L.; Funk, S.E.; Ferguson, T.W.; Collister, D.; Mathur, V. Metabolic Acidosis Is Associated with Increased Risk of Adverse Kidney Outcomes and Mortality in Patients with Non-Dialysis Dependent Chronic Kidney Disease: An Observational Cohort Study. BMC Nephrol. 2021, 22, 185. [Google Scholar] [CrossRef]
- Liu, J.; Tio, M.C.; Verma, A.; Schmidt, I.M.; Ilori, T.O.; Knauf, F.; Mc Causland, F.R.; Waikar, S.S. Determinants and Outcomes Associated With Urinary Calcium Excretion in Chronic Kidney Disease. J. Clin. Endocrinol. Metab. 2021, 107, e281. [Google Scholar] [CrossRef]
- Pfau, A.; Wytopil, M.; Chauhan, K.; Reichel, M.; Coca, S.G.; Aronson, P.S.; Eckardt, K.U.; Knauf, F. Assessment of Plasma Oxalate Concentration in Patients With CKD. Kidney Int. Rep. 2020, 5, 2013–2020. [Google Scholar] [CrossRef]
- Prochaska, M.; Taylor, E.; Ferraro, P.M.; Curhan, G. Relative Supersaturation of 24-Hour Urine and Likelihood of Kidney Stones. J. Urol. 2018, 199, 1262–1266. [Google Scholar] [CrossRef]
- Susla, O.; Bushtynska, O.; Danyliv, S.; Logoyda, L.; Gozhenko, A. The Role of Vitamins K and D in the Processes of Ectopic Calcification in Patients with Chronic Kidney Disease: The Current State of the Problem. Ukr. J. Nephrol. Dial. 2022, 3, 73–82. [Google Scholar] [CrossRef]
- Demirel, S.; Gürbüz, M. The Role of Native Vitamin D Treatment in the Clinical Assessment of Osteoporosis in Patients with Chronic Kidney Disease. Ukr. J. Nephrol. Dial. 2024, 81, 71–85. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Goettsch, C. Cardiovascular Calcification Heterogeneity in Chronic Kidney Disease. Circ. Res. 2023, 132, 993–1012. [Google Scholar] [CrossRef]
- Bargagli, M.; Ferraro, P.M.; Vittori, M.; Lombardi, G.; Gambaro, G.; Somani, B. Calcium and Vitamin D Supplementation and Their Association with Kidney Stone Disease: A Narrative Review. Nutrients 2021, 13, 4363. [Google Scholar] [CrossRef]
- Kanbay, M.; Copur, S.; Bakir, C.N.; Hatipoglu, A.; Sinha, S.; Haarhaus, M. Management of de Novo Nephrolithiasis after Kidney Transplantation: A Comprehensive Review from the European Renal Association CKD-MBD Working Group. Clin. Kidney J. 2024, 17, sfae023. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, G.; Croppi, E.; Bushinsky, D.; Jaeger, P.; Cupisti, A.; Ticinesi, A.; Mazzaferro, S.; D’Addessi, A.; Ferraro, P.M. The Risk of Chronic Kidney Disease Associated with Urolithiasis and Its Urological Treatments: A Review. J. Urol. 2017, 198, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Candela, L.; Trevisani, F.; Ventimiglia, E.; D’Arma, A.; Corsini, C.; Robesti, D.; Traxer, O.; Montorsi, F.; Salonia, A.; Villa, L. Acknowledging Acute Kidney Disease Following Ureteroscopy and Laser Lithotripsy: Results from a Tertiary Care Referral Center. Int. Urol. Nephrol. 2024, 56, 3905–3911. [Google Scholar] [CrossRef] [PubMed]
- Medina-Escobedo, M.; Sánchez-Pozos, K.; Gutiérrez-Solis, A.L.; Avila-Nava, A.; González-Rocha, L.; Lugo, R. Recurrence of Nephrolithiasis and Surgical Events Are Associated with Chronic Kidney Disease in Adult Patients. Medicina 2022, 58, 420. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, M.; Savcic-Kos, R.; Huang, J.; Rule, A.D.; Murali, N. Urological Procedures in Urolithiasis and Their Association with Chronic Kidney Disease. Clin. Med. Res. 2016, 14, 75–82. [Google Scholar] [CrossRef]
- Ertaş, K.; Temiz, M.Z.; Çolakerol, A.; Küçük, S.H.; Şahan, A.; Yürük, E. Effects of Flexible Ureteroscopy on Kidney: A Prospective Clinical Trial. Turk. J. Urol. 2020, 46, 297–302. [Google Scholar] [CrossRef]
- Ahmed, Z.Y.; Abdelrahim, A.; Gareeballah, A.; Gameraddin, M.; Elzaki, M.; Ali, S.I.; Hassan, M.A.; Mohammed, M.H.; Abouraida, R.A. Impact of Extracorporeal Shock Wave Lithotripsy (ESWL) on Kidney Length and Corticomedullary Differentiation in Patients with Renal Stones: A Case-Control Study. Cureus 2024, 16, e69760. [Google Scholar] [CrossRef]
- Clark, D.L.; Connors, B.A.; Evan, A.P.; Willis, L.R.; Handa, R.K.; Gao, S. Localization of Renal Oxidative Stress and Inflammatory Response after Lithotripsy. BJU Int. 2009, 103, 1562–1568. [Google Scholar] [CrossRef]
- Baba, D.; Çam, K.; Şenoğlu, Y.; Yüksel, A.; Erdem, H.; Başaran, E. The Efficacy of N-Acetylcysteine Against Renal Oxidative Stress After Extracorporeal Shock Wave Treatment: An Experimental Rat Model. J. Urol. Surg. 2020, 7, 8–15. [Google Scholar] [CrossRef]
- Mehra, K.; Satpathy, P.; Joshi, A.; Manikandan, R. Percutaneous Nephrolithotomy in Patients with Chronic Kidney Disease: A Systematic Review. Urol. Int. 2022, 106, 461–468. [Google Scholar] [CrossRef]
- Izol, V.; Deger, M.; Akdogan, N.; Ok, F.; Bayazit, Y.; Aridogan, I.A. The Effect of Percutaneous Nephrolithotomy on the Estimated Glomerular Filtration Rate in Patients with Chronic Kidney Disease. J. Endourol. 2021, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Watts, K.L.; Srivastava, A.; Lin, W.; Schoenfeld, D.; Abramowitz, M.; Stern, J.M. Baseline Chronic Kidney Disease Does Not Predict Long-Term Renal Functional Decline after Percutaneous Nephrolithotomy. Urolithiasis 2019, 47, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.A.; Adiyeke, E.; Ozrazgat-Baslanti, T.; Rabley, A.K.; Bozorgmehri, S.; Bihorac, A.; Bird, V.G. Clinical Considerations for Patients Experiencing Acute Kidney Injury Following Percutaneous Nephrolithotomy. Biomedicines 2023, 11, 1712. [Google Scholar] [CrossRef]
- Adiga, P.; Pudakalkatti, S.R.; Shivakumar, V.; Jain, M.; Sreenidhi, R.N.; Manohar, C.S.; Jayaram, S.; Nagabhushan, M.; Keshavamurthy, R. Is Percutaneous Nephrolithotomy Safe in Chronic Kidney Disease Patients!!! Urol. Ann. 2022, 14, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Sairam, K.; Scoffone, C.M.; Alken, P.; Turna, B.; Sodha, H.S.; Rioja, J.; Wolf, J.S.; De La Rosette, J.J.M.C.H. Percutaneous Nephrolithotomy and Chronic Kidney Disease: Results from the CROES PCNL Global Study. J. Urol. 2012, 188, 1195–1200. [Google Scholar] [CrossRef]
- Prstojević, J.K.; Hasanbegović, M.; Alić, J.; Mišanović, V.; Lujinović, A.; Metović, A.; Krupić, F.; Pokrajac, D.M.; Hadžimuratović, A.; Pašić, L.Z. Evaluation of Inflammatory Parameters Following Extracorporeal Shock Wave Lithotripsy (ESWL) and Ureteroscopy for the Treatment of Proximal Ureteral Stones. Cureus 2024, 16, e51882. [Google Scholar] [CrossRef]
- Shaker, E.K.; Chaloob, F.A. Risk Factors in Bacterial Colonization of Internal Ureteral Stent. Bionatura 2021, 6, 2022–2026. [Google Scholar] [CrossRef]
- Scotland, K.B.; Lo, J.; Grgic, T.; Lange, D. Ureteral Stent-Associated Infection and Sepsis: Pathogenesis and Prevention: A Review. Biofouling 2019, 35, 117–127. [Google Scholar] [CrossRef]
- Zumstein, V.; Betschart, P.; Albrich, W.C.; Buhmann, M.T.; Ren, Q.; Schmid, H.P.; Abt, D. Biofilm Formation on Ureteral Stents-Incidence, Clinical Impact, and Prevention. Swiss Med. Wkly. 2017, 147, w14408. [Google Scholar] [CrossRef]
- American Urological Association Kidney Stones: Surgical Management Guideline. Available online: https://www.auanet.org/guidelines-and-quality/guidelines/kidney-stones-surgical-management-guideline (accessed on 21 December 2023).
- EAU Guidelines. Edn. Presented at the EAU Annual Congress Madrid 2025. ISBN 978-94-92671-29-5. Available online: https://uroweb.org/guidelines/urolithiasis/chapter/guidelines (accessed on 26 March 2025).
- Dzięgała, M.; Krajewski, W.; Kołodziej, A.; Dembowski, J.; Zdrojowy, R. Evaluation and Physiopathology of Minor Transient Shock Wave Lithotripsy—Induced Renal Injury Based on Urinary Biomarkers Levels. Cent. Eur. J. Urol. 2018, 71, 214–220. [Google Scholar] [CrossRef]
- Bao, Y.; Tu, X.; Wei, Q. Water for Preventing Urinary Stones. Cochrane Database Syst. Rev. 2020, 2, CD004292. [Google Scholar] [CrossRef] [PubMed]
- Gamage, K.N.; Jamnadass, E.; Sulaiman, S.K.; Pietropaolo, A.; Aboumarzouk, O.; Somani, B.K. The Role of Fluid Intake in the Prevention of Kidney Stone Disease: A Systematic Review over the Last Two Decades. Turk. J. Urol. 2020, 46, S92–S103. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, C.; Wang, X.L.; Liu, T.Z.; Zeng, X.T.; Li, S.; Duan, X.W. Self-Fluid Management in Prevention of Kidney Stones: A PRISMA-Compliant Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Medicine 2015, 94, e1042. [Google Scholar] [CrossRef] [PubMed]
- Courbebaisse, M.; Travers, S.; Bouderlique, E.; Michon-Colin, A.; Daudon, M.; De Mul, A.; Poli, L.; Baron, S.; Prot-Bertoye, C. Hydration for Adult Patients with Nephrolithiasis: Specificities and Current Recommendations. Nutrients 2023, 15, 4885. [Google Scholar] [CrossRef]
- Hung, S.C.; Lai, Y.S.; Kuo, K.L.; Tarng, D.C. Volume Overload and Adverse Outcomes in Chronic Kidney Disease: Clinical Observational and Animal Studies. J. Am. Heart Assoc. 2015, 4, e001918. [Google Scholar] [CrossRef]
- Choi, H.Y.; Park, H.C.; Ha, S.K. High Water Intake and Progression of Chronic Kidney Diseases. Electrolyte Blood Press. 2015, 13, 46–51. [Google Scholar] [CrossRef]
- Wagner, S.; Merkling, T.; Metzger, M.; Bankir, L.; Laville, M.; Frimat, L.; Combe, C.; Jacquelinet, C.; Fouque, D.; Massy, Z.A.; et al. Water Intake and Progression of Chronic Kidney Disease: The CKD-REIN Cohort Study. Nephrol. Dial. Transpl. Transplant. 2022, 37, 730–739. [Google Scholar] [CrossRef]
- Clark, W.F.; Sontrop, J.M.; Huang, S.H.; Moist, L.; Bouby, N.; Bankir, L. Hydration and Chronic Kidney Disease Progression: A Critical Review of the Evidence. Am. J. Nephrol. 2016, 43, 281–292. [Google Scholar] [CrossRef]
- Paz-Graniel, I.; Valle-Hita, C.; Babio, N.; Serra-Majem, L.; Vioque, J.; Zomeño, M.D.; Corella, D.; Pintó, X.; Cano-Ibáñez, N.; Tur, J.A.; et al. Long-Term Association between Water Intake and Kidney Function in a Population at High Cardiovascular Risk. J. Nutr. Health Aging 2024, 28, 100327. [Google Scholar] [CrossRef]
- Barghouthy, Y.; Somani, B.K. Role of Citrus Fruit Juices in Prevention of Kidney Stone Disease (KSD): A Narrative Review. Nutrients 2021, 13, 4117. [Google Scholar] [CrossRef]
- Geng, J.; Qiu, Y.; Kang, Z.; Li, Y.; Li, J.; Liao, R.; Qin, Z.; Yang, Q.; Su, B. The Association between Caffeine Intake and Risk of Kidney Stones: A Population-Based Study. Front. Nutr. 2022, 9, 935820. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Inaba, M. Potassium Metabolism and Management in Patients with CKD. Nutrients 2021, 13, 1751. [Google Scholar] [CrossRef] [PubMed]
- Siener, R. Nutrition and Kidney Stone Disease. Nutrients 2021, 13, 1917. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.C.; Pearle, M.S. Diet and Stone Disease in 2022. J. Clin. Med. 2022, 11, 4740. [Google Scholar] [CrossRef]
- Seeger, H.; Kaelin, A.; Ferraro, P.M.; Weber, D.; Jaeger, P.; Ambuehl, P.; Robertson, W.G.; Unwin, R.; Wagner, C.A.; Mohebbi, N. Changes in Urinary Risk Profile after Short-Term Low Sodium and Low Calcium Diet in Recurrent Swiss Kidney Stone Formers. BMC Nephrol. 2017, 18, 349. [Google Scholar] [CrossRef]
- Kaestner, L.; Meki, S.; Moore, A.; van Woerden, C.; Lazarus, J. General and Dietary Oxalate Restriction Advice Reduces Urinary Oxalate in the Stone Clinic Setting. S. Afr. J. Surg. 2020, 58, 210–212. [Google Scholar]
- Aziz, K.; Noreen, S.; Tufail, T.; Ishaq, I.; Shah, M.A. Impact of Low-Oxalate Diet on Hyperoxaluria among Patients Suffering from Nephrolithiasis. Food Sci. Nutr. 2024, 12, 4292–4298. [Google Scholar] [CrossRef]
- Sorensen, M.D.; Hsi, R.S.; Chi, T.; Shara, N.; Wactawski-Wende, J.; Kahn, A.J.; Wang, H.; Hou, L.; Stoller, M.L. Dietary Intake of Fiber, Fruit, and Vegetables Decrease the Risk of Incident Kidney Stones in Women: A Women’s Health Initiative (WHI) Report. J. Urol. 2014, 192, 1694. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Bargagli, M.; Trinchieri, A.; Gambaro, G. Risk of Kidney Stones: Influence of Dietary Factors, Dietary Patterns, and Vegetarian–Vegan Diets. Nutrients 2020, 12, 779. [Google Scholar] [CrossRef]
- Asoudeh, F.; Talebi, S.; Jayedi, A.; Marx, W.; Najafi, M.T.; Mohammadi, H. Associations of Total Protein or Animal Protein Intake and Animal Protein Sources with Risk of Kidney Stones: A Systematic Review and Dose-Response Meta-Analysis. Adv. Nutr. 2022, 13, 821–832. [Google Scholar] [CrossRef]
- Wang, A.Y.M.; Mallamaci, F.; Zoccali, C. What Is Central to Renal Nutrition: Protein or Sodium Intake? Clin. Kidney J. 2023, 16, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.M.; Tsai, N.C.; Lin, M.Y.; Hwang, D.Y.; Lin, H.Y.H.; Lee, J.J.; Hwang, S.J.; Hung, C.C.; Chen, H.C. Hyponatremia Is Associated with Fluid Imbalance and Adverse Renal Outcome in Chronic Kidney Disease Patients Treated with Diuretics. Sci. Rep. 2016, 6, 36817. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, M.; Arrigain, S.; Schold, J.D.; Nakhoul, G.N.; Navaneethan, S.D.; Mehdi, A.; Sekar, A.; Tabbara, J.; Taliercio, J.J. Dysnatremias, Mortality, and Kidney Failure in CKD: Findings From the Chronic Renal Insufficiency Cohort (CRIC) Study. Kidney Med. 2022, 4, 100554. [Google Scholar] [CrossRef] [PubMed]
- Arzhan, S.; Lew, S.Q.; Ing, T.S.; Tzamaloukas, A.H.; Unruh, M.L. Dysnatremias in Chronic Kidney Disease: Pathophysiology, Manifestations, and Treatment. Front. Med. 2021, 8, 769287. [Google Scholar] [CrossRef]
- Crestani, T.; Crajoinas, R.O.; Jensen, L.; Dima, L.L.; Burdeyron, P.; Hauet, T.; Giraud, S.; Steichen, C. A Sodium Oxalate-Rich Diet Induces Chronic Kidney Disease and Cardiac Dysfunction in Rats. Int. J. Mol. Sci. 2021, 22, 9244. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Azizi, F. Dietary Oxalate-Calcium Balance and the Incidence of Hypertension and Chronic Kidney Disease: A Prospective Study among an Asian Population. Nutr. Metab. 2022, 19, 74. [Google Scholar] [CrossRef]
- Wu, C.L.; Tsai, W.H.; Liu, J.S.; Liu, H.W.; Huang, S.Y.; Kuo, K.L. Vegan Diet Is Associated with a Lower Risk of Chronic Kidney Disease in Patients with Hyperuricemia. Nutrients 2023, 15, 1444. [Google Scholar] [CrossRef]
- Zarantonello, D.; Brunori, G. The Role of Plant-Based Diets in Preventing and Mitigating Chronic Kidney Disease: More Light than Shadows. J. Clin. Med. 2023, 12, 6137. [Google Scholar] [CrossRef]
- MacLaughlin, H.L.; McAuley, E.; Fry, J.; Pacheco, E.; Moran, N.; Morgan, K.; McGuire, L.; Conley, M.; Johnson, D.W.; Ratanjee, S.K.; et al. Re-Thinking Hyperkalaemia Management in Chronic Kidney Disease—Beyond Food Tables and Nutrition Myths: An Evidence-Based Practice Review. Nutrients 2023, 16, 3. [Google Scholar] [CrossRef]
- Borrelli, S.; Matarazzo, I.; Lembo, E.; Peccarino, L.; Annoiato, C.; Scognamiglio, M.R.; Foderini, A.; Ruotolo, C.; Franculli, A.; Capozzi, F.; et al. Chronic Hyperkaliemia in Chronic Kidney Disease: An Old Concern with New Answers. Int. J. Mol. Sci. 2022, 23, 6378. [Google Scholar] [CrossRef]
- Takkavatakarn, K.; Wuttiputinun, T.; Phannajit, J.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. Protein-Bound Uremic Toxin Lowering Strategies in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Nephrol. 2021, 34, 1805–1817. [Google Scholar] [CrossRef]
- Windahl, K.; Faxén Irving, G.; Almquist, T.; Lidén, M.K.; van de Luijtgaarden, M.; Chesnaye, N.C.; Voskamp, P.; Stenvinkel, P.; Klinger, M.; Szymczak, M.; et al. Prevalence and Risk of Protein-Energy Wasting Assessed by Subjective Global Assessment in Older Adults With Advanced Chronic Kidney Disease: Results From the EQUAL Study. J. Ren. Nutr. 2018, 28, 165–174. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Massy, Z.A.; Drüeke, T.B. Strategies for Phosphate Control in Patients With CKD. Kidney Int. Rep. 2019, 4, 1043–1056. [Google Scholar] [CrossRef]
- Lange, J.N.; Wood, K.D.; Mufarrij, P.W.; Callahan, M.F.; Easter, L.; Knight, J.; Holmes, R.P.; Assimos, D.G. the impact of dietary calcium and oxalate ratios on stone risk. Urology 2012, 79, 1226. [Google Scholar] [CrossRef]
- Bargagli, M.; Anderegg, M.A.; Fuster, D.G. Effects of Thiazides and New Findings on Kidney Stones and Dysglycemic Side Effects. Acta Physiol. 2024, 240, e14155. [Google Scholar] [CrossRef] [PubMed]
- Dhayat, N.A.; Bonny, O.; Roth, B.; Christe, A.; Ritter, A.; Mohebbi, N.; Faller, N.; Pellegrini, L.; Bedino, G.; Venzin, R.M.; et al. Hydrochlorothiazide and Prevention of Kidney-Stone Recurrence. N. Engl. J. Med. 2023, 388, 781–791. [Google Scholar] [CrossRef]
- Agarwal, R.; Sinha, A.D.; Tu, W. Chlorthalidone for Resistant Hypertension in Advanced Chronic Kidney Disease. Circulation 2022, 146, 718–720. [Google Scholar] [CrossRef]
- Teles, F.; Peçanha de Miranda Coelho, J.A.; Albino, R.M.; Verçosa Pacheco, F.C.; Rodrigues de Oliveira, E.; Silveira, M.A.D.; Diógenes, M.; Feitosa, A.; Bezerra, R. Effectiveness of Thiazide and Thiazide-like Diuretics in Advanced Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Ren. Fail. 2023, 45, 2163903. [Google Scholar] [CrossRef]
- Vea, K.D.; Nguyen, L.A.; McGill, K.; Park, J.C.; Selevan, D. Thiazide Discontinuation in Chronic Kidney Disease Hypertension Management: A Retrospective Chart Review. Perm. J. 2024, 28, 37–45. [Google Scholar] [CrossRef]
- Lin, H.Y.H.; Chang, Y.H.; Wang, Y.T.; Liang, P.I.; Hung, C.C.; Chang, J.M.; Dai, D.F.; Lin, C.S.; Chang, K.T. Thiazide and Thiazide-like Diuretics Are Associated with Improved Cardiovascular and Renal Outcomes in Patients with Chronic Kidney Disease. Ann. Acad. Med. Singap. 2023, 52, 510–521. [Google Scholar] [CrossRef]
- Jo, W.; Koh, E.S.; Chung, S. Therapeutic Roles of Thiazides and Loop Diuretics in Blood Pressure Control and Renal Protection against Chronic Kidney Disease. Clin. Hypertens. 2023, 29, 14. [Google Scholar] [CrossRef]
- Welty, J.; Geurin, M.D. Is Potassium Citrate Effective for Preventing Kidney Stone Recurrence in Patients with Calcium-Containing Stones? Evid. Based Pract. 2018, 21, E11. [Google Scholar] [CrossRef]
- Gritter, M.; Wouda, R.D.; Yeung, S.M.H.; Wieërs, M.L.A.; Geurts, F.; de Ridder, M.A.J.; Ramakers, C.R.B.; Vogt, L.; de Borst, M.H.; Rotmans, J.I.; et al. Effects of Short-Term Potassium Chloride Supplementation in Patients with Chronic Kidney Disease. J. Am. Soc. Nephrol. 2022, 33, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.W.; Nicolaisen, S.K.; Hasvold, P.; Sanchez, R.G.; Pedersen, L.; Adelborg, K.; Egstrup, K.; Egfjord, M.; Sørensen, H.T. Elevated Potassium Levels in Patients with Chronic Kidney Disease: Occurrence, Risk Factors and Clinical Outcomes-a Danish Population-Based Cohort Study. Nephrol. Dial. Transpl. Transplant. 2018, 33, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, Y.; Zhang, J.; Zhang, Q. Safety of Potassium-Bearing Citrate in Patients with Renal Transplantation: A Case Report. Medicine 2017, 96, e6933. [Google Scholar] [CrossRef]
- Krieger, N.S.; Asplin, J.R.; Frick, K.K.; Granja, I.; Culbertson, C.D.; Ng, A.; Grynpas, M.D.; Bushinsky, D.A. Effect of Potassium Citrate on Calcium Phosphate Stones in a Model of Hypercalciuria. J. Am. Soc. Nephrol. 2015, 26, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Doizi, S.; Poindexter, J.R.; Pearle, M.S.; Blanco, F.; Moe, O.W.; Sakhaee, K.; Maalouf, N.M. Impact of Potassium Citrate vs. Citric Acid on Urinary Stone Risk in Calcium Phosphate Stone Formers. J. Urol. 2018, 200, 1278–1284. [Google Scholar] [CrossRef]
- Sorohan, B.M.; Obricǎ, B.; Jurubiǎ, R.; Lupuoru, G.; Achim, C.; Andronesi, A.; Frǎilǎ, G.; Berechet, A.; Micu, G.; Ismail, G. Sodium Citrate versus Sodium Bicarbonate for Metabolic Acidosis in Patients with Chronic Kidney Disease: A Randomized Controlled Trial. Medicine 2024, 103, E37475. [Google Scholar] [CrossRef]
- Sharbaf, F.G.; Bakhtiari, E.; Faghihi, T.; Assadi, F. Efficacy and Safety of Allopurinol on Chronic Kidney Disease Progression: A Systematic Review and Meta-Analysis. J. Pediatr. Pharmacol. Ther. 2024, 29, 359–367. [Google Scholar] [CrossRef]
- Rey, A.; Batteux, B.; Laville, S.M.; Marienne, J.; Masmoudi, K.; Gras-Champel, V.; Liabeuf, S. Acute Kidney Injury Associated with Febuxostat and Allopurinol: A Post-Marketing Study. Arthritis Res. Ther. 2019, 21, 229. [Google Scholar] [CrossRef]
- Helget, L.N.; Davis-Karim, A.; O’Dell, J.R.; Mikuls, T.R.; Newcomb, J.A.; Androsenko, M.; Brophy, M.T.; England, B.R.; Ferguson, R.; Pillinger, M.H.; et al. Efficacy and Safety of Allopurinol and Febuxostat in Patients With Gout and CKD: Subgroup Analysis of the STOP Gout Trial. Am. J. Kidney Dis. 2024, 84, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, T.; Bjazevic, J.; Kim, R.; Gryn, S.; Sultan, N.; Dresser, G.; Razvi, H. Allopurinol Hypersensitivity Syndrome: Raising Awareness of an Uncommon but Potentially Serious Adverse Event among Kidney Stone Patients. Can. Urol. Assoc. J. 2024, 18, E167–E172. [Google Scholar] [CrossRef] [PubMed]
- Yokose, C.; Lu, N.; Xie, H.; Li, L.; Zheng, Y.; McCormick, N.; Rai, S.K.; Aviña-Zubieta, J.A.; Choi, H.K. Heart Disease and the Risk of Allopurinol-Associated Severe Cutaneous Adverse Reactions: A General Population–Based Cohort Study. CMAJ 2019, 191, E1070–E1077. [Google Scholar] [CrossRef] [PubMed]
- Sahalevych, A.; Sergiychuk, R.; Ozhohin, V.; Vozianov, O.; Khrapchuk, A.; Dubovyi, Y.; Frolov, O. Mini-Percutaneous Nephrolithotomy in Surgery of Nephrolithiasis. Ukr. J. Nephrol. Dial. 2021, 44–52. [Google Scholar] [CrossRef]
- Do Truong, T.; Do Ngoc, S.; Huy, H.N.; Le Hoc, D.; Dao, U.N.; Van, L.N. Ultrasound-Guided Mini-Percutaneous Nephrolithotipsy Performed on Patients Placed in Lateral Position: A Prospective Study. Int. J. Surg. Open 2024, 62, 320–325. [Google Scholar] [CrossRef]
- Surag, K.R.; Shah, A.; Vishwanath Gali, K.; Krishnakanth, A.V.B.; Chawla, A.; Hegde, P.; Choudhary, A.; Rao, M. Severe Bleeding in Patients Following “Tubeless” Percutaneous Nephrolithotomy: Predictors of Angioembolization. Urologia 2025, 92, 89–95. [Google Scholar] [CrossRef]
- Ansari, F.M.; Para, S.A.; Wani, M.S.; Bhat, A.H.; Khawaja, A.R.; Malik, S.A.; Mehdi, S.; Ashraf, W.; Singh, S.; Maurya, M.; et al. Mini-PCNL—A Boon for CKD Patients with Nephrolithiasis. Arab. J. Urol. 2024, 22, 115–120. [Google Scholar] [CrossRef]
- Tzelves, L.; Somani, B.; Berdempes, M.; Markopoulos, T.; Skolarikos, A. Basic and Advanced Technological Evolution of Laser Lithotripsy over the Past Decade: An Educational Review by the European Society of Urotechnology Section of the European Association of Urology. Turk. J. Urol. 2021, 47, 183–192. [Google Scholar] [CrossRef]
- Kim, H.J.; Hong, S.K. Rise in Intraluminal Temperature during Ureteroscopy: Is This a Concern? Investig. Clin. Urol. 2025, 66, 1–10. [Google Scholar] [CrossRef]
- Bian, J.; Liebert, A.; Bicknell, B.; Chen, X.M.; Huang, C.; Pollock, C.A. Faecal Microbiota Transplantation and Chronic Kidney Disease. Nutrients 2022, 14, 2528. [Google Scholar] [CrossRef]
- Wigner, P.; Bijak, M.; Saluk-Bijak, J. Probiotics in the Prevention of the Calcium Oxalate Urolithiasis. Cells 2022, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, L.; Wei, W.; Fu, P. Efficacy of Probiotics/Synbiotics Supplementation in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2024, 11, 1434613. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Chen, M.J. Exploring the Preventive and Therapeutic Mechanisms of Probiotics in Chronic Kidney Disease through the Gut-Kidney Axis. J. Agric. Food Chem. 2024, 72, 8347–8364. [Google Scholar] [CrossRef] [PubMed]
- Hatch, M.; Cornelius, J.; Allison, M.; Sidhu, H.; Peck, A.; Freel, R.W. Oxalobacter Sp. Reduces Urinary Oxalate Excretion by Promoting Enteric Oxalate Secretion. Kidney Int. 2006, 69, 691–698. [Google Scholar] [CrossRef]
- Sidhu, H.; Allison, M.J.; Chow, J.M.; Clark, A.; Peck, A.B. rapid reversal of hyperoxaluria in a rat model after probiotic administration of Oxalobacter formigenes. J. Urol. 2001, 166, 1487–1491. [Google Scholar] [CrossRef]
- Verhulst, A.; Dehmel, B.; Lindner, E.; Akerman, M.E.; D’Haese, P.C. Oxalobacter Formigenes Treatment Confers Protective Effects in a Rat Model of Primary Hyperoxaluria by Preventing Renal Calcium Oxalate Deposition. Urolithiasis 2022, 50, 119–130. [Google Scholar] [CrossRef]
- Önal Darilmaz, D.; Sönmez, Ş.; Beyatli, Y. The Effects of Inulin as a Prebiotic Supplement and the Synbiotic Interactions of Probiotics to Improve Oxalate Degrading Activity. Int. J. Food Sci. Technol. 2019, 54, 121–131. [Google Scholar] [CrossRef]
- El-Kafoury, B.M.; Saleh, N.K.; Shawky, M.K.; Mehanna, N.; Ghonamy, E.; Saad, D.A. Possible Protective Role of Probiotic and Symbiotic to Limit the Progression of Chronic Kidney Disease in 5/6th Nephrectomized Albino Rats. Bull. Natl. Res. Cent. 2022, 46, 252. [Google Scholar] [CrossRef]
- Miao, H.; Liu, F.; Wang, Y.N.; Yu, X.Y.; Zhuang, S.; Guo, Y.; Vaziri, N.D.; Ma, S.X.; Su, W.; Shang, Y.Q.; et al. Targeting Lactobacillus Johnsonii to Reverse Chronic Kidney Disease. Signal Transduct. Target. Ther. 2024, 9, 195. [Google Scholar] [CrossRef]
- Bakhtiary, M.; Morvaridzadeh, M.; Agah, S.; Rahimlou, M.; Christopher, E.; Zadro, J.R.; Heshmati, J. Effect of Probiotic, Prebiotic, and Synbiotic Supplementation on Cardiometabolic and Oxidative Stress Parameters in Patients With Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Clin. Ther. 2021, 43, e71–e96. [Google Scholar] [CrossRef]
- Sohn, M.B.; Gao, B.; Kendrick, C.; Srivastava, A.; Isakova, T.; Gassman, J.J.; Fried, L.F.; Wolf, M.; Cheung, A.K.; Raphael, K.L.; et al. Targeting Gut Microbiome With Prebiotic in Patients With CKD: The TarGut-CKD Study. Kidney Int. Rep. 2024, 9, 671–685. [Google Scholar] [CrossRef]
- Yuan, T.; Xia, Y.; Li, B.; Yu, W.; Rao, T.; Ye, Z.; Yan, X.; Song, B.; Li, L.; Lin, F.; et al. Gut Microbiota in Patients with Kidney Stones: A Systematic Review and Meta-Analysis. BMC Microbiol. 2023, 23, 143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.B.; Xie, S.; Zhou, Y.; Wang, T.; Liu, Z.Y.; Li, C.S.; Gao, L.; Pan, T.J. Increased Abundance of Bacteria of the Family Muribaculaceae Achieved by Fecal Microbiome Transplantation Correlates with the Inhibition of Kidney Calcium Oxalate Stone Deposition in Experimental Rats. Front. Cell Infect. Microbiol. 2023, 13, 1145196. [Google Scholar] [CrossRef]
- An, L.; Li, S.; Chang, Z.; Lei, M.; He, Z.; Xu, P.; Zhang, S.; Jiang, Z.; Iqbal, M.S.; Sun, X.; et al. Gut Microbiota Modulation via Fecal Microbiota Transplantation Mitigates Hyperoxaluria and Calcium Oxalate Crystal Depositions Induced by High Oxalate Diet. Gut Microbes 2025, 17, 2457490. [Google Scholar] [CrossRef] [PubMed]
- Reddi, S.; Senyshyn, L.; Ebadi, M.; Podlesny, D.; Minot, S.S.; Gooley, T.; Kabage, A.J.; Hill, G.R.; Lee, S.J.; Khoruts, A.; et al. Fecal Microbiota Transplantation to Prevent Acute Graft-versus-Host Disease: Pre-Planned Interim Analysis of Donor Effect. Nat. Commun. 2025, 16, 1034. [Google Scholar] [CrossRef] [PubMed]
- Waijer, S.W.; Vart, P.; Cherney, D.Z.I.; Chertow, G.M.; Jongs, N.; Langkilde, A.M.; Mann, J.F.E.; Mosenzon, O.; McMurray, J.J.V.; Rossing, P.; et al. Effect of Dapagliflozin on Kidney and Cardiovascular Outcomes by Baseline KDIGO Risk Categories: A Post Hoc Analysis of the DAPA-CKD Trial. Diabetologia 2022, 65, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [CrossRef] [PubMed]
- Dika, Ž.; Živko, M.; Kljajić, M.; Jelaković, B. SGLT2 Inhibitors and Their Effect on Urolithiasis: Current Evidence and Future Directions. J. Clin. Med. 2024, 13, 6017. [Google Scholar] [CrossRef]
- Harmacek, D.; Pruijm, M.; Burnier, M.; Muller, M.E.; Ghajarzadeh-Wurzner, A.; Bonny, O.; Zanchi, A. Empagliflozin Changes Urine Supersaturation by Decreasing PH and Increasing Citrate. J. Am. Soc. Nephrol. 2022, 33, 1073–1075. [Google Scholar] [CrossRef]
- Kanbay, M.; Brinza, C.; Copur, S.; Sekreter, O.; Burlacu, A.; Tuttle, K.R.; Rossing, P.; Covic, A. SGLT2 Inhibitors and Nephrolithiasis Risk: A Meta-Analysis. Nephrol. Dial. Transpl. Transplant. 2025, 40, 671–678. [Google Scholar] [CrossRef]
- Paik, J.M.; Tesfaye, H.; Curhan, G.C.; Zakoul, H.; Wexler, D.J.; Patorno, E. Sodium-Glucose Cotransporter 2 Inhibitors and Nephrolithiasis Risk in Patients With Type 2 Diabetes. JAMA Intern. Med. 2024, 184, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Wanner, C.; Ferreira, J.P.; Ofstad, A.P.; Elsaesser, A.; Zinman, B.; Inzucchi, S.E. Empagliflozin and Decreased Risk of Nephrolithiasis: A Potential New Role for SGLT2 Inhibition? J. Clin. Endocrinol. Metab. 2022, 107, e3003. [Google Scholar] [CrossRef] [PubMed]
- Anan, G.; Hirose, T.; Kikuchi, D.; Takahashi, C.; Endo, A.; Ito, H.; Sato, S.; Nakayama, S.; Hashimoto, H.; Ishiyama, K.; et al. Inhibition of Sodium-Glucose Cotransporter 2 Suppresses Renal Stone Formation. Pharmacol. Res. 2022, 186, 106524. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, M.A.; Schietzel, S.; Bargagli, M.; Bally, L.; Faller, N.; Moor, M.B.; Cereghetti, G.M.; Roumet, M.; Trelle, S.; Fuster, D.G. Empagliflozin in Nondiabetic Individuals with Calcium and Uric Acid Kidney Stones: A Randomized Phase 2 Trial. Nature Medicine 2025, 31, 286–293. [Google Scholar] [CrossRef]
- Barkas, F.; Sener, Y.Z.; Golforoush, P.A.; Kheirkhah, A.; Rodriguez-Sanchez, E.; Novak, J.; Apellaniz-Ruiz, M.; Akyea, R.K.; Bianconi, V.; Ceasovschih, A.; et al. Advancements in Risk Stratification and Management Strategies in Primary Cardiovascular Prevention. Atherosclerosis 2024, 395, 117579. [Google Scholar] [CrossRef]
- Menne, J.; Dumann, E.; Haller, H.; Schmidt, B.M.W. Acute Kidney Injury and Adverse Renal Events in Patients Receiving SGLT2-Inhibitors: A Systematic Review and Meta-Analysis. PLoS Med. 2019, 16, e1002983. [Google Scholar] [CrossRef]
- Rodgers, A.L.; Siener, R. The Efficacy of Polyunsaturated Fatty Acids as Protectors against Calcium Oxalate Renal Stone Formation: A Review. Nutrients 2020, 12, 1069. [Google Scholar] [CrossRef]
- Hayashi, D.; Mouchlis, V.D.; Dennis, E.A. Omega-3 versus Omega-6 Fatty Acid Availability Is Controlled by Hydrophobic Site Geometries of Phospholipase A2s. J. Lipid Res. 2021, 62, 100113. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, H.; Wang, Y.; Ren, J.; Dai, Y.; Dai, C. Omega-3 Polyunsaturated Fatty Acids Attenuate Fibroblast Activation and Kidney Fibrosis Involving MTORC2 Signaling Suppression. Sci. Rep. 2017, 7, 46146. [Google Scholar] [CrossRef]
- Tokumaru, K.; Imafuku, T.; Satoh, T.; Inazumi, T.; Hirashima, S.; Nishinoiri, A.; Nagasaki, T.; Maeda, H.; Sugimoto, Y.; Tanaka, M.; et al. Omega 3 Fatty Acids Attenuate the Acute Kidney Injury to CKD Transition and Renal Fibrosis: Identification of Antifibrotic Metabolites. Kidney360 2024, 5, 1422. [Google Scholar] [CrossRef]
- Ong, K.L.; Marklund, M.; Huang, L.; Rye, K.A.; Hui, N.; Pan, X.F.; Rebholz, C.M.; Kim, H.; Steffen, L.M.; Van Westing, A.C.; et al. Association of Omega 3 Polyunsaturated Fatty Acids with Incident Chronic Kidney Disease: Pooled Analysis of 19 Cohorts. BMJ 2023, 380, e072909. [Google Scholar] [CrossRef] [PubMed]
- Pluta, A.; Strózecki, P.; Kȩsy, J.; Lis, K.; Sulikowska, B.; Odrowaz-Sypniewska, G.; Manitius, J. Beneficial Effects of 6-Month Supplementation with Omega-3 Acids on Selected Inflammatory Markers in Patients with Chronic Kidney Disease Stages 1–3. Biomed. Res. Int. 2017, 2017, 1680985. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Liu, M.; He, P.; Ye, Z.; Xiang, H.; Zhou, C.; Yang, S.; Zhang, Y.; Zhang, Y.; Huang, Y.; et al. Habitual Fish Oil Supplementation, Genetic Susceptibility of Kidney Stones and the Risk of New-Onset Kidney Stones. J. Clin. Lipidol. 2024, 18, e116–e124. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 Fatty Acids Supplementation and Oxidative Stress Parameters: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Z.; Zhang, H. Omega-3 Fatty Acid Supplementation as an Adjunctive Therapy in the Treatment of Chronic Kidney Disease: A Meta-Analysis. Clinics 2017, 72, 58–64. [Google Scholar] [CrossRef]
- Laranjinha, I.; Matias, P.; Dickson, J. Magnesium Supplementation to Prevent Recurrence of Renal Stones. Port. J. Nephrol. Hypert 2019, 33, 232–237. [Google Scholar] [CrossRef]
- Penniston, K.L.; Coughlin, M.M.; Jhagroo, R.A. Magnesium Supplementation Increases Urine Magnesium and Citrate in Stone Formers With Hypomagnesuria. J. Ren. Nutr. 2024, S1051-2276(24)00137-7. [Google Scholar] [CrossRef]
- Vermeulen, E.A.; Vervloet, M.G. Magnesium Administration in Chronic Kidney Disease. Nutrients 2023, 15, 547. [Google Scholar] [CrossRef]
- Panta, R.; Regmi, S. Role of Magnesium, Effects of Hypomagnesemia, and Benefits of Magnesium Supplements in Cardiovascular and Chronic Kidney Diseases. Cureus 2024, 16, e64404. [Google Scholar] [CrossRef]
- Taheri, M.; Jalali, S.; Borumandnia, N.; Tavasoli, S.; Basiri, A.; Taheri, F. Effect of Magnesium Oxide or Citrate Supplements on Metabolic Risk Factors in Kidney Stone Formers with Idiopathic Hyperoxaluria: A Randomized Clinical Trial. Magnes. Res. 2024, 37, 12–21. [Google Scholar] [CrossRef]
- Shringi, S.; Raker, C.A.; Tang, J. Dietary Magnesium Intake and Kidney Stone: The National Health and Nutrition Examination Survey 2011–2018. Rhode Isl. Med. J. 2023, 106, 20–25. [Google Scholar] [CrossRef]
- Pendón-Ruiz de Mier, M.V.; Santamaría, R.; Moyano-Peregrín, C.; Gordillo, J.E.; Salmoral-Chamizo, A.; López-López, I.; Rodelo-Haad, C.; Valle, C.; Membrives-González, C.; López-Ruiz, D.J.; et al. Bone and Vascular Effects of Magnesium Supplements in CKD Patients (the MagicalBone Pilot Study). Nefrología 2024, 44, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Evers, I.; Cruijsen, E.; Kornaat, I.; Winkels, R.M.; Busstra, M.C.; Geleijnse, J.M. Dietary Magnesium and Risk of Cardiovascular and All-Cause Mortality after Myocardial Infarction: A Prospective Analysis in the Alpha Omega Cohort. Front. Cardiovasc. Med. 2022, 9, 936772. [Google Scholar] [CrossRef] [PubMed]
- Halawa, N.; Elsaid, T.W.; El Wakeel, L.M.; Shawki, M.A. Impact of Magnesium Supplementation on Clinical Outcome and Disease Progression of Patients with Diabetic Nephropathy: A Prospective Randomized Trial. Ther. Adv. Chronic Dis. 2023, 14, 20406223231214641. [Google Scholar] [CrossRef]
- Robijn, S.; Vervaet, B.A.; Hoppe, B.; D’Haese, P.C.; Verhulst, A. Lanthanum Carbonate Inhibits Intestinal Oxalate Absorption and Prevents Nephrocalcinosis after Oxalate Loading in Rats. J. Urol. 2013, 189, 1960–1966. [Google Scholar] [CrossRef]
- Pozdzik, A.; David, C.; Vekeman, J.; Tielens, F.; Daudon, M. Lanthanum Carbonate to Control Plasma and Urinary Oxalate Level in Type 1 Primary Hyperoxaluria? IJU Case Rep. 2021, 4, 235–238. [Google Scholar] [CrossRef]
- Asnaashari, P.; Kar, P.M.; Borgan, S.; Karasik, O. Prevention of Recurrent Calcium Phosphate Stones in a Patient Undergoing Renal Replacement Therapy: A Case Report and Literature Review on Renal Stone Prevention Strategies. Hemodial. Int. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Haley, W.E.; Enders, F.T.; Vaughan, L.E.; Mehta, R.A.; Thoman, M.E.; Vrtiska, T.J.; Krambeck, A.E.; Lieske, J.C.; Rule, A.D. Kidney Function After the First Kidney Stone Event. Mayo Clin. Proc. 2016, 91, 1744–1752. [Google Scholar] [CrossRef]
| GFR Category | Dominant Stone Types | Biochemical Changes | Key Drivers |
|---|---|---|---|
| G1–G2 |
|
|
|
| G3–G5 |
|
|
|
| Strategy | AUA/EAU Guideline Recommendations | Stone Prevention Benefit | CKD Progression Impact | CKD-Specific Risks/Considerations |
|---|---|---|---|---|
| Urological Interventions | ||||
| ESWL | 1st-line for small-to-medium renal stones (<20 mm) in normal KF; no CKD-specific guidance | Fragments stones, reduces obstruction | Mixed evidence; may cause short-term kidney function changes | ↓ corticomedullary differentiation, ↓ parenchymal thickness; AKI risk tied to GFR category |
| PCNL | 1st-line for large/complex stones (>20 mm) in normal KF; lacks CKD endorsement | Clears large stones; stabilizes/improves GFR in CKD | Can improve GFR in CKD patients over time | AKI risk; ↑ bleeding/infection in GFR category G4–G5 |
| URS | 1st-line for ureteral stones in normal KF; limited CKD data | Relieves ureteral obstruction | AKI may occur, influenced by GFR category and comorbidities | Mucosal injury, fibrosis; infection risk |
| Ureteral Stenting | Adjunct to stone management; no CKD-specific guidance | Supports healing post-obstruction | Minimal long-term impact on CKD development | 2–3x UTI risk; biofilm-related infections |
| Fluid Intake | ||||
| Water | 2.5–3 L/d 1st-line for stone prevention in normal KF; CKD adjustment needed | Reduces recurrence by 50–60%; each 500 mL increase lowers risk | Slows GFR decline in early CKD; may worsen in advanced CKD with excess | Volume overload in G3–G5; tailored to 1.5–2 L/d (G3), 1–1.5 L/d (G4–G5) |
| Citrus-Based Fluids | Supports stone prevention via citrate in normal KF; no CKD-specific guidance | ↑ Urinary citrate (50–100 mL/d); may ↓ recurrence | Limited direct impact; potassium may complicate advanced CKD | Hyperkalemia risk (GFR < 30) |
| Caffeinated Beverages | May reduce stone risk in normal KF; no CKD-specific guidance | May ↓ stone risk via urine dilution/altered chemistry | Minimal direct effect; unclear in CKD | Caffeine/sugar load; requires monitoring in CKD |
| Dietary Modifications | ||||
| Sodium (<2 g/d) | 1st-line for stone prevention; aligns with CKD HTN management | ↓ Urinary Ca2+ 20–40 mg/d; ↓ Ca stone risk | Reduces BP and proteinuria, aiding early CKD | Hyponatremia (10–30% in G5); |
| Oxalate (<100 mg/d) | 1st-line for CaOx stones; CKD risks noted | ↓ Urinary oxalate 20–40% | High oxalate worsens CKD; plant-based diets may mitigate | Limits fiber/antioxidants; ↑ oxalate absorption if Ca2+ low |
| Citrate (50–100 mg/d) | 1st-line for stone inhibition; CKD K+ caution | ↓ Recurrence by 25% | Limited direct effect; supports kidney health indirectly | Hyperkalemia risk (GFR < 30); K+ ↑ 0.2–0.4 mmol/L |
| Protein (0.8 g/kg/d) | 1st-line for UA/Ca stones; CKD protein restriction supported | ↓ UA/Ca2+ | Slows progression by reducing uremic toxins | PEW (30–40% in advanced CKD) |
| Phosphate (800–1000 mg/d) | General stone prevention; KDIGO tailors for CKD-MBD | Indirect via CKD-MBD reduction | Reduces CKD-MBD progression | ↑ Oxalate absorption 10–20% if Ca2+ low |
| Pharmacological Interventions | ||||
| Thiazides (25–50 mg/d) | 1st-line for hypercalciuria in normal KF; off-label in CKD for BP | ↓ Urinary Ca2+ 100–150 mg/d; NOSTONE: no recurrence benefit | Improves BP and albuminuria, potentially renoprotective | Hypokalemia/ hyponatremia (6.6–17%); transient eGFR decline |
| Potassium Citrate (20–60 mEq/d) | 1st-line for UA/CaOx stones; CKD monitoring required | 60–75% ↓ recurrence; ↑ citrate 200–300 mg/d | Hyperkalemia risk may complicate outcomes | Hyperkalemia (11% in G3b-G4); over-alkalinization (pH > 7) |
| Allopurinol (100–300 mg/d) | 2nd-line for UA stones after diet/alkalinization; no CKD endorsement | 50–60% ↓ UA stone risk | Slows GFR decline, offers renoprotection | AKI (5–10% in G3–G4); AHS (2–3%, 20–25% mortality) |
| Step | GFR Category Consideration | Stone Prevention Priority | CKD Preservation Priority | Recommended Action |
|---|---|---|---|---|
| 1. Assess Stone Risk | G1–G2: High recurrence risk | Reduce supersaturation | Monitor GFR decline | Fluids (2.5–3.0 L/day); CaOx: thiazides (25–50 mg/day), probiotics; UA: allopurinol (100–300 mg/day), K+ citrate (20–60 mEq/day); Struvite: antibiotics, ESWL |
| G4–G5: Lower CaOx risk | Target specific stone types | Avoid overload | Fluids (1–1.5 L/day); CaOx: probiotics, magnesium (150–300 mg/day); UA: NaHCO3 (650 mg BID), allopurinol (50–100 mg/day); Struvite: antibiotics, micro-PCNL if obstructing | |
| 2. Evaluate CKD Risk | G3: Moderate progression | Balance efficacy vs. safety | Control BP, limit K+ | Fluids (1.5–2 L/day); CaOx: thiazides (12.5–25 mg/day), probiotics; UA: allopurinol (100 mg/day), K+ citrate (10–20 mEq/day); Struvite: antibiotics, URS |
| G4–G5: High progression | Minimize harm | Prioritize GFR stability | Avoid K+ citrate; CaOx: probiotics, noncalcium binders; UA: NaHCO3 (650 mg BID); Struvite: antibiotics, micro-PCNL if obstructing | |
| 3. Integrate Dual Therapy | All stages: Comorbidities present | Target shared mechanisms | Slow CKD progression | SGLT2i: 10 mg/day (G1–G4); Omega-3: 1–2 g/day; Probiotics: all stages; Magnesium: >350 mg/day (G1–G3), 150–300 mg/day (G4–G5); Noncalcium binders: for CKD-MBD, CaOx |
| 4. Monitor and Adjust | All stages: Dynamic adjustment | Track recurrence (imaging 6–12 mo) | Assess trends (GFR 3–6 mo) | Adjust if K+ > 5.2 mmol/L (G3–G5), Mg2+ > 1.2 mmol/L (G4–G5); albuminuria rises, or edema; reassess 1–3 mo if off-target |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, N. Balancing Stone Prevention and Kidney Function: A Therapeutic Dilemma. J. Clin. Med. 2025, 14, 3678. https://doi.org/10.3390/jcm14113678
Stepanova N. Balancing Stone Prevention and Kidney Function: A Therapeutic Dilemma. Journal of Clinical Medicine. 2025; 14(11):3678. https://doi.org/10.3390/jcm14113678
Chicago/Turabian StyleStepanova, Natalia. 2025. "Balancing Stone Prevention and Kidney Function: A Therapeutic Dilemma" Journal of Clinical Medicine 14, no. 11: 3678. https://doi.org/10.3390/jcm14113678
APA StyleStepanova, N. (2025). Balancing Stone Prevention and Kidney Function: A Therapeutic Dilemma. Journal of Clinical Medicine, 14(11), 3678. https://doi.org/10.3390/jcm14113678







